Abstract
Background/Aims
This study aimed to detect the expression of natural killer (NK) cell receptor natural killer group 2D (NKG2D) in the peripheral blood of patients with primary hepatocellular carcinoma and to discuss the correlation between NK cell cytotoxicity and liver function.
Methods
The number of NK cells and the expression of NK cell receptor NKG2D in peripheral blood were determined by flow cytometry in patients with primary hepatocellular carcinoma, hepatitis B cirrhosis, chronic hepatitis B, and healthy controls.
Results
When compared with patients in the healthy and the chronic hepatitis B groups, the primary hepatocellular carcinoma group showed significant decreases in all parameters, including the cytotoxicity of NK cells on K562 cells, expression rate of NKG2D in NK cells, number of NKG2D+ NK cells, expression level of NKG2D, and number of NK cells (p<0.05). The activity of NK cells showed a positive correlation, whereas the Child-Pugh scores in the primary hepatocellular carcinoma and the hepatitis B cirrhosis groups showed a negative correlation with all parameters detected above.
Conclusions
The decrease of NK cell activity in patients with primary hepatocellular carcinoma is closely related to their lower expression of NKG2D. Liver function affects the expression of NKG2D and the activity of NK cells.
Keywords: Killer cells, natural; Liver neoplasms; Natural killer group 2D
INTRODUCTION
Natural killer (NK) cells are the major component of human native immune system. They play an important role in antiviral, antitumor, and graft-rejection immune responses.1 Natural killer group 2D (NKG2D) is one of the receptors that activate NK cells. They can activate the cytotoxic effect of NK cells.2 Upon binding with the ligands on the surface of target cells, NKG2D can activate the NK cells by further binding to the adaptor molecule DAP10/DAP12. This may help the NKG2D+ NK cells to exert their antitumor effects.3 However, currently there are few reports focusing on the expression of NK cell receptor NKG2D and how NK cells interact with target cells in patients with primary hepatocellular carcinoma. This study has detected the expression of NK cell receptor NKG2D and the killing rate of NK cells in patients with primary hepatocellular carcinoma, as well as the expression of NK cell receptor NKG2D influence the activity of NK cells. The observations may provide a new strategy for adoptive immunotherapy of primary hepatocellular carcinoma.
MATERIALS AND METHODS
1. Subjects
Healthy controls and patients with primary hepatocellular carcinoma, hepatitis B cirrhosis, and chronic hepatitis B were enrolled in this study. Each group contained 20 cases. The primary hepatocellular carcinoma group contained 17 males and three females (mean age, 58 years); the hepatitis B cirrhosis group contained 16 males and four females (mean age, 53 years); the chronic hepatitis B group contained 16 males and four females (mean age, 51 years); and the healthy control 13 males and seven females (mean age, 47 years).
All the primary hepatocellular carcinoma patients were hepatitis B surface antigen positive and/or hepatitis B virus (HBV)-DNA positive. The diagnostic criteria of primary hepatocellular carcinoma were based on the 7th edition of Internal Medicine.4 They included clinical symptoms and signs, serum tumor markers, abdominal B-ultrasound, computed tomography and magnetic resonance image, and/or pathology. In this study, two primary hepatocellular carcinoma cases were confirmed by pathology. The diagnosis of hepatitis B cirrhosis and chronic hepatitis B was based on the European Association for the Study of the Liver Clinical Practice Guidelines: management of chronic hepatitis B in 2009.5 All subjects were free of other cancers and autoimmune diseases. The study was approved by the Guandong General Hospital's Ethics Committee, and all participants were provided written informed consent before being enrolled in the research.
2. Reagents
Ficoll-Hypaque density gradient (Lymphoprep) was purchased from Shanghai Biological Manufacture (Shanghai, China). Cholecystokinin (CCK-8) kit was purchased from Beyotime institute of Biotechnology (Jiangsu, China). FACS Lysing solution was purchased from BD Biosciences (San Jose, CA, USA).
