Abstract
Minimally invasive surgery, which has been extensively used to treat gastric adenocarcinoma, is now regarded as one of the standard treatments for early gastric cancer, and its suitability for advanced gastric cancer is being investigated. The use of cutting-edge techniques for minimally invasive surgery enables surgeons to deliver various treatment options to minimize a patient's distress and to maintain oncologic safety. Ongoing multicenter prospective studies aim to validate the efficacy of these surgical techniques and to expand the indications of minimally invasive surgery for the treatment of gastric cancer. In this review, we summarize the current status and issues regarding minimally invasive surgery for the treatment of gastric cancer.
Keywords: Stomach neoplasms, Gastrectomy, Laparoscopy, Robotics
INTRODUCTION
Minimally invasive surgery (MIS) for gastric cancer has gained popularity because it provides better short-term (e.g., reduced pain) and long-term (e.g., increased quality of life) results. A minimally invasive approach for the treatment of early gastric cancer (EGC) is a safe and efficient alternative to open gastrectomy.1 The proportion of patients treated using this approach is relatively smaller than the proportion of patients with EGC. However, surgeons experienced in minimally invasive gastrectomy techniques have suggested that they could be successfully applied to the treatment of advanced gastric cancer (AGC).2,3,4,5,6,7,8
To overcome technical difficulties, and to achieve more precise and effective procedures, surgeons are enthusiastically adopting emerging techniques and instruments. Advanced and improved techniques enable surgeons to endeavor to expand their indications to various types of surgical options that provide faster recovery and decrease distress after gastrectomy, while also preserving oncologic safety. In this article, we described the current status, issues of debate, and future perspectives regarding MIS for the treatment of gastric cancer.
INDICATION OF MIS FOR GASTRIC CANCER
The generally accepted indication of MIS for gastric cancer is that the patient has a clinical diagnosis of EGC without evidence of lymph node (LN) metastasis, except those who have lesions suitable for endoscopic treatment. The expanded criteria for endoscopic procedures have created some questions about when a patient should be treated using endoscopy or MIS.9,10,11 In any case, it is obvious that the role of wedge resection for EGC has been decreased due to widespread use of endoscopic approach.12 We must first reach a consensus regarding the objective risk rate of recurrence or LN metastasis compared to the results from conventional surgery, which often require additional surgical resection (including LN dissection) after the endoscopic approach is used. Determining how to increase the accuracy of the preoperative diagnosis is also crucial.
Clinical T1 cancer with perigastric LN involvement and serosa-negative gastric cancer without LN metastasis are also regarded as expanded indications for the use of MIS.4,5,13,14 These specific groups of patients could be treated in randomized controlled trials comparing different approaches. Discussions about MIS generally focus on the technical aspects. However, inaccurate preoperative staging and a subsequent limited extent of surgery is an important issue. Many studies have shown that MIS does not increase peritoneal seeding or port site metastasis, even when used in cases of AGC.3,15,16,17,18,19
MIS FOR EARLY GASTRIC CANCER
Multicenter prospective randomized clinical trials (RCTs) are awaiting the final results to confirm the oncologic safety of laparoscopic surgery for gastric cancer. However, the role of MIS for EGC is obvious and is widely accepted, especially in East Asia. The results of several RCTs have revealed the feasibility and safety of MIS for EGC.1,17,20,21,22,23 The results of recent meta-analyses indicated that laparoscopic distal gastrectomy is a safe alternative to open conventional surgery. Complication rates are lower, there is less blood loss, hospital stays are shorter, and quality of life increases without compromising long-term outcomes.24,25 However, the number of retrieved LNs was slightly fewer and the surgery time was longer.
We anticipate similar results from the ongoing multicenter RCTs in Korea and Japan (Korean Laparoscopic Gastrointestinal Surgery Study [KLASS] 01 trials and Japan Clinical Oncology Group [JCOG] 0912 trials). After the benefits of MIS are confirmed in these studies, laparoscopic surgery for EGC could be recognized, not as an investigational approach, but as a standard procedure in clinical practice. With the accumulated and extensive experiences and improved surgical instruments, some surgeons are now focusing on reducing the patient's distress by using reduced ports or a single incision approach for EGC.26,27 Extracorporeal anastomoses using minilaparotomy is being replaced by intracorporeal anastomoses and results in a totally laparoscopic gastrectomy.28,29,30,31,32 Several meta-analyses comparing laparoscopic distal gastrectomy versus open distal gastrectomy have consistently demonstrated that there is less blood loss, hospital stays are shorter, morbidity rates are lower, and postoperative mortality are similar, when laparosocpic surgery is used, although operative time is longer and there are fewer retrieved LN (Table 1).24,25,33,34,35,36 Improving the accuracy of the preoperative diagnosis is an important issue that must be resolved before this approach is used for AGC, because the numbers of cases that are actually AGC have been increasing as the use of this approach has become more prevalent.
Table 1.
Meta-Analysis of Laparoscopic versus Open Distal Gastrectomy for the Treatment of Gastric Cancer
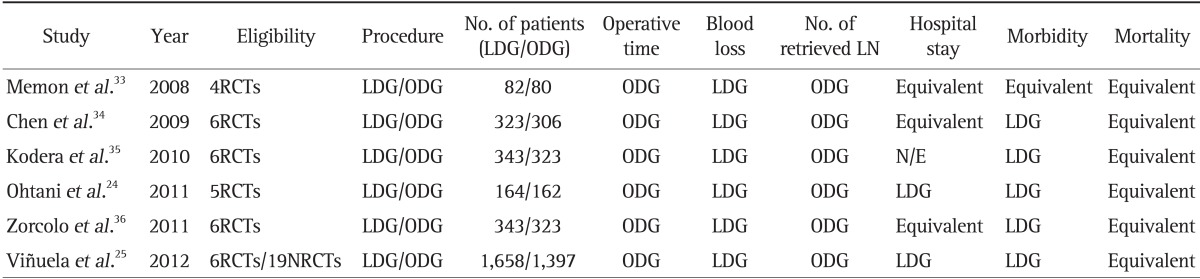
The favored group for each value is shown.
