Abstract
Background/Aims
To evaluate the diagnostic value of contrast (SonoVue®) enhancement ultrasonography (CEUS) and to compare this method with computed tomography (CT) and magnetic resonance imaging (MRI) in evaluating liver masses.
Methods
CEUS (n=50), CT (n=47), and MRI (n=43) were performed on 50 liver masses in 48 patients for baseline mass characterization. The most likely impression for each modality and the final diagnosis, based on the combined biopsy results (n=14), angiography findings (n=36), and clinical course, were determined. The diagnostic value of CEUS was compared to those of CT and MRI.
Results
The final diagnosis of the masses was hepatocellular carcinoma (n=43), hemangioma (n=3), benign adenoma (n=2), eosinophilic abscess (n=1), and liver metastasis (n=1). The overall diagnostic agreement with the final diagnosis was substantial for CEUS, CT, and MRI, with κ values of 0.621, 0.763, and 0.784, respectively. The sensitivity, specificity, and accuracy were 83.3%, 87.5%, and 84.0%, respectively, for CEUS; 95.0%, 87.5%, and 93.8%, respectively, for CT; and 94.6%, 83.3%, and 93.0%, respectively for MRI. After excluding the lesions with poor acoustic sonographic windows, the sensitivity, specificity, and accuracy for CEUS were 94.6%, 87.5%, and 93.3%, respectively, with a κ value of 0.765.
Conclusions
If an appropriate acoustic window is available, CEUS is comparable to CT and MRI for the diagnosis of liver masses.
Keywords: Contrast enhanced ultrasonography, Liver masses
INTRODUCTION
For the imaging diagnosis of liver tumors, detection, characterization, and staging of the tumor are very important. Whereas unenhanced ultrasound and color Doppler ultrasonographic examination are widely used to screen for liver lesions, these techniques have limited performance in the characterization of solid focal tumors.1
SonoVue® (Bracco SpA, Milan, Italy) is a second generation microbubble contrast agent, which allows the assessment of vascularity and enhancement patterns of focal lesions with ultrasound in real-time, using low mechanical index (MI) scanning technology. Low MI real-time ultrasound in combination with SonoVue® allows the continuous assessment of tumor vascularity and enhancement during the different vascular phases (arterial, portal, and late phase) with better temporal resolution than with computed tomography (CT) or magnetic resonance imaging (MRI). Noninvasive characterization of hepatic tumors is largely based on their enhancement patterns on contrast enhanced imaging.2,3
Contrast enhancement ultrasonography (CEUS) provides an accurate differentiation between benign and malignant liver tumors, which is critical for adequate management of these patients. CEUS is now recognized as a useful imaging modality for the noninvasive diagnosis of small newly detected liver nodules during hepatocellular carcinoma (HCC) surveillance and is also useful for guidance and follow-up after locoregional therapy of HCC.4,5
The purpose of this study is to evaluate the diagnostic value of SonoVue® CEUS, and to compare this method with CT and MRI for the evaluation of liver masses.
MATERIALS AND METHODS
1. Patients and study design
This was a single center retrospective study, at Soonchunhyang University Hospital, Seoul, Korea. We evaluated 50 consecutive naive liver masses in 48 patients using CEUS, liver dynamic CT and MRI (Primovist®; Bayer Shering Pharma, Berlin, Germany) from April 2009 to September 2011. All patients underwent screening ultrasonography for either evaluation of a known underlying liver disease including chronic viral hepatitis, and alcoholic hepatitis or a routine health check-up.
