Abstract
Objective
To investigate neuroradiological and neurophysiological characteristics of patients with dyskinetic cerebral palsy (CP), by using magnetic resonance imaging (MRI), voxel-based morphometry (VBM), diffusion tensor tractography (DTT), and motor evoked potential (MEP).
Methods
Twenty-three patients with dyskinetic CP (13 males, 10 females; mean age 34 years, range 16-50 years) were participated in this study. Functional evaluation was assessed by the Gross Motor Functional Classification System (GMFCS) and Barry-Albright Dystonia Scale (BADS). Brain imaging was performed on 3.0 Tesla MRI, and volume change of the grey matter was assessed using VBM. The corticospinal tract (CST) and superior longitudinal fasciculus (SLF) were analyzed by DTT. MEPs were recorded in the first dorsal interossei, the biceps brachii and the deltoid muscles.
Results
Mean BADS was 16.4±5.0 in ambulatory group (GMFCS levels I, II, and III; n=11) and 21.3±3.9 in non-ambulatory group (GMFCS levels IV and V; n=12). Twelve patients showed normal MRI findings, and eleven patients showed abnormal MRI findings (grade I, n=5; grade II, n=2; grade III, n=4). About half of patients with dyskinetic CP showed putamen and thalamus lesions on MRI. Mean BADS was 20.3±5.7 in normal MRI group and 17.5±4.0 in abnormal MRI group. VBM showed reduced volume of the hippocampus and parahippocampal gyrus. In DTT, no abnormality was observed in CST, but not in SLF. In MEPs, most patients showed normal central motor conduction time.
Conclusion
These results support that extrapyramidal tract, related with basal ganglia circuitry, may be responsible for the pathophysiology of dyskinetic CP rather than CST abnormality.
Keywords: Cerebral palsy, Diffusion tensor imaging, Motor evoked potentials, Magnetic resonance imaging
INTRODUCTION
Dyskinetic cerebral palsy (CP) is characterized by involuntary, uncontrolled, recurring and occasionally stereotyped movements in which the primitive reflex patterns predominate and muscle tone varies [1]. Major etiological factor in dyskinetic CP is birth asphyxia, which makes it more homogeneous characteristics compared to other types of CP. The prevalence of dyskinetic CP varies widely from 6% to 13%. However, recent trends in prevalence have been reported to be increasing or as similar to previous studies [2,3,4]. The discrepancy of dyskinetic CP prevalence among studies may be due to a lack of consistent diagnostic criteria. Dyskinetic CP involves clinical presentations, such as dystonia, which is difficult to measure quantitatively. Various measures, such as the Barry-Albright Dystonia Scale (BADS), have been developed. However, application of these tools are limited for the shortage of understanding of its pathophysiology [5,6], which makes it difficult to develop consistent diagnostic criteria. Although the pathophysiology of dyskinetic CP could be clarified through neuroradiological and neurophysiological studies [1,7,8], very few studies have attempted to investigate this. No studies to date have used the recent advanced neuroimaging technology, such as diffusion tensor tractography (DTT) and voxel-based morphometry (VBM).
In the past, brain computer tomography (CT) or conventional brain magnetic resonance imaging (MRI) was mainly used for neuroimaging to investigate the abnormality of CP. Some of the previous studies have reported that patients with dyskinetic CP typically presented with lesions in the basal ganglia and thalamus on conventional brain MRI. However, very few studies examined the exact localization of lesions within the basal ganglia or existence of multiple brain lesions [2,9,10,11,12]. Therefore, this study aimed to localize the precise brain lesions using conventional brain MRI and objectively assess the dystonic severity with BADS, to examine the correlation between existence of brain lesions and dystonic severity.
Furthermore, we applied VBM, a recent advanced neuroimaging technique, to compare and analyze the volume change of grey matter and white matter not only in a particular area of interest, but in the overall brain area between patients with dyskinetic CP and healthy controls [13]. In this study, VBM was used to examine grey matter volume change that could not be detected by conventional brain MRI.
To our best knowledge, there has been no study examining abnormalities in the corticospinal tract (CST) and surrounding white matter using DTT in patients with dyskinetic CP. There are studies using motor evoked potential (MEP), which can non-invasively assess CST function in patient with CP. However, we could not find any studies that included patients with dyskinetic CP only [14]. Therefore, this study used DTT to visualize and quantitatively measure the white matter structure abnormality in patients with dyskinetic CP and confirm the abnormality of CST and superior longitudinal fasciculus (SLF). We combined use of DTT and MEP, a gold standard for neurophysiological examination of the CST integrity.
We performed brain MRI, VBM, DTT, and MEP in patients with dyskinetic CP. The dystonic severity was assessed with BADS to examine the correlation between the severity of clinical symptoms and conventional brain MRI findings, to contribute to understanding of dyskinetic CP pathophysiology.
MATERIALS AND METHODS
Subjects
This study enrolled 23 patients with dyskinetic CP who were treated at the Department of Rehabilitation Medicine of the Chonbuk National University Hospital from October 2008 to February 2012. The diagnosis of dyskinetic CP was based on the Surveillance of Cerebral Palsy in Europe criteria [1]. Dyskinetic CP was defined as 'involuntary, uncontrolled, recurring and occasionally stereotyped movements, in which the primitive reflex patterns predominate and muscle tone varies.' We also included patients with dystonia at least in the neck and trunk on BADS and excluded patients with spastic CP [5]. This study included 13 male and 10 female patients, whose mean age was 33.7±7.8 years. For VBM and DTT, 23 healthy controls with matched age and sex were recruited. The control group included 13 men and 10 women, and their mean age was 27.5±8.4 years. This study was conducted under the approval of the Ethical Committee of the Chonbuk National University Hospital.
