Abstract
Background/Aims
A link between G protein β3 (GNB3) polymorphism and functional dyspepsia (FD) has been suggested. The aim of this study was to determine the role of GNB3 polymorphism in the long-term prognosis of FD in Koreans.
Methods
FD patients and normal healthy controls were recruited from patients who visited our center between December 2006 and June 2007. All of the subjects completed Rome III questionnaires before undergoing upper gastrointestinal endoscopy and colonoscopy. Genomic DNA was extracted for GNB3 genotyping. After 5 years, the subjects were reevaluated using the same questionnaires.
Results
GNB3 825T carrier status was significantly related to FD in Koreans (p=0.04). After 5 years, 61.0% of the initial FD patients and 12.2% of the initial normal subjects were diagnosed with FD (odds ratio [OR], 11.7; 95% confidence interval [CI], 4.3 to 31.1; p<0.001). Regardless of the GNB3 genotype (p=0.798), female sex was strongly correlated with FD after 5 years (OR, 3.3; 95% CI, 1.2 to 9.1; p=0.017).
Conclusions
The T allele of GNB3 is linked to FD in Koreans but does not predict long-term prognosis. Female sex is related to a higher prevalence of FD after 5 years.
Keywords: Functional dyspepsia, G protein β3, Polymorphism, Prognosis, Rome III
INTRODUCTION
Functional dyspepsia (FD) is a kind of functional gastrointestinal disorder (FGID) that is characterized by the presence of repetitive symptoms that are thought to originate in the gastroduodenal region in the absence of any organic, systemic disease.1 FD is a highly prevalent disease, affecting about 8% to 25% of the general population.2 Korean epidemiologic studies have demonstrated that the prevalence of FD in Koreans is between 8% and 37% based on Rome III criteria.3,4 Due to high morbidity and the prolonged duration of FD, it has a substantial impact on the patient's quality of life and medical costs.5,6
FD is a heterogeneous disorder, and multiple pathogenetic mechanisms are likely to be involved. The etiology of FD is assumed to be associated with delayed gastric emptying, altered visceral sensitivity, dysfunction of the autonomic nervous system, infection, alterations of the immune system, altered intestinal motility, and psychiatric factors.7,8,9 Recently, genetic approaches have made forward steps to identify the etiology of FD. A number of receptors, including serotonin transporter protein, interleukin-10, and heterotrimeric G proteins, have been shown to alter gastrointestinal functions related to FD.10,11,12 Among many candidate genes, the G protein β3 (GNB3) subunit polymorphism is still being studied. GNB3 is located on chromosome 12p13.31.13 It is present in all cells of the body, encodes for the βσ3 subunit of the G protein complex, and plays a key role in the downstream signaling cascade following monoamine receptor activation.12 GNB3 has a single nucleotide polymorphism, C825T, that converts a cytosine to a thymidine, and allele shifting according to each subtype shows different activity.13 Taken together, this GNB3 polymorphism can lead to an altered signal transduction and functional abnormality such as the changes in sensory function or motility that are associated with FD, as it does in depression,5 hypertension,13 and obesity.14
Intriguingly, studies on the association between GNB3 polymorphism and FGID have produced inconstant results. In recent Japanese studies, the 825T allele was related to FD.2,15 More recently, Korean studies showed that GNB3 polymorphism is not associated with FD or IBS.16,17 Such differences are probably due to ethnic variations and the multiple pathogenetic mechanisms of FGID.
In our previous study, we have shown that GNB3 825T allele is associated with irritable bowel syndrome (IBS) with constipation in Koreans.18 In this study, we aimed to uncover a link between FD and GNB3 polymorphism in a genetically homogenous Korean population. In addition, we tried to assess the role of GNB3 polymorphism on the long-term prognosis of FD and to further assess other prognostic factors of FD after 5 years.
MATERIALS AND METHODS
1. Study population
FD patients and normal healthy controls were recruited between December 2006 and June 2007 among patients who visited our center. All of the subjects completed a self-administered questionnaire based on the Rome III criteria and agreed to genetic analysis for GNB3 polymorphism. After completing the informed consent forms, the subjects underwent both an upper gastrointestinal endoscopy and a colonoscopy to exclude organic diseases such as peptic ulcer disease, reflux esophagitis, or malignancy. Subjects with FD symptoms who met the Rome III criteria were recruited as FD patients, whereas subjects without symptoms were recruited as healthy controls.
This prospective study was approved by the Institutional Review Board of the Konkuk University School of Medicine (KUH1010398), which confirmed that the study was in accordance with the ethical guidelines of the Declaration of Helsinki. Subjects were aware that their care did not in any way depend on their participation or nonparticipation in the study.