3. Specimen collection
Six milliliters peripheral blood samples were collected into tubes containing heparin from each subject. Other two milliliters peripheral blood samples of each subject were collected into tubes containing ethylenediaminetetraacetic acid. The blood routine test was performed by Automatic Blood Analyzer (Bayer 2120; Bayer, Germany). All the analyses and detections were performed within 6 hours after sample collection.
4. The detection of killing activity of NK cells
Peripheral blood mononuclear cells were isolated using a Ficoll-Hypaque density gradient (Lymphoprep) from 5 mL peripheral blood samples containing heparin. The mononuclear cells were removed by washing the lymphocytes for several times.
The effector cells (lymphocytes) and the target cells (K562 cells) were mixed and cocultured in 96-well culture plate. The best antitarget ratio was 40:1. The groups containing effector cells only, target cells only, and no cells were also set at the same time. All cells in each group were incubated in humidified incubator at 37℃ with 5% CO2 for 12 hours. After the incubation, 10 µL CCK-8 were added into each well, mixed, and continued to culture for another 4 hours. The optical density (OD) value of each well was detected by microplate reader at 450 nm wavelength. The cytotoxicity of NK cells represented as the killing rate was calculated by the following formula. Killing rate (%)=[1-(OD value of the well with mixed effector and target cells-OD value of the well containing the effector cells)/OD value of the well containing the target cells]×100%. All tests were repeated for three times.
5. Flow cytometric analysis
The fluorochrome-conjugated antibodies and the heparinized blood were mixed and incubated at 4℃ in the dark for 30 minutes. The erythrocyte lysis buffer was added to each Eppendorf tube and mixed for 10 minutes in the dark at room temperature. The phosphate buffered saline (PBS) was then added to end the lysis. The samples were centrifuged at 350 g/min for 5 minutes. After washing with cold PBS, the cells were resuspended in PBS and detected by flow cytometry.
6. Blood routine test
The routine blood test was detected by Automatic blood cell analyzer (Bayer 2120). The numbers of white blood cells (WBCs) and lymphocytes were obtained.
7. Statistical analysis
All statistical analyses were carried out using SPSS version 13.0 (SPSS Inc., Chicago, IL, USA). The data conformed to normal distribution were expressed as mean±SEM. The analysis of variance was used in multi-group comparison. Student-Newman-Keuls q test was used in two group comparison. The results conformed to skewed distribution were expressed as median (Q1 to Q3). Kruskal-Wallis H test was used in multigroup comparison, and the Nemenyi test was used in two group comparison. The linear correlation analysis was used to detect the correlations. A p<0.05 was considered as statistically significant.
RESULTS
1. The WBC and lymphocyte counts
There was no significant difference in the total number of peripheral WBCs among the four groups (p>0.05); total lymphocyte count in the cirrhotic and liver cancer patients were significantly lower than those in the normal and the hepatitis B groups (p<0.01) (Table 1).
Table 1.
A Comparison of the Blood Routine, NKG2D Expression of NK Cells, and Killing Rate of NK Cells among Groups
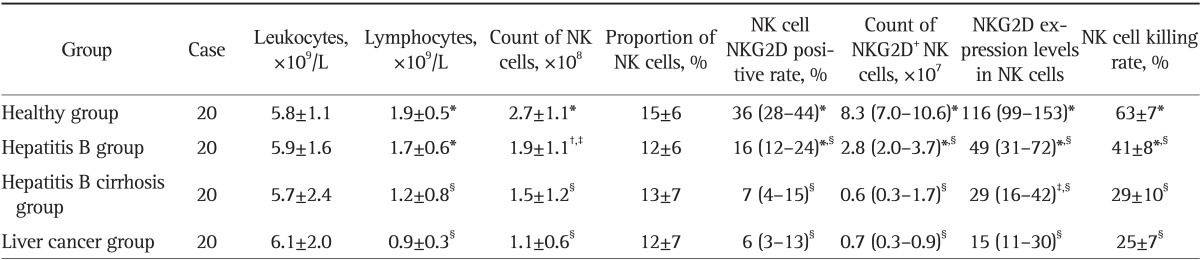
Data are presented as mean±SD or medium (25th-75th percentile). NK cell NKG2D positive rate, NKG2D+ NK cell numbers, and NK cell NKG2D expression levels are shown as skewed distribution data.