Methods
GMFCS and BADS
Gross motor function was assessed using the Gross Motor Function Classification System (GMFCS). BADS was used to assess the dystonic severity. BADS is a 5-point ordinal scale with a score range from 0 to 4 and evaluates the following eight body regions: 1) eyes: prolonged eyelid spasms and/or forced eye deviations; 2) mouth: grimacing, clenched or deviated jaw, forced open mouth and/or forceful tongue thrusting; 3) neck: pulling of the neck into any plane of motion; 4) trunk: pulling of the trunk into any plane of motion; 5) upper extremities: sustained muscle contractions, causing abnormal posturing of the upper extremities; and 6) lower extremities: sustained muscle contractions, causing abnormal posturing of the lower extremities. The total score is calculated as the sum of the eight region scores, and the maximum total score is 32, with a higher score indicating more severe dystonia [5].
Brain MRI
Using 3.0-T Siemens Verio scanner (Siemens Healthcare, Erlangen, Germany), we obtained axial T1- and T2-weighted images and coronal and sagittal T2-weighted image. Analysis of brain MRI was performed by a radiologist who was blinded to all clinical information. We modified Krageloh-Mann's classification system to grade the severity of lesions as follows [15]: level I, a single lesion involving either the thalamus or putamen; level II, lesions simultaneously involving both the thalamus and putamen; and level III, lesions simultaneously involving thalamus, putamen, and white matter.
VBM
All brain MRI data were adjusted with the anterior commissure-posterior commissure line at the center using ANALYZE ver. 7.5 (Mayo Foundation, New York, NY, USA). MATLAB 7.6 (MathWorks, Natick, MA, USA), SPM8 (http://www.fil.ion.ucl.ac.uk/spm/; Wellcome Trust Centre for Neuroimaging, London, UK) and VBM8 toolbox (http://dbm.neuro.uni-jena.de/vbm.html) were also used. The brain images of the dyskinetic CP patients and healthy controls were spatially normalized on the Montreal Neurological Institute template, provided by SPM8 to create the standard template. This standard template was convoluted with Gaussian kernel of 8-mm full width half maximum for smoothing. We then spatially normalized the original images to this standard template and converted the normalized images into the pixel unit of 2.0×2.0×2.0 mm3. These images were extracted and categorized into grey matter, white matter, and cerebrospinal fluid, using the software inherent in SPM8. Using a matrix that non-linearly normalized each image against the standard template, we calculated the Jacobian determinant to reflect the local volume increase and contraction. We then multiplied this value to each image of the grey matter. These adjusted images were convoluted with 12-mm Gaussian kernel for smoothing. We analyzed the grey matter images using VBM [16].
DTT
Diffusion tensor imaging was obtained with single-shot spin echo-planar imaging sequence with two diffusion sensitizing gradients. To shorten the scan time, the Generalized Autocalibrating Partially Parallel Acquisitions (GRAPPA) technique was applied. This technique has the advantage of reducing image distortion resulting from echo-planar imaging sequence. We also applied automated image registration program to correct image distortion as much as possible. Image parameters were set as follows: echo time=93.1 ms; repetition time=7,900 ms; field of view=230 mm2; matrix size=128×128; number of excitation=1; and b-value=1,000 s/mm2. We obtained 47 images parallel to the anterior commissure-posterior commissure line at 30 mm thickness and from 30 different diffusions without a gap. For fiber tracking, we used DTIStudio (Johns Hopkins University, Baltimore, MD, USA; http://cmrm.med.jhmi.edu), which uses continuous tracking algorithm for fiber tracking; and it was terminated when fractional anisotropy (FA) was below 0.25 or the angle was less than 70° [17]. Upper areas of interest were set in the blue section anterior to the upper pons, and lower areas of interest were set in the blue section anterior to the lower pons on a 2-dimensional FA color map to evaluate CST. Only fiber that passed the bilateral areas of interest were tracked. To evaluate SLF, the area of interest was set in the oval, green section where SLF was most clearly shown. The mean FA value and number of fibers of CST and SLF were used in DTT [18].
MEP
Medtronic Keypoint (Medtronic Inc., Skovlunde, Denmark) was used for electromyographic examinations, and Magpro (Medtronic Inc.) was used for magnetic stimulation. MEP was measured in the first dorsal interossei (FDI), biceps brachii (BB) and deltoid (Del). The international 10-20 system was used for mapping the locations of stimulation. The optimal stimulation coordinate was selected if MEP of 50 µV or more was induced at least 5 times from over 10 stimulations at the lowest excitation threshold. Latency and amplitude were obtained by averaging the values from four stimulations at 120% of excitation threshold at the optimal stimulation coordinate. Central motor conduction time (CMCT) was defined as the difference between MEP latency and MEP latency induced from cervical magnetic stimulation [19]. We used normal values of healthy adults reported in previous studies as reference [20].
Statistical analysis
SPSS ver. 18.0 for Windows (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. We assessed the difference in BADS scores between ambulatory vs. non-ambulatory groups and patients with abnormal findings on MRI vs. patients without abnormal findings on MRI, using independent t-test. The p-values below 0.05 were defined as statistically significant. Analysis of covariance (ANCOVA) was used for statistical analysis of VBM. We performed a comparative analysis of grey matter increase and decrease between the patient group and control group. To adjust for type 1 error in statistical processing, familywise error rate (Bonferroni correction) was used at the statistical significance of p=0.05. The minimum pixel count was set as 300 pixels [21]. To compare DTT and MEP findings between dyskinetic CP patients and healthy controls, independent samples t-test was used, and p<0.05 was defined as statistically significant.