2. Questionnaires
The study questionnaires consisted of items on demographics, social history, past medical history, Rome III criteria survey, and the validated Korean version of Beck's Depressive Inventory (BDI) to determine depression status.19 FD was diagnosed when there was one or more of the following symptoms based on Rome III criteria. Firstly, bothersome postprandial fullness which means one is uncomfortably full after regular sized meal for more than once a day or a week that happened more than 6 months ago. Secondly, early satiation which means one is unable to finish regular sized meal for more than once a day or a week that happened more than 6 months ago. Thirdly, epigastric pain or burning which means a pain or burning in middle of abdomen at least once a day or a week that happened more than 6 months ago. The subjects were diagnosed as FD if criteria fulfilled for the last 3 months with symptom onset at least 6 months prior to diagnosis, and if there is no evidence of structural disease (including at upper endoscopy) that is likely to explain the symptoms. FD has been subclassified into two disease categories under the Rome III criteria, postprandial distress syndrome (PDS) and epigastric pain syndrome (EPS).
PDS was diagnosed when both of the following criteria were satisfied. Firstly, bothersome postprandial fullness occurring after ordinary sized meals at least several times per week (uncomfortably full after regular sized meal, more than once a day or a week). Secondly, early satiation that prevents finishing a regular meal at least several times per week (unable to finish regular sized meal more than once a day or a week).
EPS was diagnosed when all of the following six criteria were satisfied. Firstly, pain or burning localized to the epigastrium of at least moderate severity at least once per week (pain or burning in middle of abdomen at least moderate severity that occurs at least once a day or a week). Secondly, the pain is intermittent which indicates that pain or burning often disappears completely in the same day. Thirdly, pain is not generalized or localized to other abdominal or chest regions (chest pain and heartburn occurs once a month or less often). Fourthly, pain is not relieved by defecation or passage of flatus, and never or rarely gets better after defecation. Fifthly, pain does not fulfill criteria for biliary pain. Sixthly, criteria fulfilled for the last 3 months with symptom onset at least 6 months prior to diagnosis.
Mixed type was diagnosed when the subject fulfilled both criteria of PDS and EPS subtypes.
Based on Rome III criteria, IBS was defined as recurrent abdominal pain or discomfort for at least 3 days per month in the past 3 months that is associated with two or more other symptoms such as improvement with defecation, onset of pain or discomfort is associated with a change in frequency of stool and with a change in form of stool. Four subgroups of IBS were classified according to the Bristol stool form scale: diarrhea predominant IBS, constipation predominant IBS, mixed IBS, and unclassified IBS.
3. Genetic analysis
Genomic DNA was extracted from buccal epithelial cells that were collected with cotton swabs using a QIAamp Kit (Qiagen, Hilden, Germany) as previously described in our previous study.18 Genotyping of the C825T polymorphism was performed according to the polymerase chain reaction (PCR)-based method followed by the restriction fragment length polymorphism technique. For genotypying of the GNB3 825C/T polymorphism, the DNA fragment amplification was performed with the sense primer 5'-TGA CCC ACT TGC CAC CCG TGC-3' and the antisense primer 5'-GCA CCC ACT TGC CAC CCG TGC-3'. PCR products were subsequently incubated overnight with the restriction endonuclease BseDI, which cuts at the 825 site, and the products was electrophoresed on 3% agarose gels and stained with ethidium bromide. The presence or absence of the restriction BseDI site determines whether a single 268-bp fragment (allele T) or two fragments of 116 and 152 bp (allele C) are produced.
4. Follow-up after 5 years
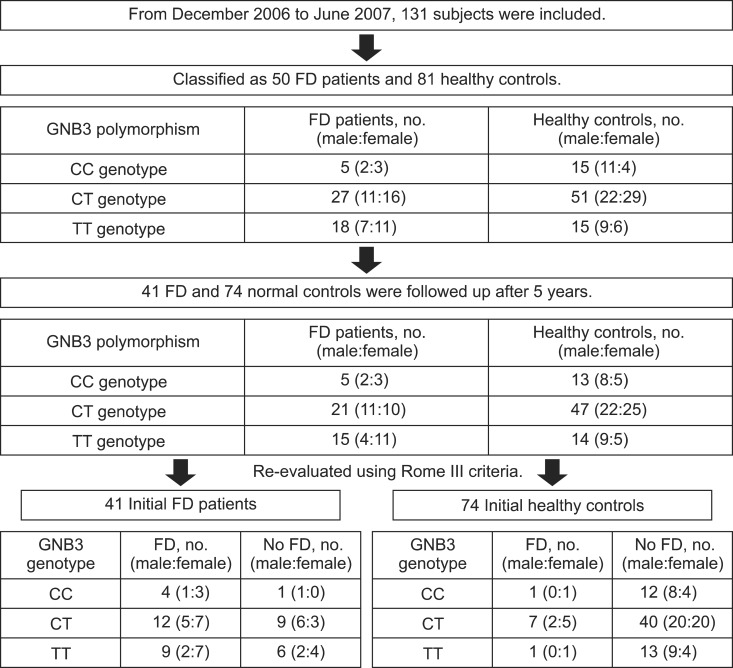
In June 2012, which was consistent with a 5-year follow-up period after the initial enrollment, the subjects were contacted by one physician (H.A.C.) for their current status. Of the 131 eligible subjects, 116 subjects completed the follow-up questionnaires, including Rome III criteria, and thus the response rate after 5 years was 88.5%. Of these 116 followed-up subjects, one was excluded from the study because of a newly developed organic disease (ulcerative colitis). Therefore, 115 subjects were analyzed for the follow-up data.