2. Contrast enhancement ultrasonography
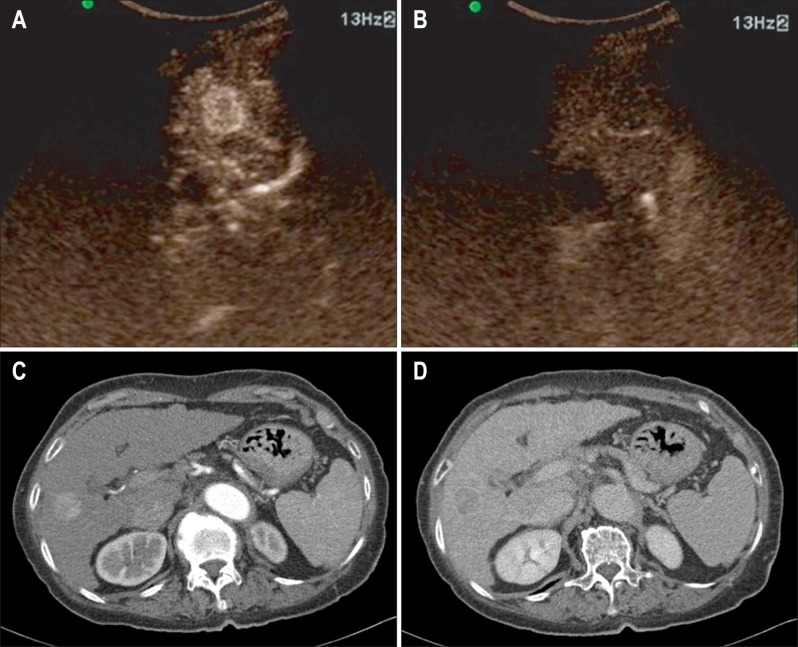
CEUS was performed by two experienced hepatologists. The most likely impression for each modality and final diagnosis were compared. Practice of CEUS was performed according to the European Federation of Societies for Ultrasound in Medicine and Biology (EFSUMB) guidelines. A CEUS examination was considered conclusive for diagnosis if the focal liver lesion (FLL) had a typical enhancement pattern after contrast injection during arterial, portal and late phases, according to the EFSUMB guidelines. For CEUS examination, a very low MI (<0.08 MHz) was used for real-time imaging. Each examination lasted about 5 minutes after bolus injection of SonoVue® (a 2.4-mL bolus for each lesion to be characterized, via a 20-gauge intravenous catheter placed in the antecubital vein, and followed by 10 mL saline flush). To characterize the lesion, the hemodynamic behavior of SonoVue® enhancement (hypoenhancing, hyperenhancing, isoenhancing) during the arterial phase (15 to 30 seconds), portal venous (30 to 120 seconds), and late vascular phases (120 to 300 seconds), were evaluated. All sonographic examinations were digitally recorded. The location and size of lesions were assessed on CEUS scans. Ultrasound diagnosis, in terms of the nature (malignant or benign) and type of the lesion (hemangiomas, focal nodular hyperplasia [FNH], liver adenoma, fatty liver alterations, HCC, or metastasis) were based on SonoVue® enhanced ultrasonography (US). Experienced hepatologists evaluated all SonoVue® enhanced images, formulating a final diagnosis (Fig. 1).
Fig. 1.
An 82-year-old female with hepatocellular carcinoma. (A) The contrast enhancement ultrasonography scan, obtained 18 seconds after injecting microbubbles, shows a slightly hypervascular nodule in the liver. (B) Washout is shown on the portal venous phase. (C) The mass shows a high attenuation on the arterial phase and (D) a washout on the delayed phase computed tomography scan.
3. CT, MRI, and final diagnosis
Both CT and MRI were performed within 2 weeks of CEUS. One experienced gastrointestinal radiologist interpreted the imaging results. All patients were followed up for at least 6 months. The final diagnosis was based on available histopathology findings from liver biopsy or surgical resection. If tissue confirmation was not performed, the final diagnosis was based on angiography and the combined clinical course of the patient. Clinical course is for example, while, we have followed up the indeterminate liver mass that was not clearly diagnosed as benign or malignancy, it has grown up or become characteristic enhanced mass. So putting together of multiple clinical situations, we conclude the final diagnosis.