RESULTS
GMFCS and BADS
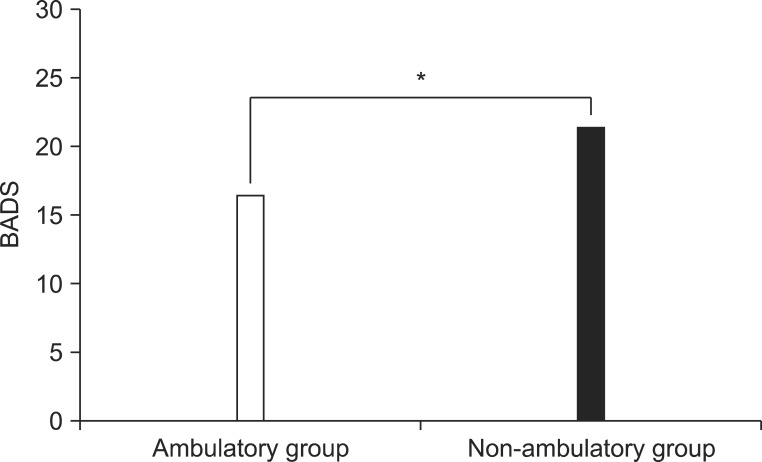
According to GMFCS classification, 1 patient was level 1, 9 were level 2, 1 was level 3, 11 were level 4, and 1 was level 5 (Table 1). The mean BADS scores of the dyskinetic CP group was 20.5±4.8, with the neck showing the highest BADS score of 3.0±0.7 (Table 2). Categorizing levels 1, 2, and 3 as ambulatory status and levels 4 and 5 as non-ambulatory status resulted in the mean BADS score of 16.4±5.0 for the ambulatory group and 21.3±3.9 for the non-ambulatory group. The mean BADS score was significantly higher in the non-ambulatory group (Fig. 1).
Table 1.
Clinical characteristics, MEPs and MRI findings in patients with dyskinetic cerebral palsy
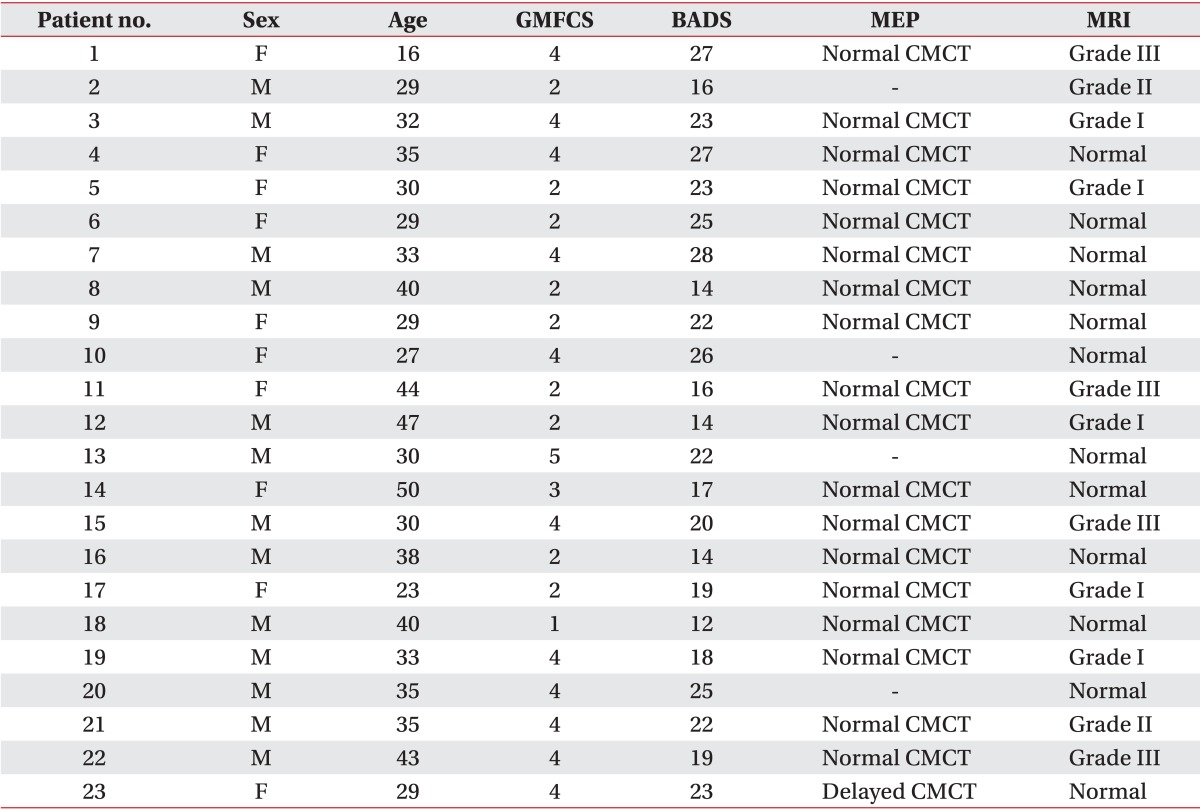
MEP, motor evoked potential; MRI, magnetic resonance imaging; GMFCS, Gross Motor Function Classification System; BADS, Barry-Albright Dystonia Scale; CMCT, central motor conduction time; grade I, one of thalamus or putamen only involved; grade II, thalamus and putamen involved; grade III, thalamus, putamen and white matter involved.
Table 2.