LDG, laparoscopic distal gastrectomy; ODG, open distal gastrectomy; LN, lymph node; RCTs, randomized controlled trials; N/E, not evaluated; NRCTs, nonrandomized controlled trials.
MIS FOR AGC
Because of an inaccurate perioperative diagnosis, a significant number of patients with AGC have been treated using minimally invasive approaches.15 Therefore, the results of treating advanced cancer with MIS should be investigated. Although there is no evidence and there are no guidelines that indicate that the use of MIS is appropriate, even for EGC, experienced surgeons have begun to treat patients with AGC using MIS, and have reported acceptable short-term outcomes.2,4,5,15,37,38 For some selected AGC cases, surgeons believe that this approach is not inferior to open conventional surgery in terms of the oncologic aspects. In addition, laparoscopic manipulation under pneumoperitoneum was a putative risk factor for peritoneal seeding and port site metastasis, but is no longer clinically problematic. The technical difficulties involved in D2 LN dissection could be an important reason to negate MIS for AGC including serosa-involved cancer. There are overwhelming technical difficulties to overcome if surgeons encounter large-size tumors or tumors that require multiorgan resection. Some experienced surgeons, however, can accurately perform D2 LN dissection with the current instruments or surgical systems.
Experienced surgeons have reported oncologic outcomes of the use of MIS for AGC.4,5,6,15,17,37,38,39,40 MIS for locally advanced cancer has a similar survival rate compared to open conventional surgery. However, the sample sizes were too small to report on stage-specific survival rates. In our experience, even serosa-positive cancer (T4a) patients treated using MIS experienced similar survival and recurrence outcomes, compared to open surgery.15 The results of a meta-analysis demonstrated that compared with open surgery, laparoscopic gastrectomy with D2 LN dissection had similar overall survival rates, comparable numbers of retrieved LNs, less blood loss, less pain, reduced postoperative complications, and shorter hospital stays.41 However, the reduced postoperative complication rate resulted from a decreased incidence of minor complications (e.g., wound infections and postoperative ileus), but the incidence of major complications was similar to open surgery.41 Recently, large-scale multicenter RCTs began enrollment of patients to assess the feasibility of MIS for AGC in Korea, Japan, and China (Table 2).
Table 2.
Multicenter, Prospective, Randomized Controlled Trials of Laparoscopic Gastrectomy for the Treatment of Advanced Gastric Cancer
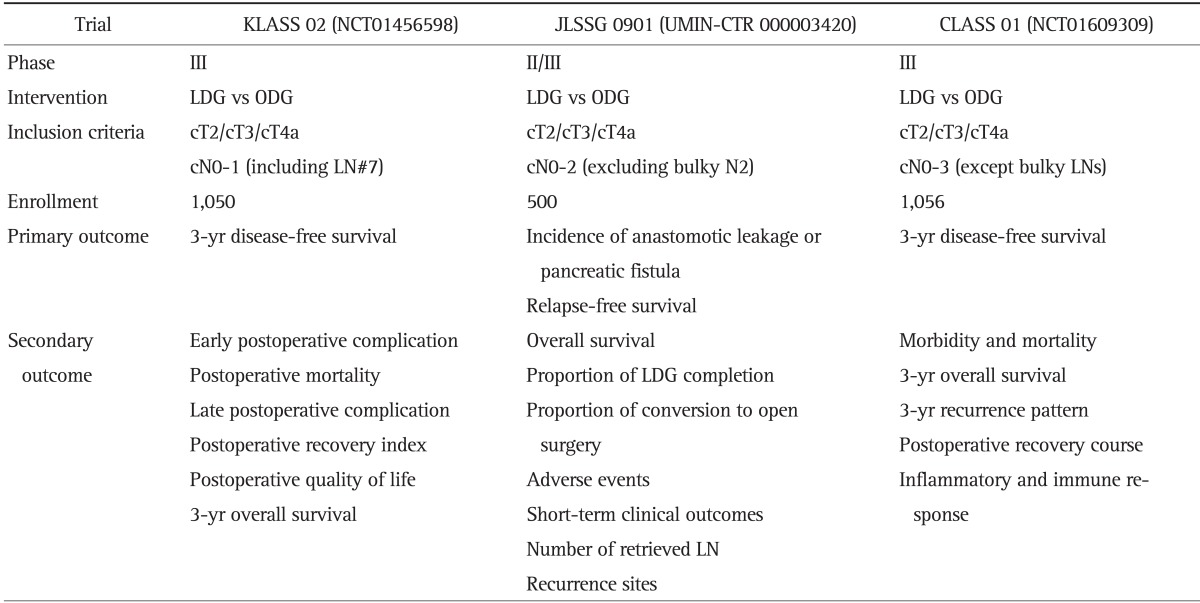
KLASS, Korean Laparoscopic Gastrointestinal Surgery Study; JLSSG, Japanese Laparoscopic Gastric Surgery Study Group; CLASS, Chinese Laparoscopic Gastrointestinal Surgery Study; LDG, laparoscopic distal gastrectomy; ODG, open distal gastrectomy; LN, lymph node.