4. Statistics
Statistical analyses were performed by using SPSS software version 18.0 (IBM Co., Armonk, NY, USA). The diagnostic level of agreement of CEUS, CT, and MRI for diagnosis of FLLs, was compared with the final diagnosis, using the κ index and Kendall Tau-b rank correlation coefficient. Agreement was regarded as poor at κ values lower than 0.4, moderate at κ values between 0.4 and 0.6, substantial at κ values between 0.6 and 0.8, and excellent at κ values greater than 0.8.
RESULTS
1. Baseline characteristics
The baseline characteristics are summarized in Table 1. The study population consisted of 48 patients (37 men and 11 women), the mean age was 58 years (range, 33 to 72 years). Chronic hepatitis B was the most common underlying liver disease in 24 of the patients (50%), followed by chronic hepatitis C in six (12%), and alcoholic liver disease in four (8%). Fourteen patients (30%) had no existing history of liver disease. Thirty-five FLLs (70%) were located in the right hepatic lobe (excluded dome), 11 (22%) in the left hepatic lobe, four (8%) in the hepatic dome. Twenty-four FLLs (48%) were measured less than or equal to 2 cm, 19 (38%) measured less than or equal to 5 cm, and seven (14%) were more than 4 cm.
Table 1.
The Clinical Characteristics of the Patients
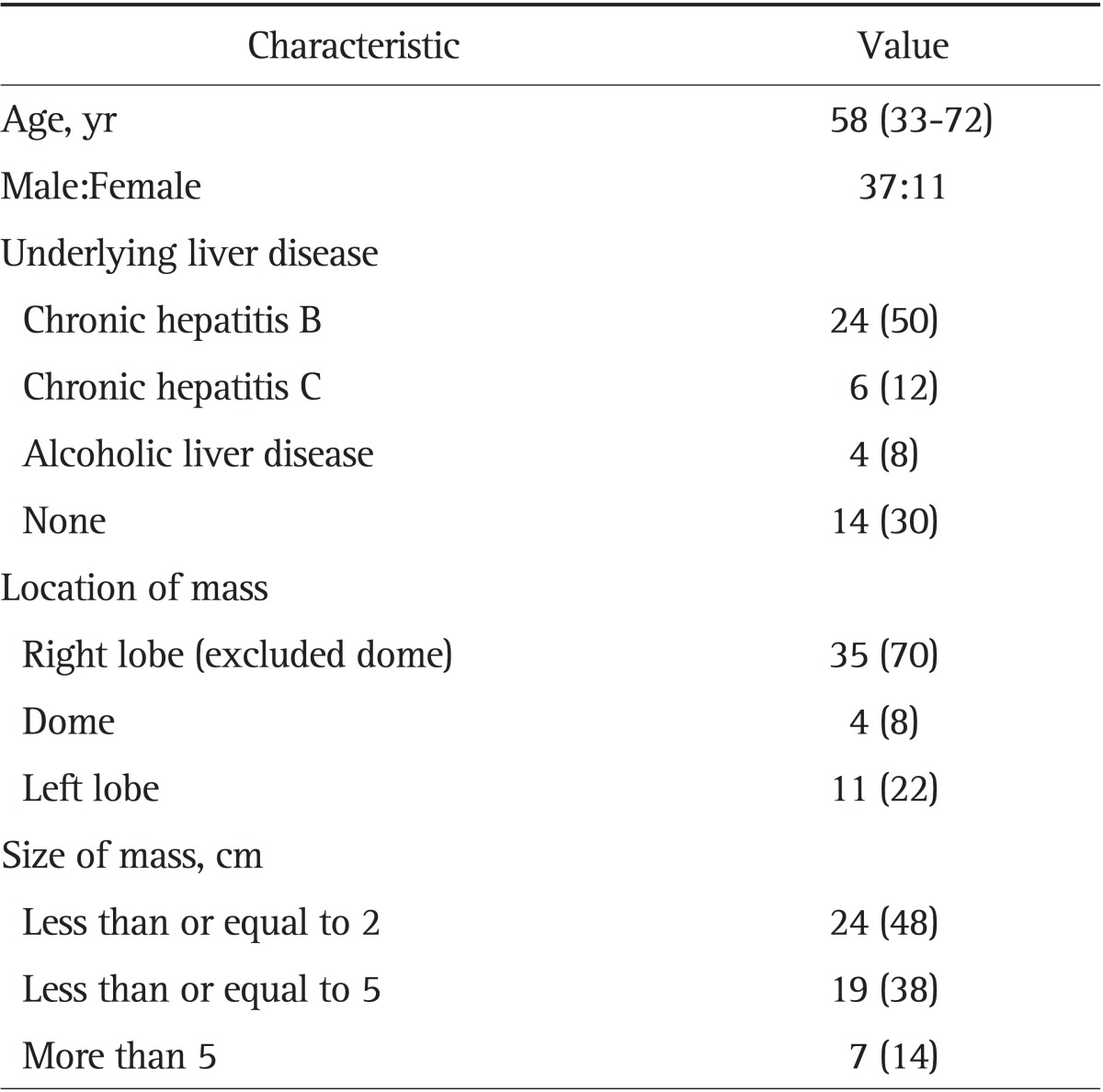
Data are presented as median (range) or number (%).