Total score and subscore of BADS in patients with dyskinetic cerebral palsy
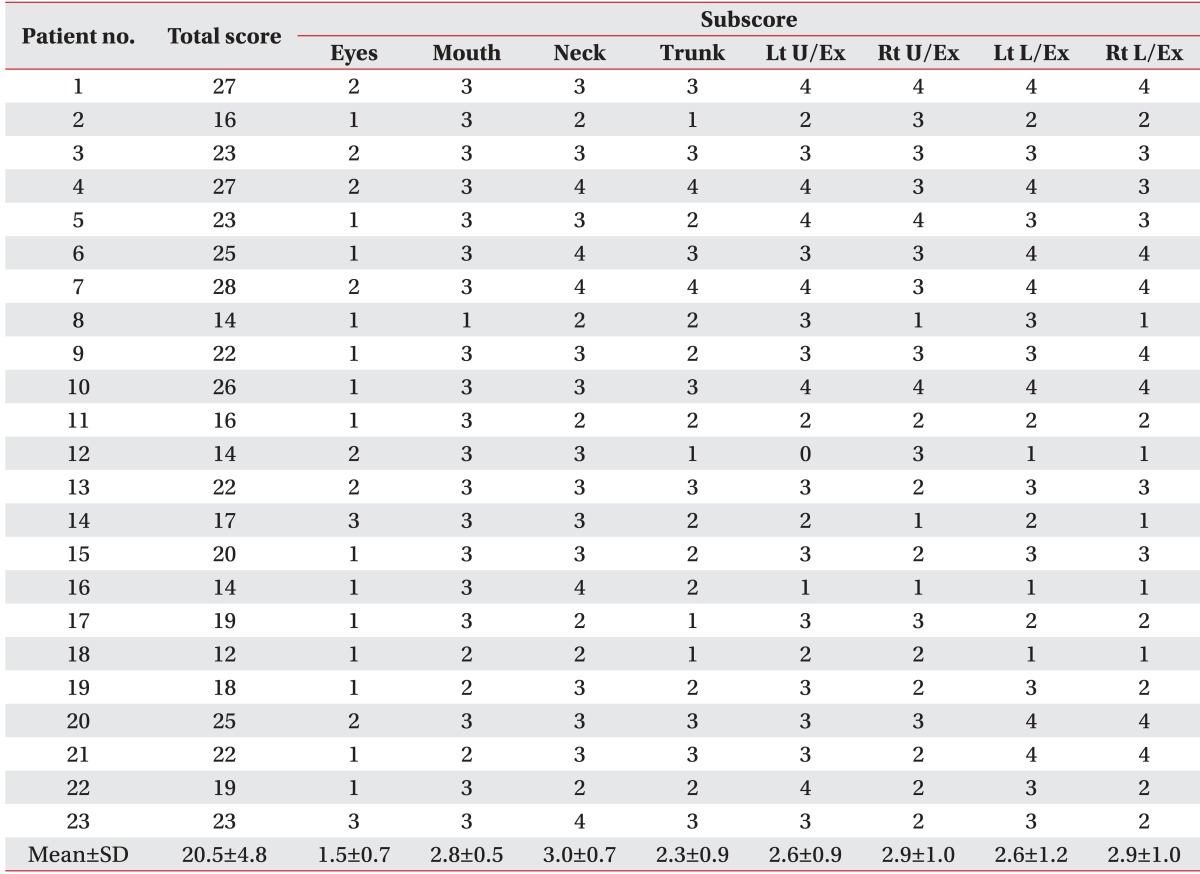
BADS, Barry-Albright Dystonia Scale; Lt, left; Rt, right; U/Ex, upper extremity; L/Ex, lower extremity.
Fig. 1.
There was a significant difference in Barry-Albright Dystonia Scale (BADS) scores between the ambulatory group and non-ambulatory group. *p<0.05.
Brain MRI
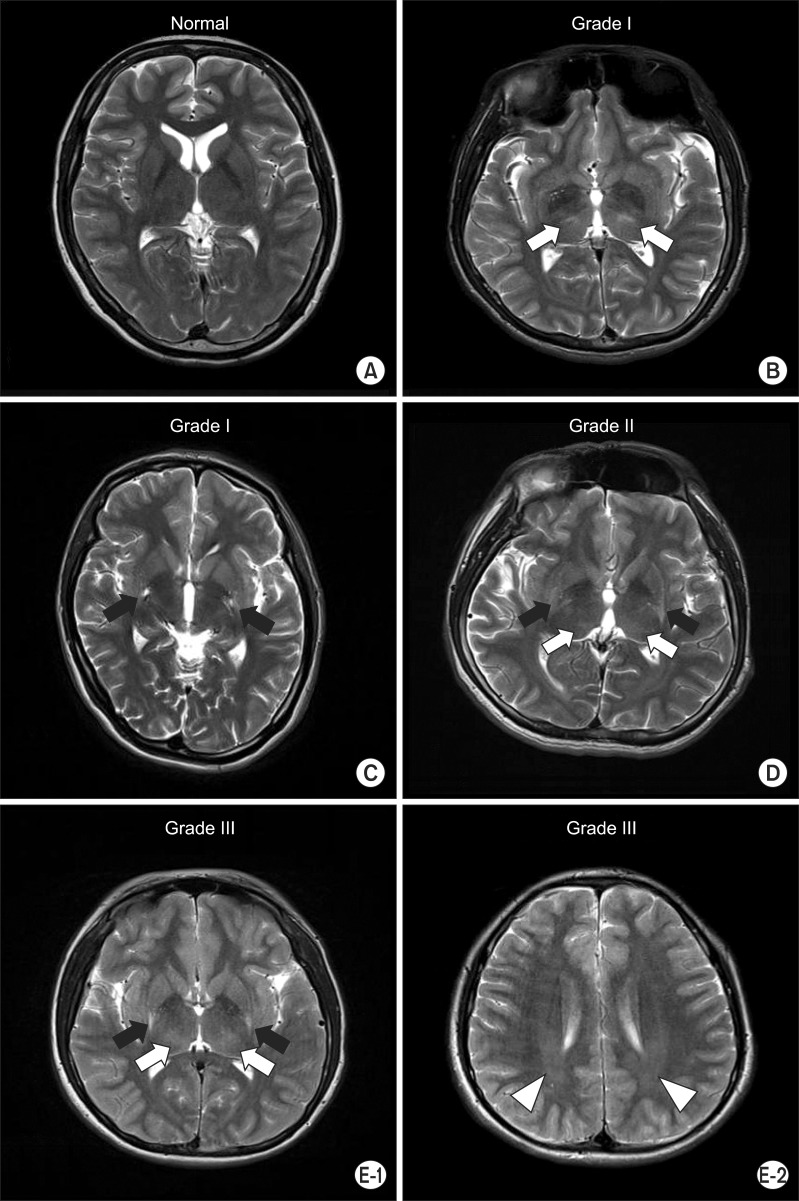
The brain MRI findings were normal in 12 out of 23 dyskinetic CP patients. Five had a single lesion that localized to either thalamus or putamen, two had lesions involving both thalamus and putamen simultaneously, and four had lesions involving thalamus, putamen, and white matter simultaneously (Table 1, Fig. 2). The mean BADS of normal brain MRI group and abnormal MRI group was 20.3±5.7 and 17.5±4.0, respectively, with no statistically significant difference.