SENTINEL LN NAVIGATION SURGERY
Sentinel node navigation surgery (SNNS) has been investigated for the purpose of increasing a patient's quality of life while maintaining oncologic safety. However, the role of SNNS for gastric cancer remains to be elucidated, even though numerous investigations have been performed to increase the sensitivity of sentinel node (SN) detection.42 The accuracy of SN detection was approximately 90%.43,44 Sensitivity using isotope and dye simultaneously (dual tracer method) was greater than the sensitivity achieved when using any single tracer.43,45 However, results of a recent multicenter phase II trial in Japan indicated that high accuracy and detection rates were achieved when a dual tracer was used for small-sized stage T1 cancer.46 The SN detection rate was 97%, and 93% of SNs were positive among patients who had metastatic LNs. The authors concluded that dual tracer endoscopic injection using tin colloid and blue dye was a safe and feasible method for selected EGCs. To make the best therapeutic decision for patients, the accuracy of the preoperative diagnosis should be such that preoperatively underestimated patients would not be treated with SNNS.47 SNNS should be used with caution because of the presence of SNs at the level II LNs and a possibility of skip metastasis.44
In a study using porcine model, laparoscopic wedge resection or segmental resection with sentinel basin dissection was safe and achievable.48 In Korea, a phase III multicenter RCT (Sentinel Node Oriented Tailored Approach [SENORITA] trial, NCT01544413) for clinical stage IA cancer is comparing laparoscopic SNNS to conventional laparoscopic gastrectomy.47 Advanced minimally invasive techniques and the concepts underlying SNNS could contribute to changing current surgical practices for gastric cancer. So far, sentinel basin LN dissection and segmental or wedge resection of the stomach for small-sized, T1N0M0 cancer could be a potential candidate for this approach.46,47
MIS FOR FUNCTION PRESERVATION
As the information about outcomes after the use of minimally invasive approaches has been accumulating, MIS has stimulated interest in function-preserving surgeries (e.g., pylorus-preserving gastrectomy, proximal gastrectomy). Initially, pylorus-preserving gastrectomy (PPG) was a surgical option for gastric ulcers.49 This method, and other limited methods, provide a better quality of life and oncologic safety when used for selected types of gastric cancer.50,51,52,53 Advantages of pylorus-preserving gastrectomy with gastrogastrostomy include less dumping syndrome, less bile reflux, less weight loss, and decreased gallstone formation.50,54,55,56,57 Because of the extremely small chance of macro- and micro-metastases at the LN stations of the supra- (#5) and infrapylroic (#6) areas, oncologic safety is thought to be guaranteed when the indication for this method is strictly limited to middle third cT1N0M0 cancer located more than 4 cm from the pylorus.50,57,58 However, use of a laparoscopic approach for this procedure is difficult for inexperienced surgeons because it should preserve the pyloric and hepatic branches of the vagus nerve and infrapyloric vessels.50,58,59 The results of recent large-volume retrospective analyses indicated that compared to conventional laparoscopic distal gastrectomy, laparoscopic pylorus-preserving gastrectomy is oncologically safe, has a similar morbidity rate, does not result in decreased patient body fat and nutritional profiles, and has a lower incidence of gallstone formation.50 Nonetheless, more RCTs that examine long-term outcomes and the incidence of gastric stasis should be performed before this procedure is widely adopted.
The results of laparoscopic proximal gastrectomy for upper third EGC have also been reported.52,53 The long-term results for patients who received a total gastrectomy were significantly worse compared with patients who received a partial gastrectomy.60 The results of a recent multicenter retrospective comparison study among patients who underwent total gastrectomy versus proximal gastrectomy with esophagogastrostomy versus proximal gastrectomy with jejunal interposition indicated that proximal gastrectomy was superior to total gastrectomy with regard to postoperative nutritional status evaluated by serum albumin and hemoglobin, but not weight loss.61 Dumping syndrome and diarrhea were also decreased, but more patients reported heartburn and a ¡°stuck feeling."61 Experienced surgeons have investigated, and reported on, various types of reconstruction methods and their technical feasibility after laparoscopic proximal gastrectomy.61,62,63 However, there is no consensus in the literature with regard to an ideal reconstruction method, and well-designed RCTs have not been performed. The number of EGC patients who are candidates for function-preserving surgeries has been increasing, and one of these surgical methods may be an useful option for these patients.
MIS FOR TECHNICALLY-DEMANDING PROCEDURES
Advanced technology has made possible the use of MIS for the above-mentioned procedures. Technically-demanding procedures such as total gastrectomy with or without splenectomy, gastrectomy with multivisceral resection, and completion total gastrectomy for remnant gastric cancer have been successfully performed by experienced surgeons using minimally invasive approaches.64,65,66,67,68 Single incision or reduced port surgery for gastric cancer also has been reported to be a safe and feasible procedure.26,27 Although this approach is safe and feasible, more evidence that demonstrates efficacy is needed. Procedures of this type are also exceedingly challenging for most surgeons. To overcome these obstacles, case-specific instruments, improved camera systems, and adequate training programs for novice surgeons should be developed. RCTs that indicate that these procedures are efficacious are also needed.
ROBOTIC SURGERY FOR GASTRIC CANCER
Surgical robotic systems have been introduced into the field of gastric cancer treatment as an improved technology that would overcome the technical limitations of laparoscopy.69,70,71 Compared with conventional laparoscopic surgery, robotic systems provide more defined movements and a better operative view. Unlike other malignancies (e.g., lower rectal cancer, prostate cancer), however, the role for these systems in the treatment of gastric cancer is unclear.70,72,73,74 Since 2005, a robotic system has been used for gastric cancer surgery in Korea, and many experienced MIS surgeons have reported that compared with laparoscopic gastrectomy, robotic gastrectomy is safe and effective.71,75,76,77 These results were achieved even though the early experience of robotic surgery was compared with laparoscopic surgery performed by experienced laparoscopic surgeons.
Results of a meta-analysis revealed that compared with laparoscopic surgery, use of robotic surgery for gastric cancer significantly decreases intraoperative blood loss, and results in comparable morbidity and mortality.78 The use of robotic gastrectomy also resulted in significantly less blood loss and a shorter hospital stay compared with open surgery. However, the operative time of robotic gastrectomy was significantly longer than the other two approaches. The current indications for robotic surgery are similar to laparoscopic surgery. Some investigators have suggested that the feasibility and advantages of robotic surgery for technically-challenging complicated cases (e.g., very advanced cancer, multiorgan resection, and function-preserving surgeries) should be investigated.2,70,79
In Korea, a multicenter prospective observational study (Multi-institutional Study on the Assessment of Robotic Surgery for Gastric Cancer, NCT01309256) that compares robotic and laparoscopic surgery for gastric cancer has begun to enroll patients. This study will investigate cost-effectiveness, patient quality of life, learning curve of the surgeon, and feasibility. The results of this study will contribute data that address questions about clinical indications for, and efficacy of, robotic surgery in the field of gastric cancer treatment.