2. Final diagnosis
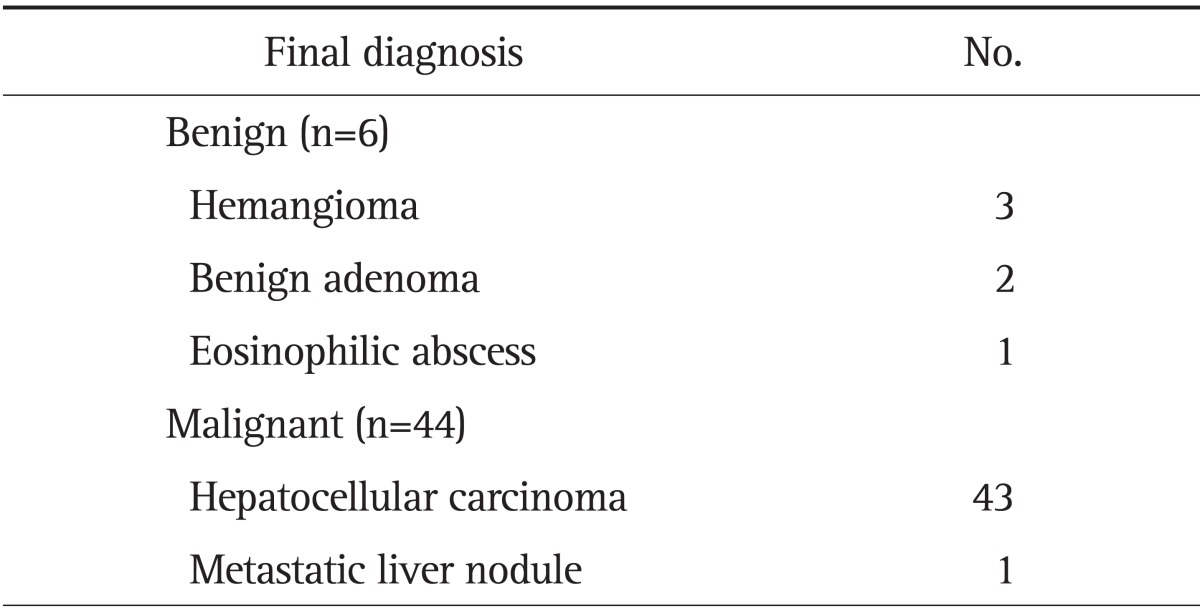
CEUS, CT, and MRI were performed for 50, 47, and 43 FLLs, respectively. Final diagnosis was determined by tissue histopathology in 14 cases (biopsy 11, surgery 3), and by angiography and clinical course in 36 cases. Table 2 shows the results of the final diagnosis. According to the final reference diagnosis, six FLLs were benign and 44 were malignant. There were 43 HCCs, three hemangiomas, two benign adenomas, one eosinophilic abscess, and one metastatic liver nodule (Table 2).
