Abstract
Background/Aims
Knowledge of the risk factors associated with adverse outcomes after percutaneous endoscopic gastrostomy (PEG) may be helpful for developing PEG recommendations. The purpose of this study was to identify the clinical risk factors associated with adverse clinical outcomes after PEG, especially regarding the use of proton pump inhibitors (PPIs).
Methods
We retrospectively reviewed the data from PEG patients at seven university hospitals between June 2006 and January 2012. All patients were followed up through February 2012 after PEG, and the clinical risk factors for adverse clinical outcomes after PEG were analyzed.
Results
Data from 1,021 PEG patients were analyzed. PPI users were more frequently included in the complication group than the noncomplication group (p=0.040). PEG-related complications (p=0.040) and mortality (p=0.003) were more frequent in the PPI group than in the control group. In the subgroup analysis of complicated PEG cases, infectious complications were more frequently found in the PPI group than in the control group (35.8% vs 27.8%). After adjustment for multiple possible confounding factors, PPI users (odds ratio, 1.531; 95% confidence interval, 1.017 to 2.305) and diabetic patients had increased mortality after PEG.
Conclusions
PPI use may be associated with adverse outcomes in patients with PEG; however, further prospective studies investigating this issue are warranted.
Keywords: Percutaneous endoscopic gastrostomy, Proton pump inhibitors, Mortality, Complication, Risk factors
INTRODUCTION
Percutaneous endoscopic gastrostomy (PEG) is an established procedure for providing nutrients through the intestine.1,2 Although PEG is simple to perform, it has a general complication rate of up to 50% and major complications occur in up to 7% of cases.3,4,5,6 Common complications reported after PEG include wound infection, aspiration pneumonia, peritonitis, or bleeding.3,4 Knowledge about the risk factors associated with adverse clinical outcomes after PEG may be helpful for the selection and recommendation of PEG.
Previously studied risk factors associated with a high mortality and complication rate after PEG include low serum albumin levels, high C-reactive protein (CRP) levels, advanced patient age, low body mass index (BMI), and comordities.7,8,9,10,11,12,13,14,15,16,17 However, little is known about whether the proton pump inhibitor (PPI) use may be associated with the adverse outcomes of PEG. PPI use may potentially increase the susceptibility to infection and pneumonia,18,19,20,21,22,23,24 which are common complications of PEG. Furthermore, PPI user in PEG patients may be associated with the higher rate of cardiovascular and cerebrovascular comorbidities.25 Therefore, it is hypothesized that PPI use in PEG patients might be associated with the adverse outcomes of PEG.
The purpose of this study was to identify the clinical predictors of adverse outcomes of PEG, especially focused on PPI use. To the best of our knowledge, this study is the first study to evaluate the association of PPI use with adverse outcomes of PEG.
MATERIALS AND METHODS
1. Patients
This study analyzed the clinical outcomes of PEG among all consecutive patients who underwent PEG at seven university hospitals in the Republic of Korea between June 2006 and January 2012. Patients with the following conditions were excluded: 1) an age of <18 years, 2) a personal history of gastrectomy, or 3) insufficient data. Simple PEG changes during the study period after an initial PEG placement were also excluded. Data were collected on patient age, gender, weight, height, BMI, diabetes mellitus (DM), indications for PEG (neurological disease, stroke, malignancy, hypoxic brain damage, or others), current medications (PPIs, H2 receptor antagonists [H2RAs], antacids, antiplatelet agents, anticoagulants, nonsteroidal anti-inflammatory drugs, and immunosuppressive agents), laboratory data (white blood cell [WBC], albumin, creatinine, and CRP) within 72 hours before PEG, complications, mortality, and duration of hospitalization after PEG. The incidence of complications and mortality of PEG were compared over the study period between a PPI group and a control group. All patients were followed up to February 2012 for complications and mortality occurred after PEG. This study was performed according to the principles of the Declaration of Helsinki, and was approved by the Institutional Review Board of each hospital.
2. Definitions
The PPI user was defined as patients who were taking standard dose of PPIs at least 48 hours before PEG placement, and all PPIs were included in the PPI group because all PPIs are similar in efficacy and potency and generally cause the hypochlorhydria at their therapeutic doses.18 As most PPIs provide hypochlorhydria from the first day of therapy,18 PPI use more than 48 hours was defined as a PPI user in this study. In contrast, the control group was defined as patients who had no use of PPI as well as no use of H2RA or antacid, which can affect the acidity of stomach. DM was defined as a fasting glucose of ≥126 mg/dL or use of insulin or hypoglycemic agents.