ONGOING MULTICENTER TRIALS OF MIS FOR GASTRIC CANCER TREATMENT
Many MIS surgeons have conducted prospective studies with a small number of patients in a single or a limited number of institutions. However, large-scale multicenter RCTs were recently designed and performed, especially in Korea and Japan. In Japan, the results of phase II and phase III clinical trials conducted by JCOG (JCOG0703 and JCOG0912 trials, respectively) have confirmed the safety. The results for long-term outcomes of laparoscopic surgery for stage I gastric cancer are pending. In Korea, a phase III, KLASS 01 trial completed patient enrollment and is awaiting final results regarding oncologic safety for use of MIS for clinical stage I gastric cancer. Results of an interim analysis of KLASS 01 trial revealed that there were no significance differences in morbidity and mortality outcomes between laparoscopic distal gastrectomy and open distal gastrectomy patients.23 Convinced that laparoscopic surgery is safe, the KLASS group has begun a multicenter phase III trial for locally advanced cancer (KLASS 02 trial, NCT01456598). The primary endpoint is noninferiority of 3-year disease-free survival for MIS compared to open conventional surgery. The Japanese Laparoscopic Gastric Surgery Study group (JLSSG) has also begun phase II and III trials (JLSSG0901 trial; UMIN-CTR number, 000003420) to investigate the technical and oncology safety of laparoscopy used for the treatment of AGC. A Chinese Laparoscopic Gastrointestinal Surgical Study Group (CLASS) trial recently initiated patient recruitment to compare the safety and oncologic feasibility of laparoscopic surgery for AGC. The primary endpoint under investigation is 3-year disease-free survival (CLASS 01 trial, NCT01609309). A KLASS 03 (NCT01584336) trial is in progress to investigate long-term outcomes and feasibility and safety of the use of laparoscopic total gastrectomy for the treatment of stage I gastric cancer.12,80,81 In Korea, a multicenter observational study (Multi-institutional Study on the Assessment of Robotic Surgery for Gastric Cancer, NCT01309256) is comparing robotic and laparoscopic gastrectomy for cost-effectiveness, quality of life, and feasibility. To examine the role of SNNS for the treatment of clinical stage IA gastric cancer, a phase III multicenter RCT (SENORITA trial, NCT01544413) is also in progress in Korea. These studies will reveal the real efficacy of MIS for gastric cancer and aid in the development of guidelines for surgeons.
CURRENT STATUS OF MIS FOR GASTRIC CANCER IN KOREA
In Korea, MIS has become a widely accepted treatment for EGC, and is performed as an alternative to conventional open surgery.82 Although the use of MIS is under investigation, several large-volume centers have also expanded their clinical indications for the use of MIS for the treatment of AGC. More limited surgeries, such as proximal gastrectomy for upper gastric cancer and pylorus-preserving gastrectomy, are actively applied and are performed with minimally invasive approaches.50,62 The use of SNNS is being examined using a multicenter RCT that includes wedge or segmental resection of the stomach with sentinel basin LN dissection.47 Some surgeons have attempted to focus on less invasive surgery such as reduced port or single incision gastrectomy. However, it is debatable whether this type of approach has any real benefit for the patient (i.e., except a cosmetic advantage).26 Nonetheless, these approaches appear to be feasible and safe when performed by experienced surgeons using innovative techniques. Korea is one of the leading countries in the use of the newest robotic surgery technology for the treatment of gastric cancer. The benefits for the patient are debatable when the high cost is considered, but the technology improves surgical outcomes for the treatment of malignant disease.46,71 Robotic surgery will have an appropriate role in the treatment of gastric cancer and could contribute to the expanding clinical indications of the use of MIS for the treatment of gastric cancer. The role of the gastric surgeon in Korea is increasing as the incidence of gastric cancer is still high, and the number of large-volume centers with experienced surgeons have increased.82 Thanks to the vigorous efforts of many gastric surgeons in Korea, many well-designed studies are being performed and their results are pending. The surgical methods and techniques are being extensively shared and are contributing to the standardization of surgical treatments among institutions.
CONCLUSIONS
The use of MIS for the treatment of gastric cancer is evolving. The clinical indications are expanding to its use in function-preserving surgery and in more extensive surgeries. Technical difficulties and lack of evidence have hindered rapid and widespread use of MIS for gastric cancer, but it seems to be promising approach. The results of the many well-designed studies that are in progress are expected to indicate that MIS is as safe and effective as open conventional surgery.
ACKNOWLEDGEMENTS
This study was supported by a grant from the National R&D Program for Cancer Control, Ministry of Health and Welfare, Republic of Korea (1320270).