Table 2.
Final Diagnosis of Liver Mass
3. Diagnostic agreement and accuracy
The overall diagnostic agreement with final diagnosis was substantial for CUES, CT, and MRI with k values of 0.621, 0.763, and 0.784, respectively. The sensitivity, specificity, and accuracy was 83.3%, 87.5%, 84.0% for CEUS, 95.0%, 87.5%, 93.8% for CT, and 94.6%, 83.3%, 93.0% for MRI. Table 3 showed eight discordance with final diagnosis of cases each CEUS, CT, and MRI. Of these eight lesions, the final diagnosis was HCC in seven cases. When the mass is found in CT or MRI, we performed CEUS. Of the five cases that showed no focal lesion on CEUS, and two cases showed atypical findings on CT or MRI. Misdiagnosis of HCC as atypical findings were metastasis and FNH. Five cases of HCC were not detected on CEUS, because of inadequate acoustic window. The five cases with inadequate sonographic acoustic windows were all located in the hepatic dome. When the lesions with poor acoustic sonographic windows were excluded, the sensitivity, specificity, and accuracy for CEUS were 94.6%, 87.5%, and 93.3% which was comparable to both CT and MRI. And κ value is 0.765. And CEUS might be more accurate diagnosis than CT and MRI in a case of hemangioma (Table 3).
Table 3.
Discordance among Contrast Enhancement Ultrasonography, Computed Tomography, and Magnetic Resonance Imaging in the Final Diagnoses
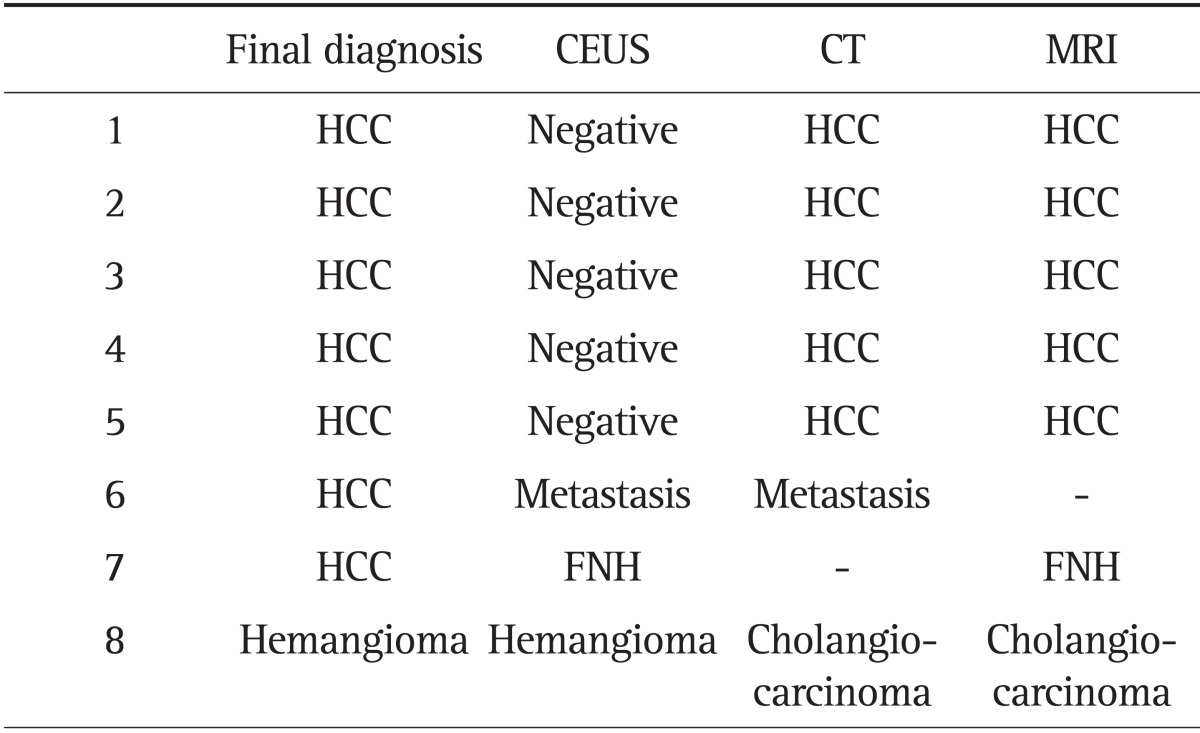
CEUS, contrast enhancement ultrasonography; CT, computed tomography; MRI, magnetic resonance imaging; HCC, hepatocellular carcinoma; FNH, focal nodular hyperplasia.