5. Statistical analysis
The distribution of the GNB3 genotypes was analyzed according to the Hardy-Weinberg equilibrium. A goodness of fit of the Hardy-Weinberg equilibrium was determined for the study groups to identify deviations from the normal genotype distribution. Thus, we compared the expected prevalence of the various alleles with the prevalence found in our study population. Deviations from the Hardy-Weinberg equilibrium were tested using chi-square tests. The chi-square tests and Student t-tests were used in the assessment of differences in variables between groups. Odds ratios (ORs) and corresponding 95% confidence intervals (CIs) were estimated by a multiple logistic regression model for the long-term follow-up data. For the statistical analysis, SPSS version 17.0 (SPSS Inc., Chicago, IL, USA) was used. A p-value less than 0.05 was considered statistically significant.
RESULTS
1. Characteristics of FD patients and normal controls
Between December 2006 and June 2007, 81 normal controls and 50 FD patients were included in the study. There were no significant differences between FD cases and healthy controls in age, gender, body mass index, smoking, drinking status, and score on the BDI (Table 1). Of the 50 FD patients, 16 patients were EPS-predominant type, 27 were PDS-predominant type, and seven were mixed type. Thirty-five of the 50 FD patients showed overlap with IBS. FD-IBS patients consisted of 20 IBS-diarrhea dominant type, three IBS-constipation dominant type, and 12 IBS-mixed type. There was no IBS-unclassified type in our study population.
Table 1.
Baseline Characteristics of the Subjects
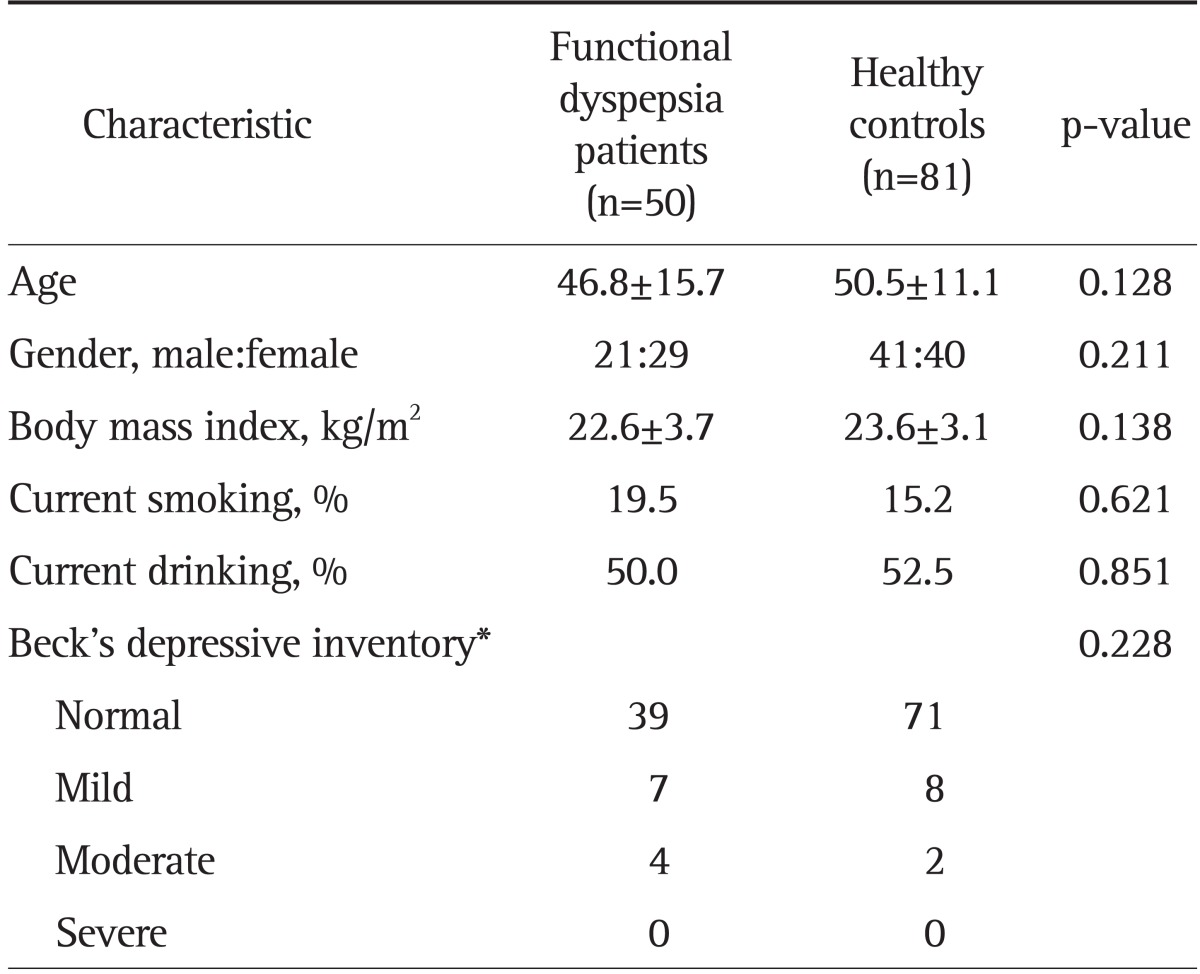
Data are presented as mean±SD or number.