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Kitano S, Shiraishi N, Fujii K, Yasuda K, Inomata M, Adachi Y. A randomized controlled trial comparing open vs laparoscopy-assisted distal gastrectomy for the treatment of early gastric cancer: an interim report. Surgery. 2002;131(1 Suppl):S306–S311. doi: 10.1067/msy.2002.120115. [DOI] [PubMed] [Google Scholar]
- 2.Uyama I, Suda K, Satoh S. Laparoscopic surgery for advanced gastric cancer: current status and future perspectives. J Gastric Cancer. 2013;13:19–25. doi: 10.5230/jgc.2013.13.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huscher CG, Mingoli A, Sgarzini G, et al. Totally laparoscopic total and subtotal gastrectomy with extended lymph node dissection for early and advanced gastric cancer: early and long-term results of a 100-patient series. Am J Surg. 2007;194:839–844. doi: 10.1016/j.amjsurg.2007.08.037. [DOI] [PubMed] [Google Scholar]
- 4.Park SS, Kim CS, Mok YJ, Kim SJ, Kim HI. Gastric cancer confined to the muscularis propria: a possible candidate for laparoscopic surgery or adjuvant therapy. Scand J Gastroenterol. 2005;40:450–454. doi: 10.1080/00365520410009302. [DOI] [PubMed] [Google Scholar]
- 5.Hur H, Jeon HM, Kim W. Laparoscopy-assisted distal gastrectomy with D2 lymphadenectomy for T2b advanced gastric cancers: three years' experience. J Surg Oncol. 2008;98:515–519. doi: 10.1002/jso.21155. [DOI] [PubMed] [Google Scholar]
- 6.Yoshimura F, Inaba K, Kawamura Y, et al. Clinical outcome and clinicopathological characteristics of recurrence after laparoscopic gastrectomy for advanced gastric cancer. Digestion. 2011;83:184–190. doi: 10.1159/000322032. [DOI] [PubMed] [Google Scholar]
- 7.Lee J, Kim W. Long-term outcomes after laparoscopy-assisted gastrectomy for advanced gastric cancer: analysis of consecutive 106 experiences. J Surg Oncol. 2009;100:693–698. doi: 10.1002/jso.21400. [DOI] [PubMed] [Google Scholar]
- 8.Pak KH, Hyung WJ, Son T, et al. Long-term oncologic outcomes of 714 consecutive laparoscopic gastrectomies for gastric cancer: results from the 7-year experience of a single institute. Surg Endosc. 2012;26:130–136. doi: 10.1007/s00464-011-1838-3. [DOI] [PubMed] [Google Scholar]
- 9.Oka S, Tanaka S, Kaneko I, et al. Endoscopic submucosal dissection for residual/local recurrence of early gastric cancer after endoscopic mucosal resection. Endoscopy. 2006;38:996–1000. doi: 10.1055/s-2006-944780. [DOI] [PubMed] [Google Scholar]
- 10.Takenaka R, Kawahara Y, Okada H, et al. Risk factors associated with local recurrence of early gastric cancers after endoscopic submucosal dissection. Gastrointest Endosc. 2008;68:887–894. doi: 10.1016/j.gie.2008.03.1089. [DOI] [PubMed] [Google Scholar]
- 11.Gotoda T. Endoscopic resection of early gastric cancer. Gastric Cancer. 2007;10:1–11. doi: 10.1007/s10120-006-0408-1. [DOI] [PubMed] [Google Scholar]
- 12.Etoh T, Inomata M, Shiraishi N, Kitano S. Minimally invasive approaches for gastric cancer: Japanese experiences. J Surg Oncol. 2013;107:282–288. doi: 10.1002/jso.23128. [DOI] [PubMed] [Google Scholar]
- 13.Volz J, Köster S, Spacek Z, Paweletz N. The influence of pneumoperitoneum used in laparoscopic surgery on an intraabdominal tumor growth. Cancer. 1999;86:770–774. [PubMed] [Google Scholar]
- 14.An JY, Heo GU, Cheong JH, Hyung WJ, Choi SH, Noh SH. Assessment of open versus laparoscopy-assisted gastrectomy in lymph node-positive early gastric cancer: a retrospective cohort analysis. J Surg Oncol. 2010;102:77–81. doi: 10.1002/jso.21554. [DOI] [PubMed] [Google Scholar]
- 15.Son T, Hyung WJ, Lee JH, Kim YM, Noh SH. Minimally invasive surgery for serosa-positive gastric cancer (pT4a) in patients with preoperative diagnosis of cancer without serosal invasion. Surg Endosc. 2014;28:866–874. doi: 10.1007/s00464-013-3236-5. [DOI] [PubMed] [Google Scholar]
- 16.Lee SW, Nomura E, Bouras G, Tokuhara T, Tsunemi S, Tanigawa N. Long-term oncologic outcomes from laparoscopic gastrectomy for gastric cancer: a single-center experience of 601 consecutive resections. J Am Coll Surg. 2010;211:33–40. doi: 10.1016/j.jamcollsurg.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 17.Huscher CG, Mingoli A, Sgarzini G, et al. Laparoscopic versus open subtotal gastrectomy for distal gastric cancer: five-year results of a randomized prospective trial. Ann Surg. 2005;241:232–237. doi: 10.1097/01.sla.0000151892.35922.f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Strong VE, Devaud N, Allen PJ, Gonen M, Brennan MF, Coit D. Laparoscopic versus open subtotal gastrectomy for adenocarcinoma: a case-control study. Ann Surg Oncol. 2009;16:1507–1513. doi: 10.1245/s10434-009-0386-8. [DOI] [PubMed] [Google Scholar]
- 19.Oh SY, Kwon S, Lee KG, et al. Outcomes of minimally invasive surgery for early gastric cancer are comparable with those for open surgery: analysis of 1,013 minimally invasive surgeries at a single institution. Surg Endosc. 2014;28:789–795. doi: 10.1007/s00464-013-3256-1. [DOI] [PubMed] [Google Scholar]
- 20.Lee JH, Han HS, Lee JH. A prospective randomized study comparing open vs laparoscopy-assisted distal gastrectomy in early gastric cancer: early results. Surg Endosc. 2005;19:168–173. doi: 10.1007/s00464-004-8808-y. [DOI] [PubMed] [Google Scholar]