DISCUSSION
The introduction of second-generation microbubble ultrasound contrast agents and the development of contrast specific ultrasound techniques have improved the ability of CEUS in detecting and characterizing liver lesions, offering new perspectives for its use in clinical hepatology.6,7 However, whereas the use of contrast agents has been established for CT or MRI, the value of contrast enhanced agents in the US of the liver is still under clinical investigation.8 Recent investigation has shown that CEUS may have a potential role in the diagnosis of FLLs. A recent prospective multicenter studies evaluated the diagnostic value of CEUS compared to spiral-CT for the characterization of FLLs in clinical practice. They concluded that CEUS proved to be of equal rank to CT scan in regard to the assessment of tumor differentiation and specification and suggested that CEUS could be used before CT for the differentiation of liver tumors, because radiation exposure and invasive biopsies may be avoided in a high number of cases.9 In another recent multicenter study including 1,349 patients with FLLs discovered by standard US, CEUS was compared with a diagnostic gold standard: biopsy in more than 75% of the lesions, spiral contrast CT or contrast MRI in the rest of the cases. The accuracy of CEUS for the diagnosis of FFLs was 90.3%. Another study showed that tumor specific vascularity pattern could be assessed by CEUS in the majority of cases, and that the diagnostic accuracy of CEUS was 83.1% for benign lesions, 95.8% for malignant lesions, 91.4% for liver metastases and 84.9% for HCC.10 In this study we showed that CEUS was comparable to CT and MRI for diagnosis FLLs. For FLLs with an appropriate acoustic ultrasonographic window, the diagnostic accuracy was 93.3%.
CEUS has several advantages over contrast-enhanced CT or MRI in the evaluation of hepatic tumors.11 First, CEUS provides real-time dynamic imaging, useful to visualize a very early or late enhancement pattern of tumors that may not occur at the predetermined timing of CT or MRI scans. Second, unique intravascular properties of the microbubbles are often beneficial for CEUS to characterize malignant tumors with increased vascular permeability and a large interstitial space; CEUS demonstrates the washout phenomenon clearly and consistently, whereas CT or MRI may show prolonged enhancement due to contrast leakage into the tumor interstitium. Third, multiple injections of microbubbles are allowed and repetitive observation of tumor enhancement pattern is possible in a single CEUS examination. Fourth, in addition to the excellent safety profile with a low rate of adverse reactions, microbubble contrast agents can be used in patients with decreased renal function who are not suitable for contrast-enhanced CT or MRI.12 For these reasons, This method is the first line imaging method used for the evaluation of FLLs, in some centers.13 In unclear lesions, a second line investigation is necessary. The advantages of this strategy are the lower price for the diagnosis of incidentally discovered FLLs and the reduction of the time interval needed for a final diagnosis in clear CEUS cases.14,15
CEUS has several intrinsic limitations. First, lesions may be difficult to observe in obese patients with abundant flatulence, or deeply situated lesions. In our study, it was difficult to assess the vascular enhancement pattern of lesions located in the hepatic dome. FLLs in the hepatic dome were often associated with a poor acoustic window resulting in inconclusive CEUS findings. This may be problematic for patients with severe cirrhosis and shrinkage of liver volume. However if the acoustic window was adequate tumor characterization with CEUS was not difficult and the agreement level was high. Therefore, for FLLs with less