PEG-related complications included all cases of PEG-related mortality, bowel perforation, post-PEG gastrointestinal bleeding, peritonitis, fever, pneumonia, peristomal leaks, or infection. PEG-related infectious complications included all cases of peristomal infection, peritonitis, and pneumonia. However, repeat procedure or second puncture at the time of PEG or asymptomatic pneumoperitoneum was not considered as a complication in this study.
3. Percutaneous endoscopic gastrostomy
PEG was performed only if the patient's physicians estimated that the patient would otherwise need a nasogastric tube feeding for more than 30 days. Patients with a current diagnosis of sepsis, ascites, coagulation disorders, or severely compromised cardiopulmonary function were excluded from indications of PEG. Most of the patients had been fed by nasogastric tube before the PEG insertion procedure. The PEG procedure was performed by either attending gastroenterologists or by experienced fellows under direct supervision of the attending gastroenterologists. The signs and symptoms of the potential complications of PEG were explained to the patients and/or their representatives before starting PEG, and written consent was obtained before the procedure. PEGs were performed with standard upper endoscopes under Ponsky-Gauderer (pull-string) technique in all study hospitals during admission.26 Patients fasted from the midnight before the procedure, and cefazolin 1.0 g was injected intravenously 20 minutes before the procedure. Most of PEGs were performed under conscious sedation by the decision of the performing endoscopists, and midazolam (0.05 to 0.1 mg/kg) or profopol (0.5 to 1 mg/kg) were used for conscious sedation. A 24 F PEG kit (PEG System, Wilson-Cook; Wilson-Cook Medical Inc., Winston-Salem, NC, USA) were used in all study hospitals.
After PEG placement, patients fasted for 12 hours, and began receiving water unless bleeding, ileus, or fever was noted. Caregivers or patients were taught PEG management methods by a regular nurse and/or dietitian of the nutrition support team on how to perform daily care of the PEG tube and wound, solutions to common PEG-related problems, and nutritional advice.
4. Statistical analysis
The primary outcome was the incidence of adverse outcomes after PEG in the PPI group and the control group. Secondary outcomes were the clinical and laboratory characteristics of both groups. Continuous variables were expressed as mean±SD, and categorical variables were expressed as numbers (percentage). Comparisons between the two groups were made using either the t-test or Fisher exact test. Tests for proportionality between two groups were made using chi-square tests. Logistic regression models were used to estimate odds ratios (ORs) and 95% confidence intervals (CIs) for mortality after PEG. Variables with p<0.1 in the univariate analysis were added to the multivariate logistic regression model to identify independent risk factors associated with mortality after PEG. Statistical significance was defined as p<0.05, and a statistical trend was defined as p<0.1. Statistical analysis was performed using the SPSS version 13.0 for Windows (SPSS Inc., Chicago, IL, USA).
RESULTS
During the study period, 1,043 patients received PEG at seven university hospitals. Of these, 22 patients were excluded from data analysis due to insufficient data (n=13) or being <18 years of age (n=9). In total, 1,021 patients were included, with a mean age of 66.1±14 years, and with males (66.7%) being slightly predominated. The median follow-up time was 136 days (range, 1 to 2,693 days).
Table 1 shows the clinical and laboratory characteristics of 1,021 patients who received PEG according to complications. Patients with malignancy were more frequently included in the complication group than noncomplication group (p=0.020). Indications of PEG can't be clarified in 39 cases due to dysphagia or refusal of oral feeding without definite causes in extreme old ages, and these data were excluded from the classification of indication. Patients being treated with PPI, antiplatelet agents or anticoagulants were more frequently included in the complication group than noncomplication group (p=0.040, p=0.016, and p=0.006, respectively). However, two groups were not significantly different in other variables.
Table 1.