Fig. 2.
Axial T2-weighted image. (A) No evidence of abnormality in patient no. 9. (B) Bilateral focal hyperintensities in the thalamus (white arrows) in patient no. 19. (C) Bilateral focal hyperintensities in the putamen (black arrows) in patient no. 17. (D) Bilateral focal hyperintensities in the putamen (black arrows) and thalamus (white arrows) in patient no. 21. (E-1) Bilateral focal hyperintensities in the putamen (black arrows) and thalamus (white arrows) in patient no. 1. (E-2) Bilateral diffuse hyperintensities in the periventricular white matter (white arrow heads) in patient no. 1.
VBM
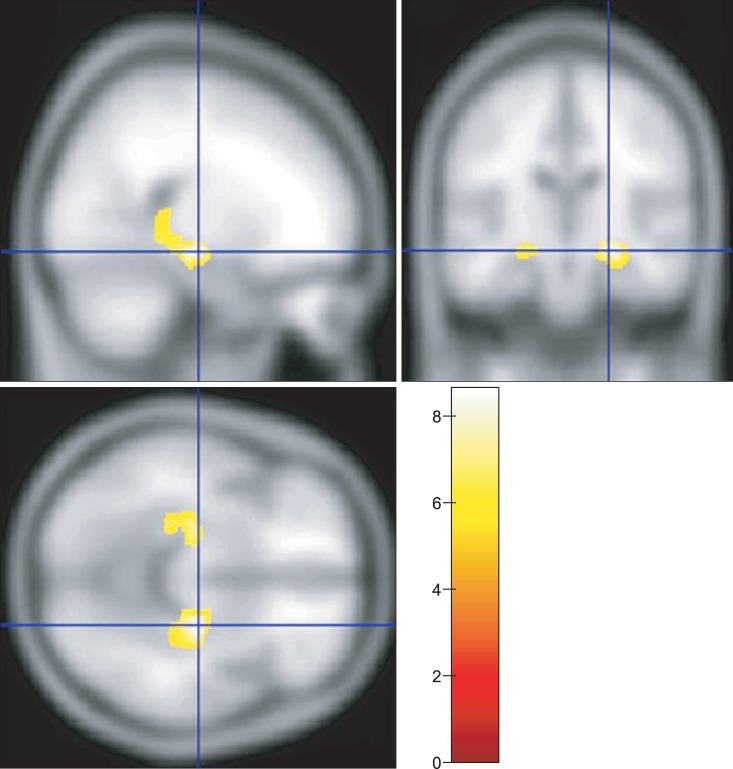
In dyskinetic CP group, areas with significant reduction in grey matter volume compared to control group were the hippocampus and parahippocampal gyrus on VBM. There were no significant difference in the basal ganglia and thalamus (Fig. 3).
Fig. 3.
Voxel-based morphometry analysis showed the regions with significantly reduced grey matter volumes in patients with dyskinetic cerebral palsy in yellow color (p<0.05, corrected for multiple comparison, familywise error rate).
DTT
The number of fiber and FA value of CST showed no significant difference between dyskinetic CP group and control group (Table 3). The FA value of SLF was significantly lower in dyskinetic CP group compared to control group. Dyskinetic CP group had less fibers compared to control group, however, the difference was not statistically significant (Table 4).
Table 3.
Fractional anisotropy and fiber numbers of corticospinal tracts in diffusion tensor tractography
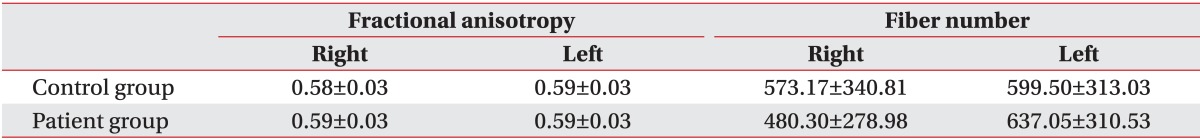
Values are presented as mean±standard deviation.
Table 4.
Fractional anisotropy and fiber numbers of superior longitudinal fasciculus in diffusion tensor tractography
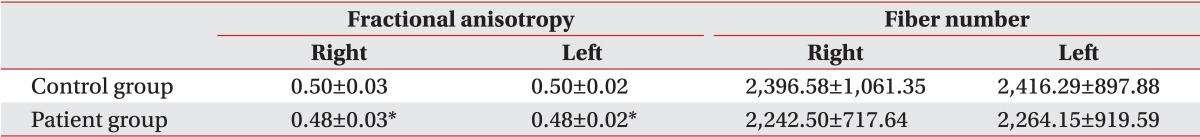
Values are presented as mean±standard deviation.
*p<0.05.
MEP
MEP was not examined in 4 out of 23 dyskinetic CP patients due to lack of cooperation. The mean latency of 19 dyskinetic CP patients who underwent MEP were 21.8±2.5, 13.7±1.8, and 13.1±2.3 ms in FDI, BB, and Del, respectively. These values did not differ significantly from reference values reported in previous studies: 20.9±0.8, 13.8±0.7, and 14.4±0.9 ms [20]. The mean amplitudes were 1,494.2±1,437.2, 1,339.7±1,220.4, and 1,556.5±1,694.2 µV in FDI, BB, and Del, respectively. Compared to the reference values of 3,443.8±1,190.5, 1,977.2±927.2, and 1,767.6±647.3 µV, only the FDI amplitude showed a reduction [20]. CMCT was 8.3±2.3 ms and did not differ significantly from the reference value of 7.6±0.9 ms [20]. When comparing the CMCT of each patient with reference values, abnormal findings were over 2.5 SD higher than the reference value, difference between left and right in CMCT being at least 1.5 ms, or MEP not appearing, only 1 patient showed delayed left CMCT.