NKG2D, natural killer group 2D; NK, natural killer.
*Compared with the hepatocellular carcinoma (HCC), p<0.01; †Compared with the healthy group, p<0.05; ‡Compared with the HCC, p<0.05; §Compared with the healthy group, p<0.01.
2. The number of NK cells in each group
There was no significant difference in the proportion of lymphocytes among the four groups. Compared with the normal group, the total number of NK cells in the hepatitis B, the liver cirrhosis, and the liver cancer groups reduced sequentially, and the NK cells in the liver cancer group decreased significantly when compared with those in the hepatitis B and the healthy control groups (p<0.05) (Table 1).
3. NKG2D expression and killer function of NK cell in peripheral blood in each group
NKG2D positive rate, number of NKG2D positive cells, NKG2D expression levels (represented as mean fluorescence density), and NK cell killing rate among the four groups were ranked as the following: normal control>hepatitis B>cirrhosis>liver cancer. These indices in the liver cancer and the cirrhotic groups were significantly lower than those in the hepatitis B and the control groups (p<0.01) (Table 1, Figs 1, 2, 3, 4).
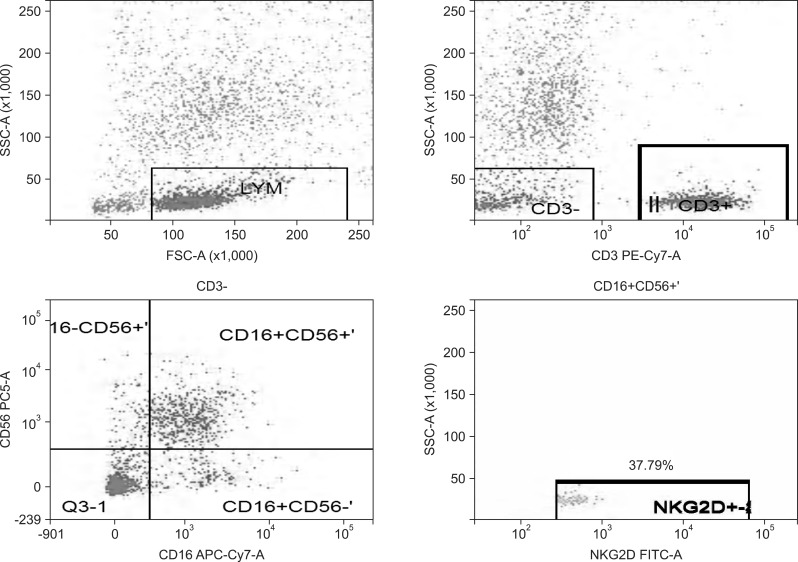
Fig. 1.
Natural killer group 2D (NKG2D) expression rate of NK cells in the healthy group.
SSC-A, side scatter (A); FSC-A, forward scatter (A); CD3 PE-Cy7-A, the CD3 antibody marked by PE-Cy7 (A); CD56 PC5-A, the CD56 antibody marked by Percp-cy5.5 (A); CD16 APC-Cy7-A, the CD16 antibody marked by APC-Cy7 (A); NKG2D FITC-A, the NKG2D antibody marked by FITC (A).