- 21.Hayashi H, Ochiai T, Shimada H, Gunji Y. Prospective randomized study of open versus laparoscopy-assisted distal gastrectomy with extraperigastric lymph node dissection for early gastric cancer. Surg Endosc. 2005;19:1172–1176. doi: 10.1007/s00464-004-8207-4. [DOI] [PubMed] [Google Scholar]
- 22.Kim YW, Baik YH, Yun YH, et al. Improved quality of life outcomes after laparoscopy-assisted distal gastrectomy for early gastric cancer: results of a prospective randomized clinical trial. Ann Surg. 2008;248:721–727. doi: 10.1097/SLA.0b013e318185e62e. [DOI] [PubMed] [Google Scholar]
- 23.Kim HH, Hyung WJ, Cho GS, et al. Morbidity and mortality of laparoscopic gastrectomy versus open gastrectomy for gastric cancer: an interim report: a phase III multicenter, prospective, randomized Trial (KLASS Trial) Ann Surg. 2010;251:417–420. doi: 10.1097/SLA.0b013e3181cc8f6b. [DOI] [PubMed] [Google Scholar]
- 24.Ohtani H, Tamamori Y, Noguchi K, et al. Meta-analysis of laparoscopy-assisted and open distal gastrectomy for gastric cancer. J Surg Res. 2011;171:479–485. doi: 10.1016/j.jss.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 25.Viñuela EF, Gonen M, Brennan MF, Coit DG, Strong VE. Laparoscopic versus open distal gastrectomy for gastric cancer: a meta-analysis of randomized controlled trials and high-quality nonrandomized studies. Ann Surg. 2012;255:446–456. doi: 10.1097/SLA.0b013e31824682f4. [DOI] [PubMed] [Google Scholar]
- 26.Park do J, Lee JH, Ahn SH, Eng AK, Kim HH. Single-port laparoscopic distal gastrectomy with D1+β lymph node dissection for gastric cancers: report of 2 cases. Surg Laparosc Endosc Percutan Tech. 2012;22:e214–e216. doi: 10.1097/SLE.0b013e318253df9b. [DOI] [PubMed] [Google Scholar]
- 27.Kunisaki C, Ono HA, Oshima T, Makino H, Akiyama H, Endo I. Relevance of reduced-port laparoscopic distal gastrectomy for gastric cancer: a pilot study. Dig Surg. 2012;29:261–268. doi: 10.1159/000341677. [DOI] [PubMed] [Google Scholar]
- 28.Song KY, Park CH, Kang HC, et al. Is totally laparoscopic gastrectomy less invasive than laparoscopy-assisted gastrectomy? Prospective, multicenter study. J Gastrointest Surg. 2008;12:1015–1021. doi: 10.1007/s11605-008-0484-0. [DOI] [PubMed] [Google Scholar]
- 29.Kim JJ, Song KY, Chin HM, et al. Totally laparoscopic gastrectomy with various types of intracorporeal anastomosis using laparoscopic linear staplers: preliminary experience. Surg Endosc. 2008;22:436–442. doi: 10.1007/s00464-007-9446-y. [DOI] [PubMed] [Google Scholar]
- 30.Kanaya S, Gomi T, Momoi H, et al. Delta-shaped anastomosis in totally laparoscopic Billroth I gastrectomy: new technique of intraabdominal gastroduodenostomy. J Am Coll Surg. 2002;195:284–287. doi: 10.1016/s1072-7515(02)01239-5. [DOI] [PubMed] [Google Scholar]
- 31.Okabe H, Obama K, Tanaka E, et al. Intracorporeal esophagojejunal anastomosis after laparoscopic total gastrectomy for patients with gastric cancer. Surg Endosc. 2009;23:2167–2171. doi: 10.1007/s00464-008-9987-8. [DOI] [PubMed] [Google Scholar]
- 32.Kim MG, Kim KC, Kim BS, et al. A totally laparoscopic distal gastrectomy can be an effective way of performing laparoscopic gastrectomy in obese patients (body mass index≥30) World J Surg. 2011;35:1327–1332. doi: 10.1007/s00268-011-1034-6. [DOI] [PubMed] [Google Scholar]
- 33.Memon MA, Khan S, Yunus RM, Barr R, Memon B. Meta-analysis of laparoscopic and open distal gastrectomy for gastric carcinoma. Surg Endosc. 2008;22:1781–1789. doi: 10.1007/s00464-008-9925-9. [DOI] [PubMed] [Google Scholar]
- 34.Chen XZ, Hu JK, Yang K, Wang L, Lu QC. Short-term evaluation of laparoscopy-assisted distal gastrectomy for predictive early gastric cancer: a meta-analysis of randomized controlled trials. Surg Laparosc Endosc Percutan Tech. 2009;19:277–284. doi: 10.1097/SLE.0b013e3181b080d3. [DOI] [PubMed] [Google Scholar]
- 35.Kodera Y, Fujiwara M, Ohashi N, et al. Laparoscopic surgery for gastric cancer: a collective review with meta-analysis of randomized trials. J Am Coll Surg. 2010;211:677–686. doi: 10.1016/j.jamcollsurg.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 36.Zorcolo L, Rosman AS, Pisano M, et al. A meta-analysis of prospective randomized trials comparing minimally invasive and open distal gastrectomy for cancer. J Surg Oncol. 2011;104:544–551. doi: 10.1002/jso.21980. [DOI] [PubMed] [Google Scholar]
- 37.Zhao Y, Yu P, Hao Y, et al. Comparison of outcomes for laparoscopically assisted and open radical distal gastrectomy with lymphadenectomy for advanced gastric cancer. Surg Endosc. 2011;25:2960–2966. doi: 10.1007/s00464-011-1652-y. [DOI] [PubMed] [Google Scholar]
- 38.Gordon AC, Kojima K, Inokuchi M, Kato K, Sugihara K. Long-term comparison of laparoscopy-assisted distal gastrectomy and open distal gastrectomy in advanced gastric cancer. Surg Endosc. 2013;27:462–470. doi: 10.1007/s00464-012-2459-1. [DOI] [PubMed] [Google Scholar]
- 39.Pugliese R, Maggioni D, Sansonna F, et al. Subtotal gastrectomy with D2 dissection by minimally invasive surgery for distal adenocarcinoma of the stomach: results and 5-year survival. Surg Endosc. 2010;24:2594–2602. doi: 10.1007/s00464-010-1014-1. [DOI] [PubMed] [Google Scholar]