DISCUSSION
The brain MRI of patients with dyskinetic CP showed abnormality in the putamen of the basal ganglia, thalamus, and periventricular white matter, and there was no difference in BADS score between normal MRI group and abnormal MRI group. VBM also showed volume reduction of hippocampus and parahippocampal gyrus in dyskinetic CP group compared to control group. In contrast, MEP and DTT results showed normal functional integrity of CST in dyskinetic CP group. However, in the SLF adjacent to CST, dyskinetic CP group showed low FA value on DTT compared to control group.
In the current study, eleven patients with dyskinetic CP (47.8%) showed abnormality in the putamen, thalamus, and periventricular white matter on brain MRI. This finding is consistent with previous studies which reported that patients with dyskinetic CP typically show lesions in the basal ganglia and thalamus on conventional brain MRI [2,9,10,11,12]. In the paucity of studies examining the exact localization of basal ganglia lesions, we slightly modified Krageloh-Mann's classification system to better localize lesions within the basal ganglia [10]. We were then able to detect lesions in the putamen within the basal ganglia. This is consistent with the report of Yokochi et al. [15] that all patients with athetoid CP had putamen lesions on the conventional brain MRI. All seven patients with basal ganglia lesions had putamen abnormalities in our study. It is known that putamen lesions are mainly caused by perinatal, hypoxic-ischemic brain injury, and globus pallidus lesions generally result from hyperbilirubinemia-related encephalopathy [2,15,22]. Therefore, dyskinetic CP examined in this study is likely to have been caused by perinatal asphyxia. The mechanism in which perinatal asphyxia leads to injury in the basal ganglia and thalamus may be that these two brain structures are particularly vulnerable to hypoxic-ischemic injury due to high metabolic demands during the perinatal period [23].
A number of patients (12 patients, 52.2%) in this study showed normal brain MRI, as similarly reported in some previous studies. Yokochi et al. [15] reported that 7 out of 22 patients with athetoid CP did not show abnormal brain MRI; and Maegaki et al. [24] reported that 3 patients with athetoid CP showed normal brain CT or MRI findings. When interpreting the results of the above studies, one considers that previous studies have used brain CT techniques which are unlikely to have accurately reflected intracerebral abnormalities on brain MRI, as they are less advanced and therefore susceptible to a relatively high false-negative rate. However, this study showed a similar proportion of patients with abnormal findings on brain MRI as reported in previous studies. Considering the superiority of the 3.0-T brain MRI used in this study, such similar outcomes may suggest that brain MRI may not be the optimal diagnostic tool for dyskinetic CP.
Analysis of the relation between presence of lesions on brain MRI and dystonic severity showed no significant difference in BADS. Such a result is in contrast with the study of Krageloh-Mann et al. [10], who reported a correlation between the degree of lesions on brain MRI and clinical symptoms. This may be due to the fact that we only examined patients with dyskinetic CP and excluded those with spastic CP. Krageloh-Mann et al. [10] also reported that purely dyskinetic CP was only seen with the mild pattern. We assume, therefore, that the degree of lesions on brain MRI of patients with dyskinetic CP may not correlate to dystonic severity. We focused on the correlation between the BADS score and ambulation, and the BADS score was significantly higher in the non-ambulatory group compared to the ambulatory group. This may suggest that the more severe dystonia is related to the worse prognosis of the ambulatory status.
We could not analyze the correlation between the grade of lesion on brain MRI and BADS score due to a small number of subjects in this study. Further studies with a larger number of subjects on this topic will be needed.
This study showed a volume reduction in the hippocampus and parahippocampal gyrus in dyskinetic CP group compared to control group on VBM, whereas, the basal ganglia and thalamus showed no volume reduction. Considering that the hippocampus and parahippocampal gyrus are vulnerable to hypoxic-ischemic injury, just as the basal ganglia and thalamus, this volume reduction may have been caused by hypoxic-ischemic injury. However, there is another possibility that the volume reduction may have resulted from secondary developmental atrophy, as a volume change is confined to these structures. In other words, considering that the hippocampus and parahippocampal gyrus are structures related with learning, memory and emotions, etc. [25,26], this volume change may be caused by cognitive impairments and delayed development as well as abnormal social interactions. Therefore, in a neuroimaging study in dyskinetic CP, VBM will be a useful technique for detecting subtle structural brain lesions in patients with normal findings on conventional MRI.
Reviewing previous studies that examined the volume change of brain using VBM, there was no study including patients with dyskinetic CP. Some VBM studies carried out in dystonic patients reported volume reduction in the putamen, primary, sensory motor cortex, thalamus and cerebellum, etc. [27,28].
Based on DTT results, CST integrity was preserved in dyskinetic CP group, but SLF was not. These findings suggest underlying pathology of the extrapyramidal tract in dyskinetic CP group. The extrapyramidal tracts such as the cortex-pons-cerebellum-thalamus-cortex circuit or cortex-striatum-globus pallidus-thalamus-cortex circuit control precision, timing, compensatory muscle tone and balance, etc., in motor control. SLF connects the prefrontal lobe, premotor cortex and posterior parietal with anterior putamen and caudate nucleus, and is generally activated by performing a new activity under controlled attention [29]. This may be related not only to the abnormal motor control but also language problems, as commonly seen in patients with dyskinetic CP [2]. Further research is needed to investigate the abnormal brain structure detected on VBM using DTT.
Using MEP, we compared the latency, amplitude and CMCT in dyskinetic CP group with normal references in our previous studies [20], and found no significant differences except for reduced amplitude in FDI. Eighteen out of 19 patients with dyskinetic CP (94.7%) showed normal CMCT, and this is consistent with the report of Muller et al. [14] that 16 patients with extrapyramidal disorder presenting with dystonia and chorea still showed normal CMCT on MEP despite motor impairment. Normal CMCT despite lesions found on brain MRI may indicate that CST integrity is preserved in patients with dyskinetic CP. This result also supports that the pathology of extrapyramidal tract seems to be related with abnormal motor control in dyskinetic CP group. One patient (patient no. 23) with abnormal findings on MEP showed delayed left CMCT. This patient presented with muscle weakness and paresthesia of the left upper limb and was diagnosed with cervical myelopathy on cervical spine MRI and underwent a surgical treatment. This may suggest that MEP study in dyskinetic CP patient is available for follow-up examination to detect cervical myelopathy and carpal tunnel syndrome, as being common musculoskeletal complications in dyskinetic CP patients. The reduced amplitude of FDI may be due to secondary disuse muscular atrophy from impaired hand control in dyskinetic CP group.