*Cutoff values for the total Beck's depressive inventory score in males were as follows: 1) normal (≤15 points), 2) mild depressive mood (16 to 19 points), 3) moderate depressive mood (20 to 23 points), and 4) severe depressive mood (≥24 points). The cutoff values in females were 1 point higher than those in males: 1) normal (≤16 points), 2) mild depressive mood (17 to 20 points), 3) moderate depressive mood (21 to 24 points), and 4) severe depressive mood (≥25 points).
2. GNB3 genotype distributions in FD patients and normal controls
GNB3 polymorphism was investigated at the time of the initial enrollment, and GNB3 825T carrier status was significantly related to FD (p=0.040). Although homogenous GNB3 825T carrier status showed a tendency to be related to FD, the statistical difference was not significant (p=0.060). There was no difference on GNB3 genotype between the EPS-predominant FD patients and the PDS-predominant FD patients (p=0.571) (Table 2).
Table 2.
Genotype and Allele Frequencies of the G Protein β3 Gene
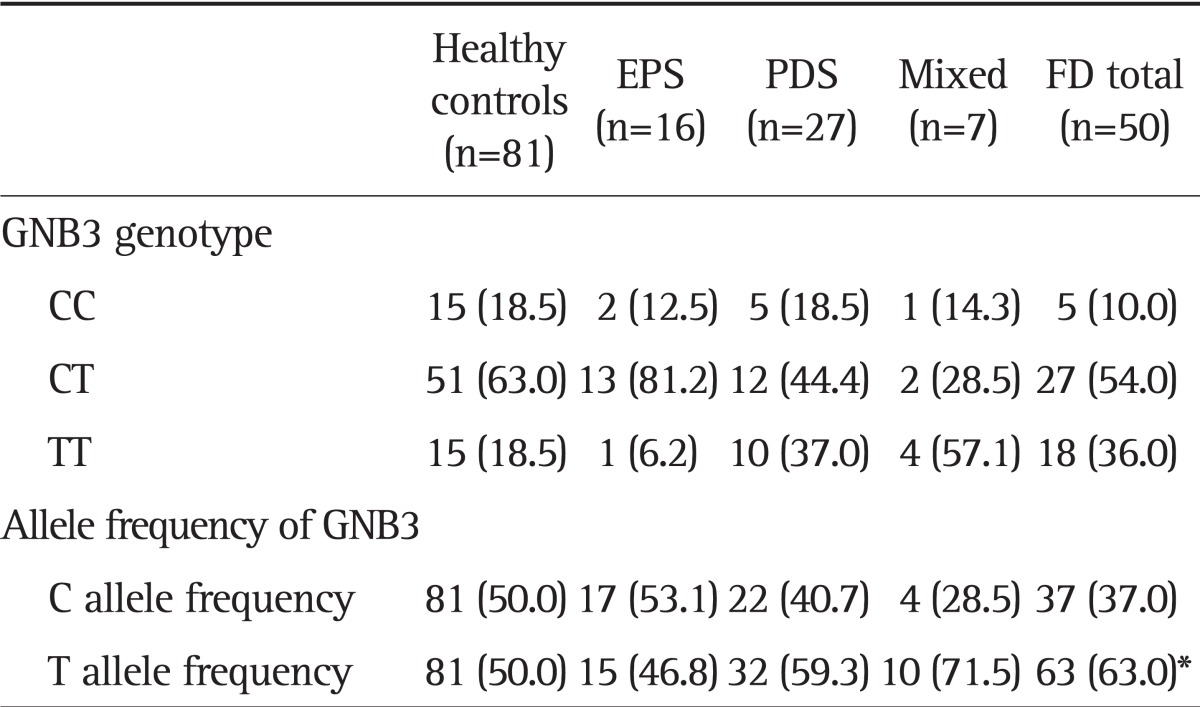
Data are presented as number (%).