Clinical and Laboratory Characteristics of 1,021 Percutaneous Endoscopic Gastrostomy Patients with or without Complications
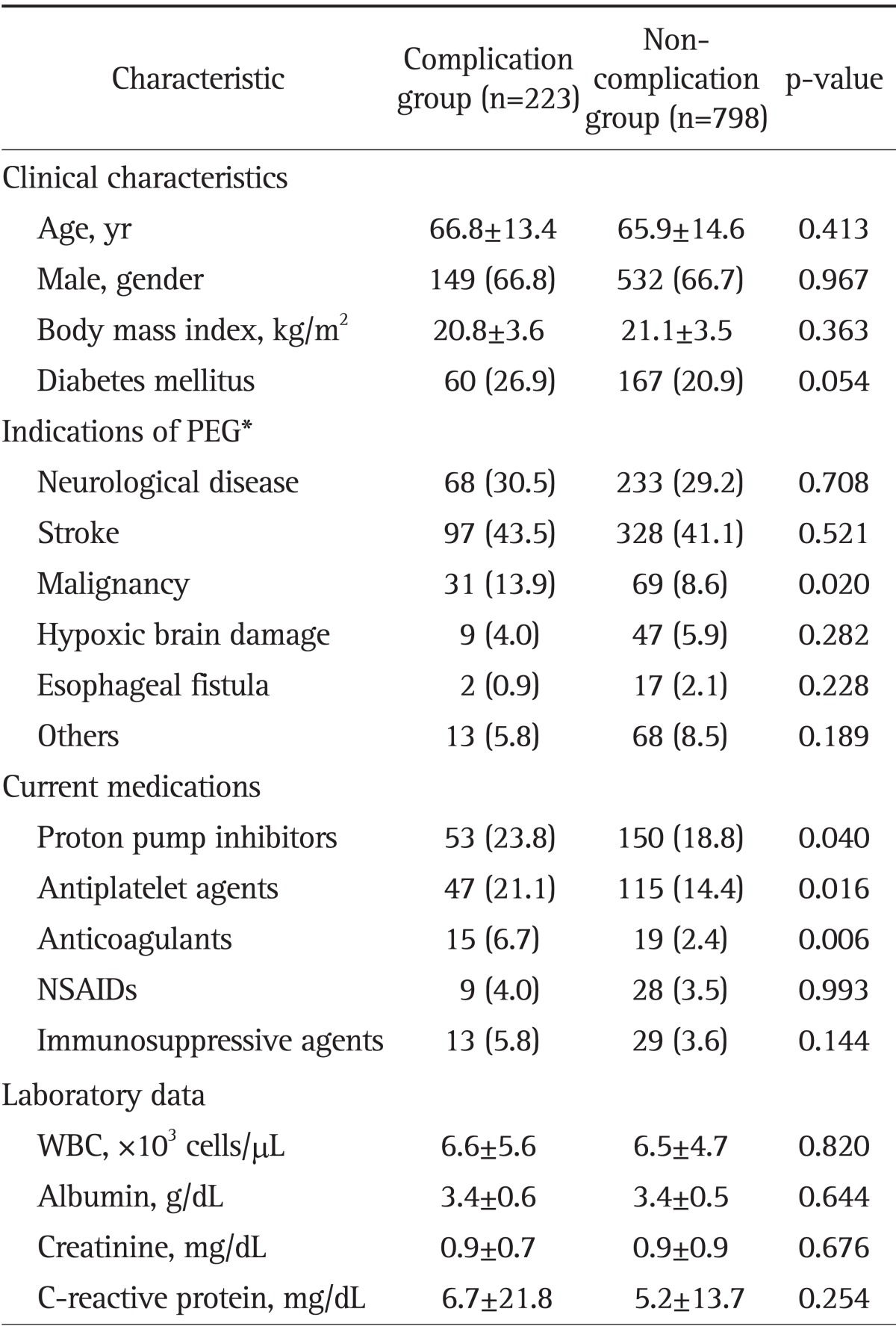
Data are presented as mean±SD or number (%).
PEG, percutaneous endoscopic gastrostomy; NSAID, nonsteroidal anti-inflammatory drug; WBC, white blood cell.
*Indications of PEG could not be clarified in 39 cases, and these cases were excluded from the classification of indication.
Table 2 shows the clinical and laboratory characteristics of 675 PEG patients according to PPI use: a PPI group (n=203) and a control group (n=472). The remaining 346 patients were excluded from data analysis as they did not satisfy the criteria of a PPI group and a control group, which was described in the Materials and Methods. Two groups were not significantly different in age, gender, BMI, or DM. Patients with neurological disease or stroke were more frequently included in the PPI group (p=0.000, respectively), and patients under antiplatelet agents and anticoagulants were more frequently included in the PPI group than in the control group (p=0.001 and p=0.039). Laboratory findings of WBCs and albumin were significantly lower in the PPI group than in the control group (all p<0.05). Other variables were equally included in both groups. Patients in the PPI group showed a higher rate of PEG-related complications (p=0.040) and mortality (p=0.003) than those in the control group. In subgroup analysis of complicated PEG cases, infectious complications were more frequently detected in the PPI group than in the control group (35.8% vs 27.8%), however, this difference was not statistically significant (p=0.312). Among infectious complications, occurrences of pneumonia were also more frequently detected in the PPI group than in the control group (20.8% vs 13.3%; p=0.243). The cure rate of complications and duration of hospitalization after PEG were not significantly different between two groups, either.
Table 2.