This study has following limitations. First, this study only considered dystonia to assess symptom severity of dyskinetic CP. Dyskinetic CP can be subdivided into the dystonia type and choreoathetosis type, and these two subtypes are simultaneously present in many patients [30]. As there was little data on quantitative assessment of choreoathetosis, it was difficult to clinically evaluate this condition as opposed to dystonia. Sanger [31] has mentioned that the pathophysiology of choreoathetosis is more closely related to dystonia than to chorea or athetosis, suggesting that the choreoathetosis type is a form of hyperkinetic dystonia. Therefore, it can be said that objective measures of dystonia represent the clinical symptom severity of dyskinetic CP.
The second limitation of this study is that as pathophysiology of dystonia is known to include sensory impairment as well as motor impairment [31,32,33], it would have been more helpful if SEP was also conducted along with MEP. Future studies examining SEP results of patients with dyskinetic CP will be needed.
The third limitation of this study is that this study was a preliminary study on pathophysiology of dyskinetic CP and as such could not perform sufficient analysis of each imaging technology. The results of this study can provide a basis for future studies comparing characteristics of each brain structure through VBM and DTT between patients and healthy controls. It may contribute to future studies on functional impact of motor nerve control.
In conclusion, about half of the patients with dyskinetic CP showed normal brain MRI findings, and those with abnormal brain MRI showed lesions in the putamen, thalamus, and periventricular white matter. VBM results showed volume reduction of the hippocampus and parahippocampal gyrus. CST was normal on DTT, and CMCT was normal on MEP. Therefore, we thought if a patient with dyskinetic CP shows normal brain MRI finding, VBM can help to detect subtle structural brain lesions. Moreover, as CMCT of patients with dyskinetic CP are shown to be normal on MEP, this will be particularly helpful for follow-up observation of changes in clinical presentation. Using advanced neuroimaging techniques, such as VBM and DTT, will help further our understanding of pathophysiology of dyskinetic CP where conventional brain MRI falls short in identification.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Christine C, Dolk H, Platt MJ, Colver A, Prasauskiene A, Krageloh-Mann I, et al. Recommendations from the SCPE collaborative group for defining and classifying cerebral palsy. Dev Med Child Neurol Suppl. 2007;109:35–38. doi: 10.1111/j.1469-8749.2007.tb12626.x. [DOI] [PubMed] [Google Scholar]
- 2.Himmelmann K, Hagberg G, Wiklund LM, Eek MN, Uvebrant P. Dyskinetic cerebral palsy: a population-based study of children born between 1991 and 1998. Dev Med Child Neurol. 2007;49:246–251. doi: 10.1111/j.1469-8749.2007.00246.x. [DOI] [PubMed] [Google Scholar]
- 3.Himmelmann K, McManus V, Hagberg G, Uvebrant P, Krageloh-Mann I, Cans C, et al. Dyskinetic cerebral palsy in Europe: trends in prevalence and severity. Arch Dis Child. 2009;94:921–926. doi: 10.1136/adc.2008.144014. [DOI] [PubMed] [Google Scholar]
- 4.Andersen GL, Irgens LM, Haagaas I, Skranes JS, Meberg AE, Vik T. Cerebral palsy in Norway: prevalence, subtypes and severity. Eur J Paediatr Neurol. 2008;12:4–13. doi: 10.1016/j.ejpn.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 5.Barry MJ, VanSwearingen JM, Albright AL. Reliability and responsiveness of the Barry-Albright Dystonia Scale. Dev Med Child Neurol. 1999;41:404–411. doi: 10.1017/s0012162299000870. [DOI] [PubMed] [Google Scholar]
- 6.Monbaliu E, Ortibus E, Roelens F, Desloovere K, Deklerck J, Prinzie P, et al. Rating scales for dystonia in cerebral palsy: reliability and validity. Dev Med Child Neurol. 2010;52:570–575. doi: 10.1111/j.1469-8749.2009.03581.x. [DOI] [PubMed] [Google Scholar]
- 7.Ashwal S, Russman BS, Blasco PA, Miller G, Sandler A, Shevell M, et al. Practice parameter: diagnostic assessment of the child with cerebral palsy: report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology. 2004;62:851–863. doi: 10.1212/01.wnl.0000117981.35364.1b. [DOI] [PubMed] [Google Scholar]
- 8.Seo JS, Kim TM, Chae JM, Kim YK, Kim BO. Comparative analysis of developmental assessment, evoked potentials, electroencephalography, and brain MRI in children with cerebral palsy. J Korean Acad Rehabil Med. 2000;24:645–656. [Google Scholar]
- 9.Park CI, Kim SW, Kim YC, Shin JC, Lee JD. Brain MRI and SPECT findings in children with cerebral palsy. J Korean Acad Rehabil Med. 1997;21:1060–1067. [Google Scholar]
- 10.Krageloh-Mann I, Helber A, Mader I, Staudt M, Wolff M, Groenendaal F, et al. Bilateral lesions of thalamus and basal ganglia: origin and outcome. Dev Med Child Neurol. 2002;44:477–484. doi: 10.1017/s0012162201002389. [DOI] [PubMed] [Google Scholar]
- 11.Bax M, Tydeman C, Flodmark O. Clinical and MRI correlates of cerebral palsy: the European Cerebral Palsy Study. JAMA. 2006;296:1602–1608. doi: 10.1001/jama.296.13.1602. [DOI] [PubMed] [Google Scholar]