EPS, epigastric pain syndrome; PDS, postprandial distress syndrome; FD, functional dyspepsia; GNB3, G protein β3.
*p=0.04; odds ratio, 1.70; 95% confidence interval, 1.02 to 2.83.
3. Long-term follow-up after the 5 years
Of the 115 individuals, 34 subjects matched the FD criteria after 5 years of the follow-up period (Fig. 1). There was no link between the GNB3 genotype and the presence of FD after 5 years (p=0.798). Regardless of the GNB3 genotype, female subjects (40.7%) showed a higher prevalence of FD than male subjects (17.9%) after 5 years (OR, 3.3; 95% CI, 1.2 to 9.1; p=0.008). In addition, subjects who were initially diagnosed as FD showed higher prevalence of FD after 5 years (61.0%) than those without FD at the time of study enrollment (12.2%) (OR, 11.7; 95% CI, 4.3 to 31.1; p<0.001). Age and subtypes of FD were not related to long-term prognosis (Table 3).
Fig. 1.
Flow diagram for the subjects according to G protein β3 (GNB3) polymorphism and sex. The flow diagram shows that 25 (61.0%) of the 41 functional dyspepsia (FD) patients and 9 (12.2%) of the 74 normal controls were diagnosed with FD after 5 years.
Table 3.
Logistic Regression Results for Independent Prognostic Factors Associated with Functional Dyspepsia after 5 Years
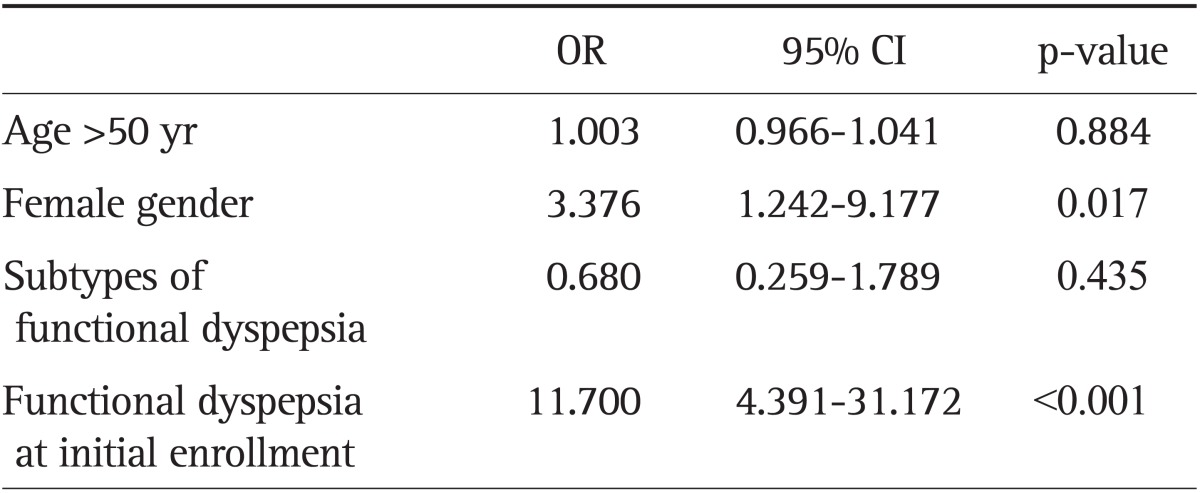
OR, odds ratio; CI, confidence interval.
4. FD-IBS overlap and the prognosis after 5 years
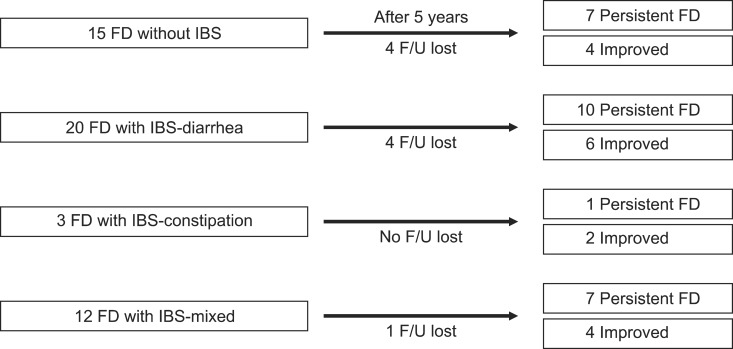
An FD-IBS overlap was diagnosed in 35 (70.0%) of the FD patients at the time of enrollment, while 15 of the patients were classified as FD alone. Of the 35 FD-IBS overlap patients, 30 were followed-up after 5 years. Ten (62.5%) of the 16 IBS-diarrhea dominant type patients presented with FD after 5 years, whereas one (33.3%) of the three IBS-constipation dominant type patients presented with FD after 5 years. Seven (63.6%) of the 11 IBS-mixed type patients presented with FD after 5 years. In total, seven (63.6%) of the 11 FD-only patients and 18 (60.0%) of the 30 FD-IBS overlap patients were diagnosed with FD after 5 years (Fig. 2).