Comparative Analysis of the Clinical Characteristics and Outcomes of 675 Percutaneous Endoscopic Gastrostomy Patients between the Proton Pump Inhibitor Group versus the Control Group
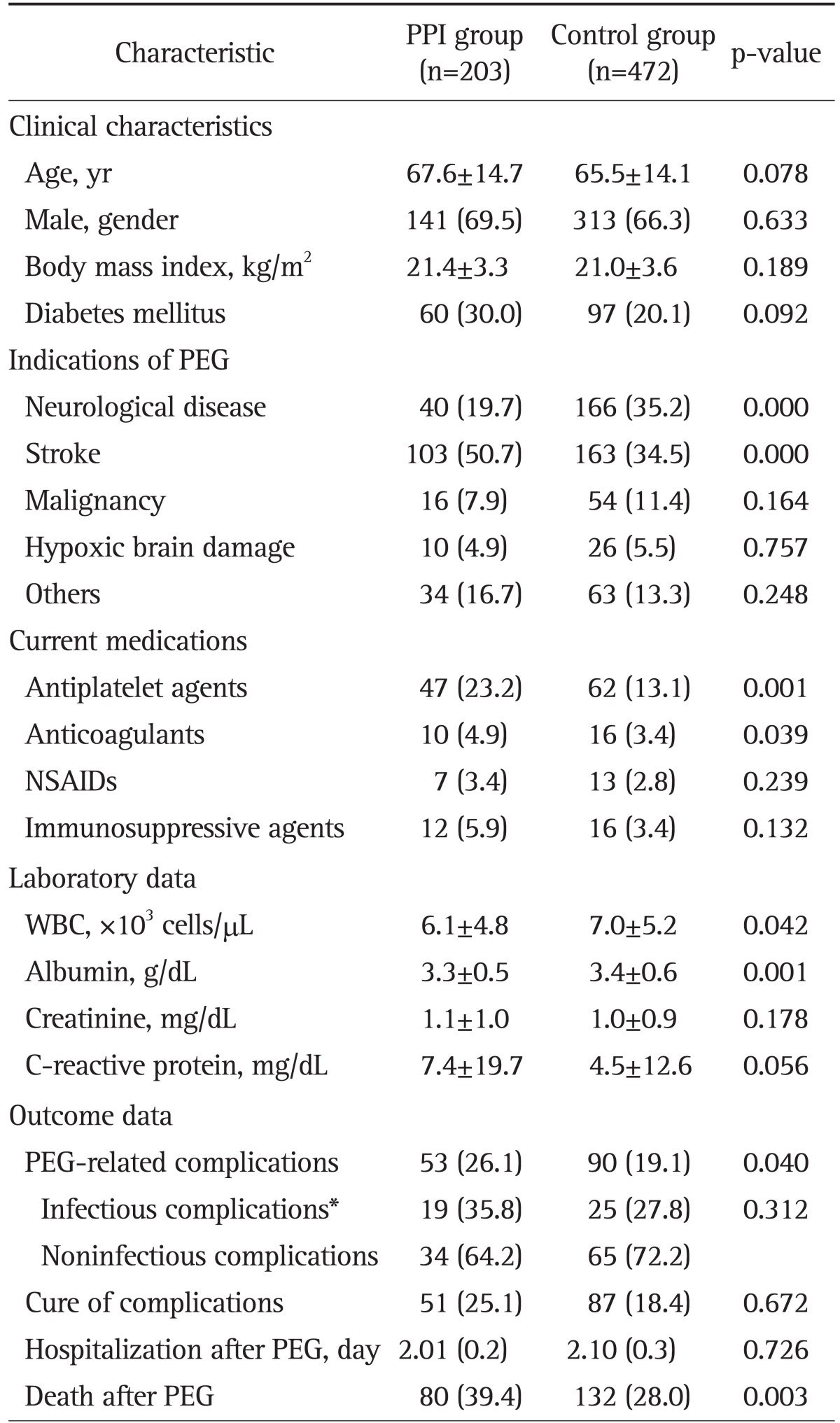
Data are presented as mean±SD or number (%).
PEG, percutaneous endoscopic gastrostomy; NSAID, nonsteroidal anti-inflammatory drug; WBC, white blood cell.
*PEG-related infectious complications included all cases of peristomal infection, peritonitis, and pneumonia.
To identify independent risk factors related to mortality after PEG placement, multivariate analysis was performed after adjusting for age, DM, neurological disease, stroke, use of PPIs, antiplatelet agents or anticoagulants, WBC level, albumin level, and CRP level (Table 3). In this analysis, PPI use (OR, 1.712; 95% CI, 1.147 to 2.555; p=0.009) as well as the presence of DM (OR, 1.853; 95% CI, 1.210 to 2.837; p=0.005) were found to be independent risk factors for increased mortality after PEG placement.