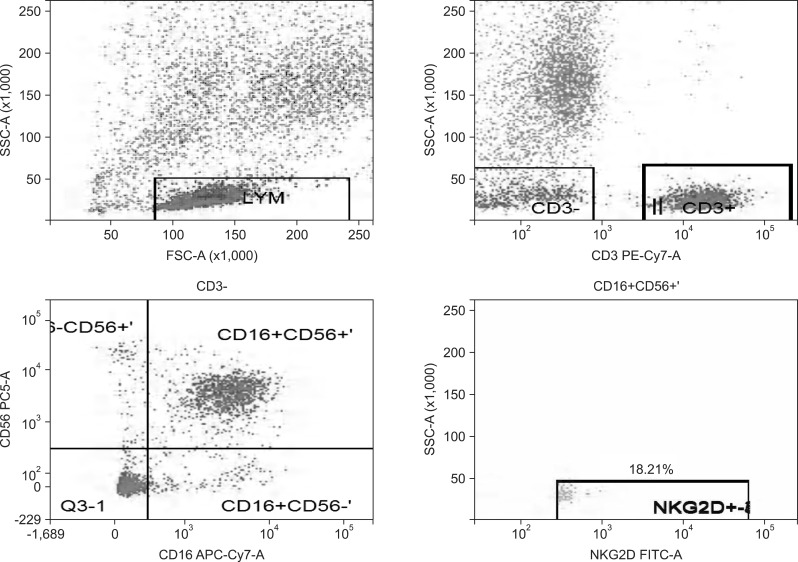
Fig. 2.
Natural killer group 2D (NKG2D) expression rate of NK cells in the hepatitis B group.
SSC-A, side scatter (A); FSC-A, forward scatter (A); CD3 PE-Cy7-A, the CD3 antibody marked by PE-Cy7 (A); CD56 PC5-A, the CD56 antibody marked by Percp-cy5.5 (A); CD16 APC-Cy7-A, the CD16 antibody marked by APC-Cy7 (A); NKG2D FITC-A, the NKG2D antibody marked by FITC (A).
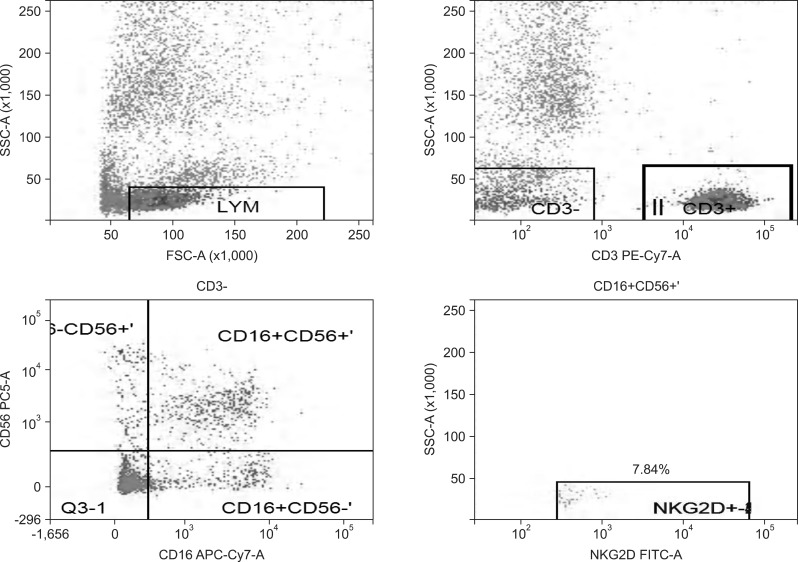
Fig. 3.
Natural killer group 2D (NKG2D) expression rate of NK cells in the hepatitis B cirrhosis group.
SSC-A, side scatter (A); FSC-A, forward scatter (A); CD3 PE-Cy7-A, the CD3 antibody marked by PE-Cy7 (A); CD56 PC5-A, the CD56 antibody marked by Percp-cy5.5 (A); CD16 APC-Cy7-A, the CD16 antibody marked by APC-Cy7 (A); NKG2D FITC-A, the NKG2D antibody marked by FITC (A).