- 40.Pugliese R, Maggioni D, Sansonna F, et al. Outcomes and survival after laparoscopic gastrectomy for adenocarcinoma: analysis on 65 patients operated on by conventional or robot-assisted minimal access procedures. Eur J Surg Oncol. 2009;35:281–288. doi: 10.1016/j.ejso.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 41.Wei HB, Wei B, Qi CL, et al. Laparoscopic versus open gastrectomy with D2 lymph node dissection for gastric cancer: a meta-analysis. Surg Laparosc Endosc Percutan Tech. 2011;21:383–390. doi: 10.1097/SLE.0b013e31822d02dc. [DOI] [PubMed] [Google Scholar]
- 42.Miyashiro I. What is the problem in clinical application of sentinel node concept to gastric cancer surgery? J Gastric Cancer. 2012;12:7–12. doi: 10.5230/jgc.2012.12.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee JH, Ryu KW, Kim CG, et al. Sentinel node biopsy using dye and isotope double tracers in early gastric cancer. Ann Surg Oncol. 2006;13:1168–1174. doi: 10.1245/s10434-006-9038-4. [DOI] [PubMed] [Google Scholar]
- 44.Kim MC, Kim HH, Jung GJ, et al. Lymphatic mapping and sentinel node biopsy using 99mTc tin colloid in gastric cancer. Ann Surg. 2004;239:383–387. doi: 10.1097/01.sla.0000114227.70480.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Park do J, Kim HH, Park YS, et al. Simultaneous indocyanine green and (99m)Tc-antimony sulfur colloid-guided laparoscopic sentinel basin dissection for gastric cancer. Ann Surg Oncol. 2011;18:160–165. doi: 10.1245/s10434-010-1221-y. [DOI] [PubMed] [Google Scholar]
- 46.Kitagawa Y, Takeuchi H, Takagi Y, et al. Sentinel node mapping for gastric cancer: a prospective multicenter trial in Japan. J Clin Oncol. 2013;31:3704–3710. doi: 10.1200/JCO.2013.50.3789. [DOI] [PubMed] [Google Scholar]
- 47.Park JY, Ryu KW, Eom BW, et al. Proposal of the surgical options for primary tumor control during sentinel node navigation surgery based on the discrepancy between preoperative and postoperative early gastric cancer diagnoses. Ann Surg Oncol. 2014;21:1123–1129. doi: 10.1245/s10434-013-3427-2. [DOI] [PubMed] [Google Scholar]
- 48.Lee JH, Lee MS, Kim HH, et al. Feasibility of laparoscopic partial gastrectomy with sentinel node basin dissection in a porcine model. Surg Endosc. 2011;25:1070–1075. doi: 10.1007/s00464-010-1318-1. [DOI] [PubMed] [Google Scholar]
- 49.Maki T, Shiratori T, Hatafuku T, Sugawara K. Pylorus-preserving gastrectomy as an improved operation for gastric ulcer. Surgery. 1967;61:838–845. [PubMed] [Google Scholar]
- 50.Suh YS, Han DS, Kong SH, et al. Laparoscopy-assisted pylorus-preserving gastrectomy is better than laparoscopy-assisted distal gastrectomy for middle-third early gastric cancer. Ann Surg. 2014;259:485–493. doi: 10.1097/SLA.0b013e318294d142. [DOI] [PubMed] [Google Scholar]
- 51.Kim BH, Hong SW, Kim JW, Choi SH, Yoon SO. Oncologic safety of pylorus-preserving gastrectomy in the aspect of micrometastasis in lymph nodes at stations 5 and 6. Ann Surg Oncol. 2014;21:533–538. doi: 10.1245/s10434-013-3252-7. [DOI] [PubMed] [Google Scholar]
- 52.Tanimura S, Higashino M, Fukunaga Y, et al. Laparoscopic gastrectomy with regional lymph node dissection for upper gastric cancer. Br J Surg. 2007;94:204–207. doi: 10.1002/bjs.5542. [DOI] [PubMed] [Google Scholar]
- 53.Matsui H, Okamoto Y, Nabeshima K, Kondoh Y, Ogoshi K, Makuuchi H. Endoscopy-assisted gastric resection: a safe and reliable procedure for tumor clearance during laparoscopic high distal or proximal gastrectomy. Surg Endosc. 2009;23:1146–1149. doi: 10.1007/s00464-009-0354-1. [DOI] [PubMed] [Google Scholar]
- 54.Sano T, Hollowood A. Early gastric cancer: diagnosis and less invasive treatments. Scand J Surg. 2006;95:249–255. doi: 10.1177/145749690609500407. [DOI] [PubMed] [Google Scholar]
- 55.Nunobe S, Sasako M, Saka M, Fukagawa T, Katai H, Sano T. Symptom evaluation of long-term postoperative outcomes after pylorus-preserving gastrectomy for early gastric cancer. Gastric Cancer. 2007;10:167–172. doi: 10.1007/s10120-007-0434-7. [DOI] [PubMed] [Google Scholar]
- 56.Shinohara H, Sonoda T, Niki M, Nomura E, Nishiguchi K, Tanigawa N. Laparoscopically-assisted pylorus-preserving gastrectomy with preservation of the vagus nerve. Eur J Surg. 2002;168:55–58. doi: 10.1080/110241502317307580. [DOI] [PubMed] [Google Scholar]
- 57.Hiki N, Kaminishi M. Pylorus-preserving gastrectomy in gastric cancer surgery: open and laparoscopic approaches. Langenbecks Arch Surg. 2005;390:442–447. doi: 10.1007/s00423-005-0573-4. [DOI] [PubMed] [Google Scholar]
- 58.Takeuchi H, Kitagawa Y. Is pylorus-preserving gastrectomy universally applicable to early gastric cancer of the mid stomach? Ann Surg Oncol. 2014;21:356–357. doi: 10.1245/s10434-013-3256-3. [DOI] [PubMed] [Google Scholar]
- 59.Morita S, Katai H, Saka M, Fukagawa T, Sano T, Sasako M. Outcome of pylorus-preserving gastrectomy for early gastric cancer. Br J Surg. 2008;95:1131–1135. doi: 10.1002/bjs.6295. [DOI] [PubMed] [Google Scholar]
- 60.Ogoshi K, Okamoto Y, Nabeshima K, et al. Focus on the conditions of resection and reconstruction in gastric cancer: what extent of resection and what kind of reconstruction provide the best outcomes for gastric cancer patients? Digestion. 2005;71:213–224. doi: 10.1159/000087046. [DOI] [PubMed] [Google Scholar]