Table 3.
Multivariate Analysis of the Associated Variables for Mortality after Percutaneous Endoscopic Gastrostomy Placement
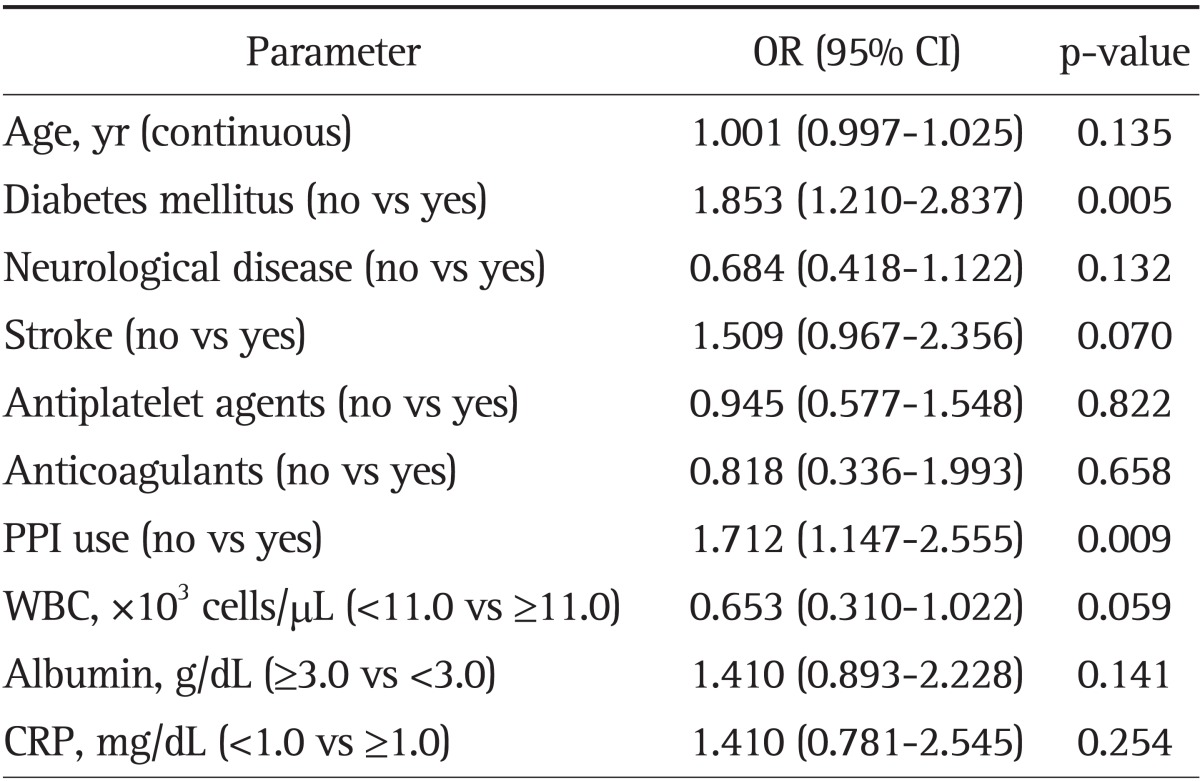
OR, odds ratio; CI, confidence interval; PPI, proton pump inhibitor; WBC, white blood cell; CRP, C-reactive protein.
DISCUSSION
To our knowledge, this is the first study examining the association between PPI use and adverse clinical outcomes of PEG. In this study, patients being treated with PPI were more frequently included in the complication group than noncomplication group (p=0.040). PPI users before PEG placement more frequently experienced PEG-related complications (p=0.040) and overall mortality (p=0.003) than those in the control group. Although the difference did not reach statistical significance, infectious complications, and pneumonia were more frequently detected in the PPI group than in the control group (35.8% vs 27.8%, p=0.312 and 20.8% vs 13.3%, p=0.243, respectively) in subgroup analysis of PEG cases with complications. Furthermore, the data indicated that PPI user (OR, 1.531; 95% CI, 1.017 to 2.305) as well as diabetic patients were associated with an increased mortality of PEG, even after adjusting for multiple possible confounding factors with multivariate analysis.
Most previous studies on outcomes of PEG suggested low albumin levels, high CRP levels, advanced patient age, low BMI, dementia, and comorbidities as potential risk factors for high mortality and complication rates of PEG.7,8,9,10,11,12,13,14,15,16,17 Lang et al.7 reported that the presence of DM, chronic obstructive pulmonary disease, and a low serum albumin level each increased the early mortality risk among PEG patients. In accordance with this report, we also found that the presence of DM was associated with an increased mortality after PEG. However, it is interesting that PPI use before PEG placement was associated with adverse outcomes after PEG procedure, which is a novel finding of this study.