Fig. 2.
Prognosis of patients with functional dyspepsia (FD)-irritable bowel syndrome (IBS) overlaps after 5 years. Of the 35 overlapping patients, 30 were followed up after 5 years.
IBS, irritable bowel syndrome; F/U, follow-up.
DISCUSSION
In this case-control association study, we found that the GNB3 allele frequency with T is linked to FD in Koreans, but it does not predict the long-term prognosis of FD. Regardless of the GNB3 genotype, the presence of FD after 5 years was related to female gender and to those who had been diagnosed as having FD.
GNB3 825T carrier status was significantly higher in FD patients than in healthy controls at the baseline of our study. This is consistent with a Japanese study that found an association between homozygous GNB3 825T status and Japanese dyspeptic subjects without Helicobacter pylori infection.2 Another Japanese study had the similar finding that the GNB3 825 TT genotype is associated with EPS.15 In addition, we showed that the GNB3 825T allele is associated with Korean IBS patients who have constipation in a recent study.18 These findings can be explained by the fact that GNB3 T allele increases signal transduction upon G protein-coupled receptor activation, which is associated with a visceral hypersensitivity that can lead to symptomatic generation.20 In contrast, a study from Germany found that homozygous GNB3 825C is associated with upper abdominal symptoms that are unrelated to meals.21 Furthermore, a study from the US revealed that meal-unrelated dyspepsia is associated with both the homozygous GNB3 825T and C genotypes.22 Such various results can be partially explained by differences in genotypic composition of different populations in different countries, which comprise different racial groups. In fact, T allele frequency in the GNB3 polymorphism is known to be higher in Asians (42% to 53%) than in Caucasians (27% to 42%).23,24
The prognosis of FD might differ according to various pathophysiological disturbances. Studies showed clustering of FGID within families, and studies of twins suggest that both genetic and environmental factors influence FD.9,25 Therefore, one suspects that the long-term prognosis of FD can be influenced both by genetic and environmental factors. Notably, in the present study, there was no association between GNB3 polymorphism and the presence of FD after 5 years from initial diagnosis. Based on our finding, it is clear that FD is not caused by a single genetic factor, hence, further research is required to elucidate the specific signal transduction pathways affected by these genetic factors and environmental factors. In other words, potential risk factors for FD, including age, gender, diet, lifestyle, H. pylori infection, smoking, drinking, or psychological disturbances, should be considered significant when predicting the prognosis of FD.26,27 Unfortunately, we were not able to investigate on environmental factors including food and stress in the current study.
Predictive factors, including GNB3 genotypes, for the long-term prognosis of FD are still uncertain. A recent population-based study showed that FGID decreased upon aging,28 and another study showed that psychic vulnerability is a predictive factor for a poor prognosis of FD,29 these studies but did not perform genetic analysis. In our study, regardless of the GNB3 genotype, females showed higher prevalence of FD than males after 5 years. It is well known that women have more severe nausea, satiety, constipation, and overall gastroparesis symptoms than men.30 Another study confirmed that prolonged gastric emptying in patients with FD is related to the female gender, while the abnormalities of the meal intragastric distribution appear to occur in dyspeptic males and females.31 They found that anxiety is frequent in FD and appears to be related to abnormal antral retention of food in females as observed in delayed gastric emptying and poor proximal gastric motor function. There also appear to be gender differences in the psychosocial realm, with dyspeptic women experiencing a lesser sense of well-being than dyspeptic men, as well as an association between a history of abuse and FD.32 Therefore, we can assume that such gender-related differences have led to a higher prevalence of FD in our female subjects than in male subjects by decreasing the pain tolerance.