- 12.Einspieler C, Cioni G, Paolicelli PB, Bos AF, Dressler A, Ferrari F, et al. The early markers for later dyskinetic cerebral palsy are different from those for spastic cerebral palsy. Neuropediatrics. 2002;33:73–78. doi: 10.1055/s-2002-32368. [DOI] [PubMed] [Google Scholar]
- 13.Baron JC, Chetelat G, Desgranges B, Perchey G, Landeau B, de la Sayette V, et al. In vivo mapping of gray matter loss with voxel-based morphometry in mild Alzheimer's disease. Neuroimage. 2001;14:298–309. doi: 10.1006/nimg.2001.0848. [DOI] [PubMed] [Google Scholar]
- 14.Muller K, Homberg V, Aulich A, Lenard HG. Magnetoelectrical stimulation of motor cortex in children with motor disturbances. Electroencephalogr Clin Neurophysiol. 1992;85:86–94. doi: 10.1016/0168-5597(92)90073-k. [DOI] [PubMed] [Google Scholar]
- 15.Yokochi K, Aiba K, Kodama M, Fujimoto S. Magnetic resonance imaging in athetotic cerebral palsied children. Acta Paediatr Scand. 1991;80:818–823. doi: 10.1111/j.1651-2227.1991.tb11955.x. [DOI] [PubMed] [Google Scholar]
- 16.Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14(1 Pt 1):21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- 17.Mori S, Crain BJ, Chacko VP, van Zijl PC. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol. 1999;45:265–269. doi: 10.1002/1531-8249(199902)45:2<265::aid-ana21>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 18.Vernooij MW, Smits M, Wielopolski PA, Houston GC, Krestin GP, van der Lugt A. Fiber density asymmetry of the arcuate fasciculus in relation to functional hemispheric language lateralization in both right- and left-handed healthy subjects: a combined fMRI and DTI study. Neuroimage. 2007;35:1064–1076. doi: 10.1016/j.neuroimage.2006.12.041. [DOI] [PubMed] [Google Scholar]
- 19.Dominkus M, Grisold W, Jelinek V. Transcranial electrical motor evoked potentials as a prognostic indicator for motor recovery in stroke patients. J Neurol Neurosurg Psychiatry. 1990;53:745–748. doi: 10.1136/jnnp.53.9.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yook SW, Park SH, Ko MH, Seo JH. Motor evoked potentials of the upper extremities in healthy children. Ann Rehabil Med. 2011;35:759–764. doi: 10.5535/arm.2011.35.6.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC. A unified statistical approach for determining significant signals in images of cerebral activation. Hum Brain Mapp. 1996;4:58–73. doi: 10.1002/(SICI)1097-0193(1996)4:1<58::AID-HBM4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 22.Kretzschmar K, Ludwig B, Kramer G, Collmann H, Kazner E. Bilateral lesions of the putamina. Neuroradiology. 1986;28:87–91. doi: 10.1007/BF00341775. [DOI] [PubMed] [Google Scholar]
- 23.Krageloh-Mann I. Imaging of early brain injury and cortical plasticity. Exp Neurol. 2004;190(Suppl 1):S84–S90. doi: 10.1016/j.expneurol.2004.05.037. [DOI] [PubMed] [Google Scholar]
- 24.Maegaki Y, Maeoka Y, Ishii S, Eda I, Ohtagaki A, Kitahara T, et al. Central motor reorganization in cerebral palsy patients with bilateral cerebral lesions. Pediatr Res. 1999;45(4 Pt 1):559–567. doi: 10.1203/00006450-199904010-00016. [DOI] [PubMed] [Google Scholar]
- 25.Kohler S, Black SE, Sinden M, Szekely C, Kidron D, Parker JL, et al. Memory impairments associated with hippocampal versus parahippocampal-gyrus atrophy: an MR volumetry study in Alzheimer's disease. Neuropsychologia. 1998;36:901–914. doi: 10.1016/s0028-3932(98)00017-7. [DOI] [PubMed] [Google Scholar]
- 26.Richardson MP, Strange BA, Dolan RJ. Encoding of emotional memories depends on amygdala and hippocampus and their interactions. Nat Neurosci. 2004;7:278–285. doi: 10.1038/nn1190. [DOI] [PubMed] [Google Scholar]
- 27.Obermann M, Yaldizli O, De Greiff A, Lachenmayer ML, Buhl AR, Tumczak F, et al. Morphometric changes of sensorimotor structures in focal dystonia. Mov Disord. 2007;22:1117–1123. doi: 10.1002/mds.21495. [DOI] [PubMed] [Google Scholar]
- 28.Delmaire C, Vidailhet M, Elbaz A, Bourdain F, Bleton JP, Sangla S, et al. Structural abnormalities in the cerebellum and sensorimotor circuit in writer's cramp. Neurology. 2007;69:376–380. doi: 10.1212/01.wnl.0000266591.49624.1a. [DOI] [PubMed] [Google Scholar]
- 29.Riva D, Njiokiktjien C. Brain lesion localization and developmental functions. Montrouge: John Libbey Eurotext; 2010. [Google Scholar]
- 30.Monbaliu E, Ortibus E, De Cat J, Dan B, Heyrman L, Prinzie P, et al. The Dyskinesia Impairment Scale: a new instrument to measure dystonia and choreoathetosis in dyskinetic cerebral palsy. Dev Med Child Neurol. 2012;54:278–283. doi: 10.1111/j.1469-8749.2011.04209.x. [DOI] [PubMed] [Google Scholar]
- 31.Sanger TD. Pathophysiology of pediatric movement disorders. J Child Neurol. 2003;18(Suppl 1):S9–S24. doi: 10.1177/0883073803018001S0401. [DOI] [PubMed] [Google Scholar]
- 32.Hallett M. Neurophysiology of dystonia: the role of inhibition. Neurobiol Dis. 2011;42:177–184. doi: 10.1016/j.nbd.2010.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berardelli A, Rothwell JC, Hallett M, Thompson PD, Manfredi M, Marsden CD. The pathophysiology of primary dystonia. Brain. 1998;121(Pt 7):1195–1212. doi: 10.1093/brain/121.7.1195. [DOI] [PubMed] [Google Scholar]