With the widespread use of PPIs, the possible association of PPI use with adverse outcomes after PEG may be considered. Although little is known about the association between PPI use and adverse outcomes of PEG, there might be several possible explanations for this association. First of all, PPI use may simply be a clinical predictor for severe comorbid patients and not the direct cause of these complications. In our study, stroke was more frequently indicated in a PPI group than a control group, and patients using antiplatelet agents or anticoagulants, which may represent the cardiovascular or cerebrovascular comorbidities, were more frequently included in the PPI group. Therefore, the higher mortality rate of PEG in the PPI group may be associated with the higher rate of cardiovascular or cerebrovascular comorbidities in this group, because PPIs are frequently prescribed in these conditions.25 Secondly, the complications of PEG may be potentially increased by the use of PPI itself. In the literature review, the use of PPIs may inhibit the bactericidal activity of neutrophils,27,28,29,30,31 increase bacterial translocation32 and encourage growth of gut microflora.33 As a result, PPI use may increase the susceptibility of infectious complications of PEG, such as wound infection or pneumonia. Ono et al.34 demonstrated that the wound infection after PEG is increased by gastric hypochlorhydria. In our study, infectious complications including pneumonia, wound infection, and peritonitis were more frequently detected in the PPI group than in the control group, however, the difference did not reach a statistical significance possibly due to a small sample size of cases with infectious complications. Therefore, further large prospective studies on this issue may be warranted to confirm this association.
Our study has an important clinical implication, as it is the first large study to evaluate the association between PPI use and adverse outcomes of PEG. PPIs are available over the counter in many countries and overused,35 and majority of PPI use had no documented indication for their use.36 Therefore, physicians may consider the proper indications of PPI use before PEG placement based on our findings. The other advantage of our study was that we included multiple possible predictors of adverse outcome of PEG and many clinical data including medication history and laboratory findings, which were obtained from multiple endoscopic centers.
However, our study has some limitations. First of all, the study design was retrospective and we can't answer whether PPI users are prone to adverse clinical outcomes unrelated to PPI use (i.e., PPI is simply a clinical predicator) or PPI use itself has the causal relationship with the adverse clinical outcomes. Retrospective study can't answer the cause and effect relationship, and this limitation is not related to our study but inherent in all retrospective study. However, our study may call attention the possible relationship of PPI use and adverse clinical outcomes in patients with PEG, and may draw further prospective studies on this issue. The causal relationship of PPI use and adverse clinical outcomes in patient with PEG will be more clearly elucidated in the future prospective studies. Secondly, the causes of mortality were not clearly demonstrated in our study because of its retrospective nature and multiple comorbid conditions in our study patients. However, the exact causes of mortality in PEG patients might not be clearly identified, either, even in a prospective data collection as PEG patients often have multiple, chronic, debilitating conditions. Thirdly, 346 patients were excluded from data analysis as they did not fulfill the criteria of either the PPI group or the control group. However, our classification scheme was necessary for a clear division of the state of hypochlorhydria because any use of H2RAs or antacids may affect the acidity of stomach, even though they did not use any PPIs.