In conclusion, we demonstrated that the GNB3 T allele frequency is linked to FD in Koreans, but that it does not predict the long-term prognosis of FD. Regardless of the GNB3 genotype, recurrence of FD is higher in women. Taken as a whole, gender should be considered more significantly than genetic polymorphisms when taking care of FD patients over a long-term period.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Drossman DA. The functional gastrointestinal disorders and the Rome III process. Gastroenterology. 2006;130:1377–1390. doi: 10.1053/j.gastro.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 2.Tahara T, Arisawa T, Shibata T, et al. Homozygous 825T allele of the GNB3 protein influences the susceptibility of Japanese to dyspepsia. Dig Dis Sci. 2008;53:642–646. doi: 10.1007/s10620-007-9923-0. [DOI] [PubMed] [Google Scholar]
- 3.Noh YW, Jung HK, Kim SE, Jung SA. Overlap of erosive and non-erosive reflux diseases with functional gastrointestinal disorders according to Rome III criteria. J Neurogastroenterol Motil. 2010;16:148–156. doi: 10.5056/jnm.2010.16.2.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim JS, Lee KJ, Kim JH, Hahm KB, Cho SW. Functional gastrointestinal disorders in patients referred to specialist gastroenterologists in a tertiary hospital. Korean J Neurogastroenterol Motil. 2004;10:111–117. [Google Scholar]
- 5.Lee HJ, Lee SY, Kim JH, Sung IK, et al. Depressive mood and quality of life in functional gastrointestinal disorders: differences between functional dyspepsia, irritable bowel syndrome and overlap syndrome. Gen Hosp Psychiatry. 2010;32:499–502. doi: 10.1016/j.genhosppsych.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 6.Brook RA, Kleinman NL, Choung RS, Melkonian AK, Smeeding JE, Talley NJ. Functional dyspepsia impacts absenteeism and direct and indirect costs. Clin Gastroenterol Hepatol. 2010;8:498–503. doi: 10.1016/j.cgh.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 7.Tack J, Piessevaux H, Coulie B, Caenepeel P, Janssens J. Role of impaired gastric accommodation to a meal in functional dyspepsia. Gastroenterology. 1998;115:1346–1352. doi: 10.1016/s0016-5085(98)70012-5. [DOI] [PubMed] [Google Scholar]
- 8.Holtmann G, Goebell H, Jockenhoevel F, Talley NJ. Altered vagal and intestinal mechanosensory function in chronic unexplained dyspepsia. Gut. 1998;42:501–506. doi: 10.1136/gut.42.4.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morris-Yates A, Talley NJ, Boyce PM, Nandurkar S, Andrews G. Evidence of a genetic contribution to functional bowel disorder. Am J Gastroenterol. 1998;93:1311–1317. doi: 10.1111/j.1572-0241.1998.440_j.x. [DOI] [PubMed] [Google Scholar]
- 10.Heils A, Teufel A, Petri S, et al. Allelic variation of human serotonin transporter gene expression. J Neurochem. 1996;66:2621–2624. doi: 10.1046/j.1471-4159.1996.66062621.x. [DOI] [PubMed] [Google Scholar]
- 11.Rad R, Dossumbekova A, Neu B, et al. Cytokine gene polymorphisms influence mucosal cytokine expression, gastric inflammation, and host specific colonisation during Helicobacter pylori infection. Gut. 2004;53:1082–1089. doi: 10.1136/gut.2003.029736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ford CE, Skiba NP, Bae H, et al. Molecular basis for interactions of G protein beta gamma subunits with effectors. Science. 1998;280:1271–1274. doi: 10.1126/science.280.5367.1271. [DOI] [PubMed] [Google Scholar]
- 13.Siffert W, Rosskopf D, Siffert G, et al. Association of a human G-protein beta3 subunit variant with hypertension. Nat Genet. 1998;18:45–48. doi: 10.1038/ng0198-45. [DOI] [PubMed] [Google Scholar]
- 14.Siffert W, Forster P, Jöckel KH, et al. Worldwide ethnic distribution of the G protein beta3 subunit 825T allele and its association with obesity in Caucasian, Chinese, and Black African individuals. J Am Soc Nephrol. 1999;10:1921–1930. doi: 10.1681/ASN.V1091921. [DOI] [PubMed] [Google Scholar]
- 15.Oshima T, Nakajima S, Yokoyama T, et al. The G-protein beta3 subunit 825 TT genotype is associated with epigastric pain syndrome-like dyspepsia. BMC Med Genet. 2010;11:13. doi: 10.1186/1471-2350-11-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park HY, Jahng JH, Lee YJ, Park H, Lee SI. Serotonin transporter gene and G-protein beta3 C825T gene polymorphism in patients with functional dyspepsia and irritable bowel syndrome. Korean J Neurogastroenterol Motil. 2009;15:58–64. [Google Scholar]