than optimal acoustic windows on ultrasonography, CT or MRI may be the optimal modality for diagnosis. Second, compared with CT or MRI, the performance of CEUS is more strongly influenced by the experience of the investigator, by patient-related factors (cooperativeness), by nodules dimensions and by nodule location. Another limitation of CEUS in comparison to multiphase CT and MR imaging is the fact that only one liver lesion can be examined at a time as the transducer has to be kept still during the examination, and further contrast injections are necessary to characterize other additional primary liver tumors. Finally, the arterial phase in CEUS examination is about 30 seconds, which make it challenging to scan the entire liver for detection of multiple hypervascular HCC lesions.16
The limitations of this study are first, the retrospective nature and small number of patients. It might have a chance to make a bias with communication or discussion in the process of decision of clinical final diagnosis between the CEUS examiners and radiologists who interpreted CT or MRI. Secondly, due to the relatively high prevalence of chronic hepatitis B related HCC in Korea, there is a possibility of selection bias. Thirdly, European Association for the Study of the Liver and American Association for the Study of Liver diseases guidelines showed that HCC can be diagnosed with imaging modality. Majority of HCC in this study was diagnosed with CT or MRI and biopsy proven HCC was 14 cases only. If the final diagnosis was not confirmed as histopatholgy, it was based on angiography. But, angiographic image is not accepted as definite diagnosis in many HCC guidelines, because angiography could not show dynamic triphase images.17,18 In addition, among the evaluated 50 masses, the final diagnosis was 43 HCCs, and the others are benign lesions (three hemangiomas, two benign adenomas, one eosinophilic abscess). It seems to take CEUS more frequently among the patients who are suspected malignancy than benign. It is too small sample size to make conclusion that CEUS is more accurate tool for benign lesions. So, this study have less representative in diagnosis benign lesions like hemangioma.
In this study, five HCC cases of false negative in CEUS with inadequate sonographic acoustic windows were all located in the hepatic dome. It is important point of our study that emphasize the locational factor of focal lesion to detect by CEUS. Additionally, among the evaluated 50 masses, the final diagnosis was 43 HCCs, and the others are benign lesions (three hemangiomas, two benign adenomas, one eosinophilic abscess). Therefore, this study's result and opinions might be applicable very useful as a first line screening tool of HCC, especially high prevalence rate of chronic hepatitis B induced HCC in South Korea.
In conclusion, the authors believe that if an appropriate acoustic window is available, CEUS is comparable to CT and MRI for the diagnosis of liver masses. However further prospective studies using histology as a reference standard are needed.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Murakami T, Imai Y, Okada M, et al. Ultrasonography, computed tomography and magnetic resonance imaging of hepatocellular carcinoma: toward improved treatment decisions. Oncology. 2011;81(Suppl 1):86–99. doi: 10.1159/000333267. [DOI] [PubMed] [Google Scholar]
- 2.Jang JY, Kim MY, Jeong SW, et al. Current consensus and guidelines of contrast enhanced ultrasound for the characterization of focal liver lesions. Clin Mol Hepatol. 2013;19:1–16. doi: 10.3350/cmh.2013.19.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trillaud H, Bruel JM, Valette PJ, et al. Characterization of focal liver lesions with SonoVue-enhanced sonography: international multicenter-study in comparison to CT and MRI. World J Gastroenterol. 2009;15:3748–3756. doi: 10.3748/wjg.15.3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schneider M. Characteristics of SonoVuetrade mark. Echocardiography. 1999;16(7, Pt 2):743–746. doi: 10.1111/j.1540-8175.1999.tb00144.x. [DOI] [PubMed] [Google Scholar]