- 61.Masuzawa T, Takiguchi S, Hirao M, et al. Comparison of perioperative and long-term outcomes of total and proximal gastrectomy for early gastric cancer: a multi-institutional retrospective study. World J Surg. 2014;38:1100–1106. doi: 10.1007/s00268-013-2370-5. [DOI] [PubMed] [Google Scholar]
- 62.Ahn SH, Jung DH, Son SY, Lee CM, Park DJ, Kim HH. Laparoscopic double-tract proximal gastrectomy for proximal early gastric cancer. Gastric Cancer. doi: 10.1007/s10120-013-0303-5. Epub 2013 Sep 20. http://dx.doi.org/10.1007/s10120-013-0303-5. [DOI] [PubMed] [Google Scholar]
- 63.Kinoshita T, Gotohda N, Kato Y, Takahashi S, Konishi M, Kinoshita T. Laparoscopic proximal gastrectomy with jejunal interposition for gastric cancer in the proximal third of the stomach: a retrospective comparison with open surgery. Surg Endosc. 2013;27:146–153. doi: 10.1007/s00464-012-2401-6. [DOI] [PubMed] [Google Scholar]
- 64.Shinohara T, Uyama I, Kanaya S, et al. Totally laparoscopic pancreaticoduodenectomy for locally advanced gastric cancer. Langenbecks Arch Surg. 2009;394:733–737. doi: 10.1007/s00423-009-0492-x. [DOI] [PubMed] [Google Scholar]
- 65.Song J, Kim JY, Kim S, et al. Laparoscopic completion total gastrectomy in remnant gastric cancer: technical detail and experience of two cases. Hepatogastroenterology. 2009;56:1249–1252. [PubMed] [Google Scholar]
- 66.Hyung WJ, Lim JS, Song J, Choi SH, Noh SH. Laparoscopic spleen-preserving splenic hilar lymph node dissection during total gastrectomy for gastric cancer. J Am Coll Surg. 2008;207:e6–e11. doi: 10.1016/j.jamcollsurg.2008.04.027. [DOI] [PubMed] [Google Scholar]
- 67.Uyama I, Sugioka A, Matsui H, Fujita J, Komori Y, Hasumi A. Laparoscopic pancreas-preserving total gastrectomy for proximal gastric cancer: a case and technical report. Surg Endosc. 2001;15:217–218. doi: 10.1007/s004640040037. [DOI] [PubMed] [Google Scholar]
- 68.Shinohara T, Kanaya S, Taniguchi K, Fujita T, Yanaga K, Uyama I. Laparoscopic total gastrectomy with D2 lymph node dissection for gastric cancer. Arch Surg. 2009;144:1138–1142. doi: 10.1001/archsurg.2009.223. [DOI] [PubMed] [Google Scholar]
- 69.Maeso S, Reza M, Mayol JA, et al. Efficacy of the Da Vinci surgical system in abdominal surgery compared with that of laparoscopy: a systematic review and meta-analysis. Ann Surg. 2010;252:254–262. doi: 10.1097/SLA.0b013e3181e6239e. [DOI] [PubMed] [Google Scholar]
- 70.Marano A, Choi YY, Hyung WJ, Kim YM, Kim J, Noh SH. Robotic versus laparoscopic versus open gastrectomy: a meta-analysis. J Gastric Cancer. 2013;13:136–148. doi: 10.5230/jgc.2013.13.3.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Song J, Oh SJ, Kang WH, Hyung WJ, Choi SH, Noh SH. Robot-assisted gastrectomy with lymph node dissection for gastric cancer: lessons learned from an initial 100 consecutive procedures. Ann Surg. 2009;249:927–932. doi: 10.1097/01.sla.0000351688.64999.73. [DOI] [PubMed] [Google Scholar]
- 72.Gutt CN, Oniu T, Mehrabi A, Kashfi A, Schemmer P, Büchler MW. Robot-assisted abdominal surgery. Br J Surg. 2004;91:1390–1397. doi: 10.1002/bjs.4700. [DOI] [PubMed] [Google Scholar]
- 73.Baek SJ, Lee DW, Park SS, Kim SH. Current status of robot-assisted gastric surgery. World J Gastrointest Oncol. 2011;3:137–143. doi: 10.4251/wjgo.v3.i10.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wall J, Marescaux J. Robotic gastrectomy is safe and feasible, but real benefits remain elusive. Arch Surg. 2011;146:1092. doi: 10.1001/archsurg.2011.198. [DOI] [PubMed] [Google Scholar]
- 75.Kim MC, Heo GU, Jung GJ. Robotic gastrectomy for gastric cancer: surgical techniques and clinical merits. Surg Endosc. 2010;24:610–615. doi: 10.1007/s00464-009-0618-9. [DOI] [PubMed] [Google Scholar]
- 76.Yoon HM, Kim YW, Lee JH, et al. Robot-assisted total gastrectomy is comparable with laparoscopically assisted total gastrectomy for early gastric cancer. Surg Endosc. 2012;26:1377–1381. doi: 10.1007/s00464-011-2043-0. [DOI] [PubMed] [Google Scholar]
- 77.Woo Y, Hyung WJ, Pak KH, et al. Robotic gastrectomy as an oncologically sound alternative to laparoscopic resections for the treatment of early-stage gastric cancers. Arch Surg. 2011;146:1086–1092. doi: 10.1001/archsurg.2011.114. [DOI] [PubMed] [Google Scholar]
- 78.Xiong B, Ma L, Zhang C. Robotic versus laparoscopic gastrectomy for gastric cancer: a meta-analysis of short outcomes. Surg Oncol. 2012;21:274–280. doi: 10.1016/j.suronc.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 79.Coratti A, Annecchiarico M, Di Marino M, Gentile E, Coratti F, Giulianotti PC. Robot-assisted gastrectomy for gastric cancer: current status and technical considerations. World J Surg. 2013;37:2771–2781. doi: 10.1007/s00268-013-2100-z. [DOI] [PubMed] [Google Scholar]
- 80.Park do J, Han SU, Hyung WJ, et al. Long-term outcomes after laparoscopy-assisted gastrectomy for advanced gastric cancer: a large-scale multicenter retrospective study. Surg Endosc. 2012;26:1548–1553. doi: 10.1007/s00464-011-2065-7. [DOI] [PubMed] [Google Scholar]
- 81.Yang HK, Suh YS, Lee HJ. Minimally invasive approaches for gastric cancer-Korean experience. J Surg Oncol. 2013;107:277–281. doi: 10.1002/jso.23179. [DOI] [PubMed] [Google Scholar]
- 82.Kim YW, Yoon HM, Eom BW, Park JY. History of minimally invasive surgery for gastric cancer in Korea. J Gastric Cancer. 2012;12:13–17. doi: 10.5230/jgc.2012.12.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]