- 17.Kim HG, Lee KJ, Lim SG, Jung JY, Cho SW. G-protein Beta3 subunit C825T polymorphism in patients with overlap syndrome of functional dyspepsia and irritable bowel syndrome. J Neurogastroenterol Motil. 2012;18:205–210. doi: 10.5056/jnm.2012.18.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee HJ, Lee SY, Choi JE, et al. G protein beta3 subunit, interleukin-10, and tumor necrosis factor-alpha gene polymorphisms in Koreans with irritable bowel syndrome. Neurogastroenterol Motil. 2010;22:758–763. doi: 10.1111/j.1365-2982.2010.01496.x. [DOI] [PubMed] [Google Scholar]
- 19.Rhee MK, Lee YH, Park SH, et al. A standardization study of Beck depression inventory 1: Korean version (K-BDI): reliability and factor analysis. Korean J Psychopathol. 1995;4:77–95. [Google Scholar]
- 20.Lindemann M, Virchow S, Ramann F, et al. The G protein beta3 subunit 825T allele is a genetic marker for enhanced T cell response. FEBS Lett. 2001;495:82–86. doi: 10.1016/s0014-5793(01)02339-0. [DOI] [PubMed] [Google Scholar]
- 21.Holtmann G, Siffert W, Haag S, et al. G-protein beta 3 subunit 825 CC genotype is associated with unexplained (functional) dyspepsia. Gastroenterology. 2004;126:971–979. doi: 10.1053/j.gastro.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 22.Camilleri CE, Carlson PJ, Camilleri M, et al. A study of candidate genotypes associated with dyspepsia in a U.S. community. Am J Gastroenterol. 2006;101:581–592. doi: 10.1111/j.1572-0241.2006.00481.x. [DOI] [PubMed] [Google Scholar]
- 23.Hauner H, Meier M, Jöckel KH, Frey UH, Siffert W. Prediction of successful weight reduction under sibutramine therapy through genotyping of the G-protein beta3 subunit gene (GNB3) C825T polymorphism. Pharmacogenetics. 2003;13:453–459. doi: 10.1097/00008571-200308000-00003. [DOI] [PubMed] [Google Scholar]
- 24.Rosskopf D, Manthey I, Siffert W. Identification and ethnic distribution of major haplotypes in the gene GNB3 encoding the G-protein beta3 subunit. Pharmacogenetics. 2002;12:209–220. doi: 10.1097/00008571-200204000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Locke GR, 3rd, Zinsmeister AR, Talley NJ, Fett SL, Melton LJ., 3rd Familial association in adults with functional gastrointestinal disorders. Mayo Clin Proc. 2000;75:907–912. doi: 10.4065/75.9.907. [DOI] [PubMed] [Google Scholar]
- 26.Mahadeva S, Goh KL. Epidemiology of functional dyspepsia: a global perspective. World J Gastroenterol. 2006;12:2661–2666. doi: 10.3748/wjg.v12.i17.2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wildner-Christensen M, Hansen JM, De Muckadell OB. Risk factors for dyspepsia in a general population: non-steroidal anti-inflammatory drugs, cigarette smoking and unemployment are more important than Helicobacter pylori infection. Scand J Gastroenterol. 2006;41:149–154. doi: 10.1080/00365520510024070. [DOI] [PubMed] [Google Scholar]
- 28.Agréus L, Svärdsudd K, Talley NJ, Jones MP, Tibblin G. Natural history of gastroesophageal reflux disease and functional abdominal disorders: a population-based study. Am J Gastroenterol. 2001;96:2905–2914. doi: 10.1111/j.1572-0241.2001.04680.x. [DOI] [PubMed] [Google Scholar]
- 29.Sloth H, Jørgensen LS. Chronic non-organic upper abdominal pain: diagnostic safety and prognosis of gastrointestinal and non-intestinal symptoms. A 5- to 7-year follow-up study. Scand J Gastroenterol. 1988;23:1275–1280. doi: 10.3109/00365528809090204. [DOI] [PubMed] [Google Scholar]
- 30.Parkman HP, Yates K, Hasler WL, et al. Clinical features of idiopathic gastroparesis vary with sex, body mass, symptom onset, delay in gastric emptying, and gastroparesis severity. Gastroenterology. 2011;140:101–115. doi: 10.1053/j.gastro.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lorena SL, Tinois E, Brunetto SQ, Camargo EE, Mesquita MA. Gastric emptying and intragastric distribution of a solid meal in functional dyspepsia: influence of gender and anxiety. J Clin Gastroenterol. 2004;38:230–236. doi: 10.1097/00004836-200403000-00007. [DOI] [PubMed] [Google Scholar]
- 32.Flier SN, Rose S. Is functional dyspepsia of particular concern in women? A review of gender differences in epidemiology, pathophysiologic mechanisms, clinical presentation, and management. Am J Gastroenterol. 2006;101(12 Suppl):S644–S653. doi: 10.1111/j.1572-0241.2006.01015.x. [DOI] [PubMed] [Google Scholar]