In conclusion, the use of PPI may be associated with adverse clinical outcomes in patients with PEG, however, further prospective studies on this issue may be warranted to elucidate this association.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Angus F, Burakoff R. The percutaneous endoscopic gastrostomy tube medical and ethical issues in placement. Am J Gastroenterol. 2003;98:272–277. doi: 10.1111/j.1572-0241.2003.07267.x. [DOI] [PubMed] [Google Scholar]
- 2.Guglielmi FW, Panella C, Losco A, et al. Clinical nutrition practice in Italian Gastroenterology Units. Dig Liver Dis. 2000;32:473–479. doi: 10.1016/s1590-8658(00)80003-1. [DOI] [PubMed] [Google Scholar]
- 3.Mamel JJ. Percutaneous endoscopic gastrostomy. Am J Gastroenterol. 1989;84:703–710. [PubMed] [Google Scholar]
- 4.Safadi BY, Marks JM, Ponsky JL. Percutaneous endoscopic gastrostomy. Gastrointest Endosc Clin N Am. 1998;8:551–568. [PubMed] [Google Scholar]
- 5.Grant DG, Bradley PT, Pothier DD, et al. Complications following gastrostomy tube insertion in patients with head and neck cancer: a prospective multi-institution study, systematic review and meta-analysis. Clin Otolaryngol. 2009;34:103–112. doi: 10.1111/j.1749-4486.2009.01889.x. [DOI] [PubMed] [Google Scholar]
- 6.Sheehan JJ, Hill AD, Fanning NP, et al. Percutaneous endoscopic gastrostomy: 5 years of clinical experience on 238 patients. Ir Med J. 2003;96:265–267. [PubMed] [Google Scholar]
- 7.Lang A, Bardan E, Chowers Y, et al. Risk factors for mortality in patients undergoing percutaneous endoscopic gastrostomy. Endoscopy. 2004;36:522–526. doi: 10.1055/s-2004-814400. [DOI] [PubMed] [Google Scholar]
- 8.Sanders DS, Carter MJ, D'Silva J, James G, Bolton RP, Bardhan KD. Survival analysis in percutaneous endoscopic gastrostomy feeding: a worse outcome in patients with dementia. Am J Gastroenterol. 2000;95:1472–1475. doi: 10.1111/j.1572-0241.2000.02079.x. [DOI] [PubMed] [Google Scholar]
- 9.Figueiredo FA, da Costa MC, Pelosi AD, Martins RN, Machado L, Francioni E. Predicting outcomes and complications of percutaneous endoscopic gastrostomy. Endoscopy. 2007;39:333–338. doi: 10.1055/s-2007-966198. [DOI] [PubMed] [Google Scholar]
- 10.Janes SE, Price CS, Khan S. Percutaneous endoscopic gastrostomy: 30-day mortality trends and risk factors. J Postgrad Med. 2005;51:23–28. [PubMed] [Google Scholar]
- 11.Blomberg J, Lagergren P, Martin L, Mattsson F, Lagergren J. Albumin and C-reactive protein levels predict short-term mortality after percutaneous endoscopic gastrostomy in a prospective cohort study. Gastrointest Endosc. 2011;73:29–36. doi: 10.1016/j.gie.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 12.Smith BM, Perring P, Engoren M, Sferra JJ. Hospital and long-term outcome after percutaneous endoscopic gastrostomy. Surg Endosc. 2008;22:74–80. doi: 10.1007/s00464-007-9372-z. [DOI] [PubMed] [Google Scholar]
- 13.Higaki F, Yokota O, Ohishi M. Factors predictive of survival after percutaneous endoscopic gastrostomy in the elderly: is dementia really a risk factor? Am J Gastroenterol. 2008;103:1011–1016. doi: 10.1111/j.1572-0241.2007.01719.x. [DOI] [PubMed] [Google Scholar]
- 14.Chong VH, Vu C. Percutaneous endoscopic gastrostomy outcomes: can patient profiles predict mortality and weaning? Singapore Med J. 2006;47:383–387. [PubMed] [Google Scholar]
- 15.Tokunaga T, Kubo T, Ryan S, et al. Long-term outcome after placement of a percutaneous endoscopic gastrostomy tube. Geriatr Gerontol Int. 2008;8:19–23. doi: 10.1111/j.1447-0594.2008.00442.x. [DOI] [PubMed] [Google Scholar]
- 16.Abuksis G, Mor M, Segal N, et al. Percutaneous endoscopic gastrostomy: high mortality rates in hospitalized patients. Am J Gastroenterol. 2000;95:128–132. doi: 10.1111/j.1572-0241.2000.01672.x. [DOI] [PubMed] [Google Scholar]
- 17.Fox VL, Abel SD, Malas S, Duggan C, Leichtner AM. Complications following percutaneous endoscopic gastrostomy and subsequent catheter replacement in children and young adults. Gastrointest Endosc. 1997;45:64–71. doi: 10.1016/s0016-5107(97)70304-3. [DOI] [PubMed] [Google Scholar]
- 18.Bavishi C, Dupont HL. Systematic review: the use of proton pump inhibitors and increased susceptibility to enteric infection. Aliment Pharmacol Ther. 2011;34:1269–1281. doi: 10.1111/j.1365-2036.2011.04874.x. [DOI] [PubMed] [Google Scholar]
- 19.McCarthy DM. Adverse effects of proton pump inhibitor drugs: clues and conclusions. Curr Opin Gastroenterol. 2010;26:624–631. doi: 10.1097/MOG.0b013e32833ea9d9. [DOI] [PubMed] [Google Scholar]