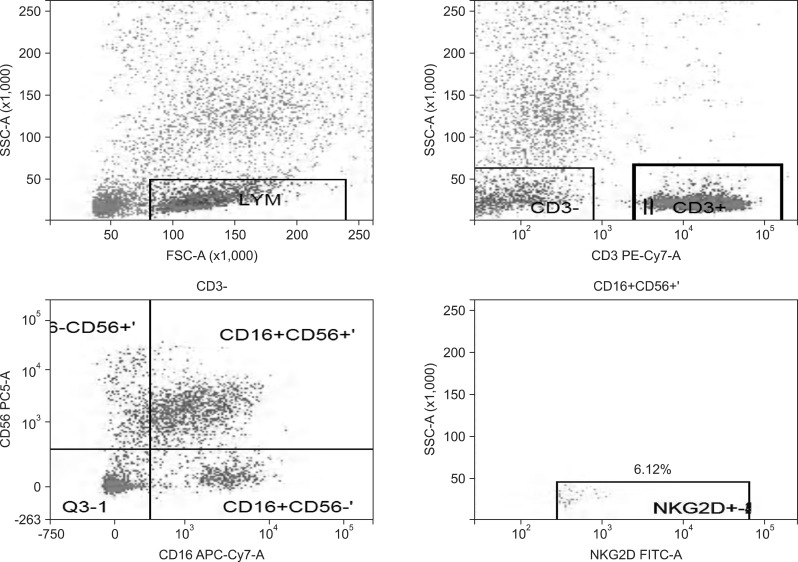
Fig. 4.
Natural killer group 2D (NKG2D) expression rate of NK cells in the liver cancer group.
SSC-A, side scatter (A); FSC-A, forward scatter (A); CD3 PE-Cy7-A, the CD3 antibody marked by PE-Cy7 (A); CD56 PC5-A, the CD56 antibody marked by Percp-cy5.5 (A); CD16 APC-Cy7-A, the CD16 antibody marked by APC-Cy7 (A); NKG2D FITC-A, the NKG2D antibody marked by FITC (A).
4. The impact of liver function on NK cell NKG2D expression and killer function in cirrhosis and liver cancer patients
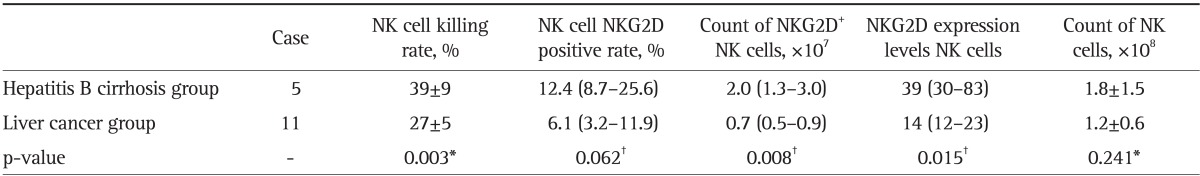
The NK cell killing rate, NKG2D positive rate, number of NKG2D+ NK cells, and expression levels of NKG2D in NK cells were lower in Child-Pugh B and C liver cancer patients, when parallel compared with those in Child-Pugh B and C liver cirrhosis patients (p<0.05, except for NK cell NKG2D positive rate with p>0.05). These indices in Child-Pugh C both cirrhosis and liver cancer patients were significantly lower than those in Child-Pugh B patients (p<0.05, except for NK cell NKG2D positive rate with p>0.05). However, the liver function showed no effect on the count of NK cells (p>0.05) (Tables 2, 3, 4, 5).
Table 2.
Comparison of the NKG2D Expression of NK Cells and the Killing Rate of NK Cells between the Hepatitis B Cirrhosis and Liver Cancer Groups in Child-Pugh B Degree Patients
Data are presented as mean±SD or medium (25th-75th percentile).
NKG2D, natural killer group 2D; NK, natural killer.
*t-test; †Mann-Whitney U test.
Table 3.