- 5.Nicolau C, Vilana R, Catalá V, et al. Importance of evaluating all vascular phases on contrast-enhanced sonography in the differentiation of benign from malignant focal liver lesions. AJR Am J Roentgenol. 2006;186:158–167. doi: 10.2214/AJR.04.1009. [DOI] [PubMed] [Google Scholar]
- 6.Kim TK, Lee KH, Khalili K, Jang HJ. Hepatocellular nodules in liver cirrhosis: contrast-enhanced ultrasound. Abdom Imaging. 2011;36:244–263. doi: 10.1007/s00261-011-9686-0. [DOI] [PubMed] [Google Scholar]
- 7.Bolondi L, Correas JM, Lencioni R, Weskott HP, Piscaglia F. New perspectives for the use of contrast-enhanced liver ultrasound in clinical practice. Dig Liver Dis. 2007;39:187–195. doi: 10.1016/j.dld.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 8.von Herbay A, Westendorff J, Gregor M. Contrast-enhanced ultrasound with SonoVue: differentiation between benign and malignant focal liver lesions in 317 patients. J Clin Ultrasound. 2010;38:1–9. doi: 10.1002/jcu.20626. [DOI] [PubMed] [Google Scholar]
- 9.Seitz K, Strobel D, Bernatik T, et al. Contrast-Enhanced Ultrasound (CEUS) for the characterization of focal liver lesions: prospective comparison in clinical practice: CEUS vs. CT (DEGUM multicenter trial). Parts of this manuscript were presented at the Ultrasound Dreilandertreffen 2008, Davos. Ultraschall Med. 2009;30:383–389. doi: 10.1055/s-0028-1109673. [DOI] [PubMed] [Google Scholar]
- 10.Strobel D, Seitz K, Blank W, et al. Tumor-specific vascularization pattern of liver metastasis, hepatocellular carcinoma, hemangioma and focal nodular hyperplasia in the differential diagnosis of 1,349 liver lesions in contrast-enhanced ultrasound (CEUS) Ultraschall Med. 2009;30:376–382. doi: 10.1055/s-0028-1109672. [DOI] [PubMed] [Google Scholar]
- 11.Wilson SR, Kim TK, Jang HJ, Burns PN. Enhancement patterns of focal liver masses: discordance between contrast-enhanced sonography and contrast-enhanced CT and MRI. AJR Am J Roentgenol. 2007;189:W7–W12. doi: 10.2214/AJR.06.1060. [DOI] [PubMed] [Google Scholar]
- 12.Piscaglia F, Bolondi L Italian Society for Ultrasound in Medicine and Biology (SIUMB) Study Group on Ultrasound Contrast Agents. The safety of Sonovue in abdominal applications: retrospective analysis of 23188 investigations. Ultrasound Med Biol. 2006;32:1369–1375. doi: 10.1016/j.ultrasmedbio.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 13.Sporea I, Sirli R, Martie A, Popescu A, Danila M. How useful is contrast enhanced ultrasonography for the characterization of focal liver lesions? J Gastrointestin Liver Dis. 2010;19:393–398. [PubMed] [Google Scholar]
- 14.Sirli R, Sporea I, Martie A, Popescu A, Dănilă M. Contrast enhanced ultrasound in focal liver lesions: a cost efficiency study. Med Ultrason. 2010;12:280–285. [PubMed] [Google Scholar]
- 15.Romanini L, Passamonti M, Aiani L, et al. Economic assessment of contrast-enhanced ultrasonography for evaluation of focal liver lesions: a multicentre Italian experience. Eur Radiol. 2007;17(Suppl 6):F99–F106. doi: 10.1007/s10406-007-0234-5. [DOI] [PubMed] [Google Scholar]
- 16.Martie A, Sporea I, Popescu A, et al. Contrast enhanced ultrasound for the characterization of hepatocellular carcinoma. Med Ultrason. 2011;13:108–113. [PubMed] [Google Scholar]
- 17.European Association for Study of Liver; European Organisation for Research and Treatment of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. Eur J Cancer. 2012;48:599–641. doi: 10.1016/j.ejca.2011.12.021. [DOI] [PubMed] [Google Scholar]
- 18.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]