- 20.Dial MS. Proton pump inhibitor use and enteric infections. Am J Gastroenterol. 2009;104(Suppl 2):S10–S16. doi: 10.1038/ajg.2009.46. [DOI] [PubMed] [Google Scholar]
- 21.Leonard J, Marshall JK, Moayyedi P. Systematic review of the risk of enteric infection in patients taking acid suppression. Am J Gastroenterol. 2007;102:2047–2056. doi: 10.1111/j.1572-0241.2007.01275.x. [DOI] [PubMed] [Google Scholar]
- 22.Howell MD, Novack V, Grgurich P, et al. Iatrogenic gastric acid suppression and the risk of nosocomial Clostridium difficile infection. Arch Intern Med. 2010;170:784–790. doi: 10.1001/archinternmed.2010.89. [DOI] [PubMed] [Google Scholar]
- 23.Linsky A, Gupta K, Lawler EV, Fonda JR, Hermos JA. Proton pump inhibitors and risk for recurrent Clostridium difficile infection. Arch Intern Med. 2010;170:772–778. doi: 10.1001/archinternmed.2010.73. [DOI] [PubMed] [Google Scholar]
- 24.Sultan N, Nazareno J, Gregor J. Association between proton pump inhibitors and respiratory infections: a systematic review and meta-analysis of clinical trials. Can J Gastroenterol. 2008;22:761–766. doi: 10.1155/2008/821385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blackburn DF, Lamb DA, Mcleod MM, Eurich DT. Increased use of acid-suppressing drugs before the occurrence of ischemic events: a potential source of confounding in recent observational studies. Pharmacotherapy. 2010;30:985–993. doi: 10.1592/phco.30.10.985. [DOI] [PubMed] [Google Scholar]
- 26.Gauderer MW, Ponsky JL, Izant RJ., Jr Gastrostomy without laparotomy: a percutaneous endoscopic technique. J Pediatr Surg. 1980;15:872–875. doi: 10.1016/s0022-3468(80)80296-x. [DOI] [PubMed] [Google Scholar]
- 27.Suzuki M, Mori M, Miura S, et al. Omeprazole attenuates oxygen-derived free radical production from human neutrophils. Free Radic Biol Med. 1996;21:727–731. doi: 10.1016/0891-5849(96)00180-3. [DOI] [PubMed] [Google Scholar]
- 28.Zedtwitz-Liebenstein K, Wenisch C, Patruta S, Parschalk B, Daxböck F, Graninger W. Omeprazole treatment diminishes intra- and extracellular neutrophil reactive oxygen production and bactericidal activity. Crit Care Med. 2002;30:1118–1122. doi: 10.1097/00003246-200205000-00026. [DOI] [PubMed] [Google Scholar]
- 29.Wandall JH. Effects of omeprazole on neutrophil chemotaxis, super oxide production, degranulation, and translocation of cytochrome b-245. Gut. 1992;33:617–621. doi: 10.1136/gut.33.5.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luciani F, Spada M, De Milito A, et al. Effect of proton pump inhibitor pretreatment on resistance of solid tumors to cytotoxic drugs. J Natl Cancer Inst. 2004;96:1702–1713. doi: 10.1093/jnci/djh305. [DOI] [PubMed] [Google Scholar]
- 31.Handa O, Yoshida N, Fujita N, et al. Molecular mechanisms involved in anti-inflammatory effects of proton pump inhibitors. Inflamm Res. 2006;55:476–480. doi: 10.1007/s00011-006-6056-4. [DOI] [PubMed] [Google Scholar]
- 32.Hopkins AM, McDonnell C, Breslin NP, O'Morain CA, Baird AW. Omeprazole increases permeability across isolated rat gastric mucosa pre-treated with an acid secretagogue. J Pharm Pharmacol. 2002;54:341–347. doi: 10.1211/0022357021778583. [DOI] [PubMed] [Google Scholar]
- 33.Lewis SJ, Franco S, Young G, O'Keefe SJ. Altered bowel function and duodenal bacterial overgrowth in patients treated with omeprazole. Aliment Pharmacol Ther. 1996;10:557–561. doi: 10.1046/j.1365-2036.1996.d01-506.x. [DOI] [PubMed] [Google Scholar]
- 34.Ono H, Ito S, Yamazaki Y, Otaki Y, Otaki H. Effects of gastric acidity on peristomal infection after percutaneous endoscopic gastrostomy placement. J Hosp Infect. 2010;76:42–45. doi: 10.1016/j.jhin.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 35.Heidelbaugh JJ, Goldberg KL, Inadomi JM. Overutilization of proton pump inhibitors: a review of cost-effectiveness and risk [corrected] Am J Gastroenterol. 2009;104(Suppl 2):S27–S32. doi: 10.1038/ajg.2009.49. [DOI] [PubMed] [Google Scholar]
- 36.Rotman SR, Bishop TF. Proton pump inhibitor use in the U.S. ambulatory setting, 2002-2009. PLoS One. 2013;8:e56060. doi: 10.1371/journal.pone.0056060. [DOI] [PMC free article] [PubMed] [Google Scholar]