Comparison of the NKG2D Expression of NK Cells and the Killing Rate of NK Cells between the Hepatitis B Cirrhosis and Liver Cancer Groups in Child-Pugh C Degree Patients
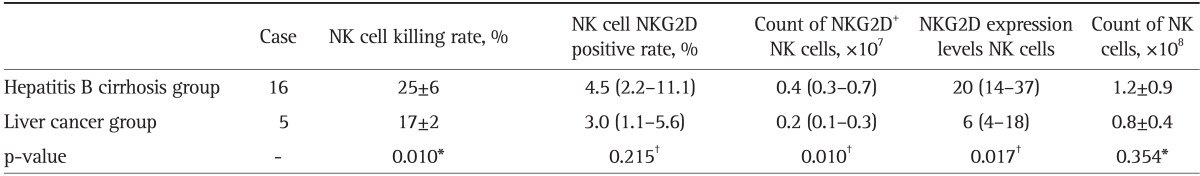
Data are presented as mean±SD or medium (25th-75th percentile).
NKG2D, natural killer group 2D; NK, natural killer.
*t-test; †Mann-Whitney U test.
Table 4.
A Comparison of the NKG2D Expression of NK Cells and the Killing Rate of NK Cells between the Child-Pugh B and C Classes in the Hepatitis B Cirrhosis Group
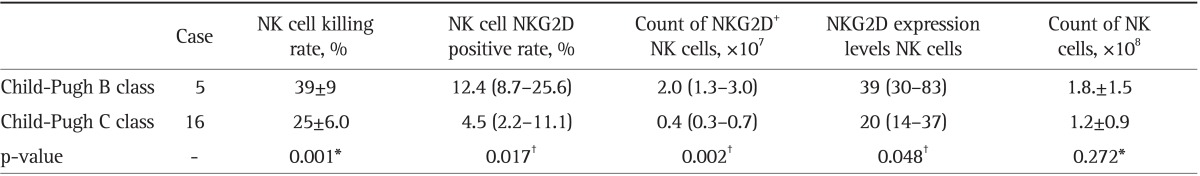
Data are presented as mean±SD or medium (25th-75th percentile).
NKG2D, natural killer group 2D; NK, natural killer.
*t-test; †Mann-Whitney U test.
Table 5.
A Comparison of the NKG2D Expression of NK Cells and the Killing Rate of NK Cells between the Child-Pugh B and C Classes in the Liver Cancer Group
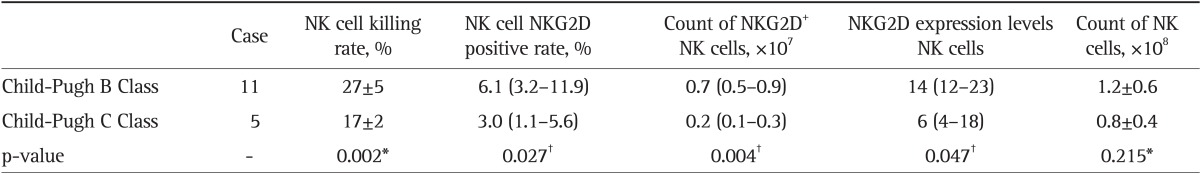
Data are presented as mean±SD or medium (25th-75th percentile).
NKG2D, natural killer group 2D; NK, natural killer.
*t-test; †Mann-Whitney U test.
5. The correlation analysis
The activity of NK cells exhibited a positive correlation with the number of NK cells, the positive rate of NKG2D, the number of NKG2D+ NK cells, and the NKG2D expression level (r=0.657, r=0.770, r=0.927, and r=0.734, respectively; all p<0.01) (Table 6). The Child-Pugh score in the liver cancer and cirrhosis groups showed a negative correlation with the NK cell activity, NK cell NKG2D positive rate, NKG2D+ NK cell count, and the level of NKG2D expression (Tables 7 and 8).
Table 6.
The Correlation between the Activity of NK Cells and the NKG2D Expression of NK Cells or NK Cell Subpopulations
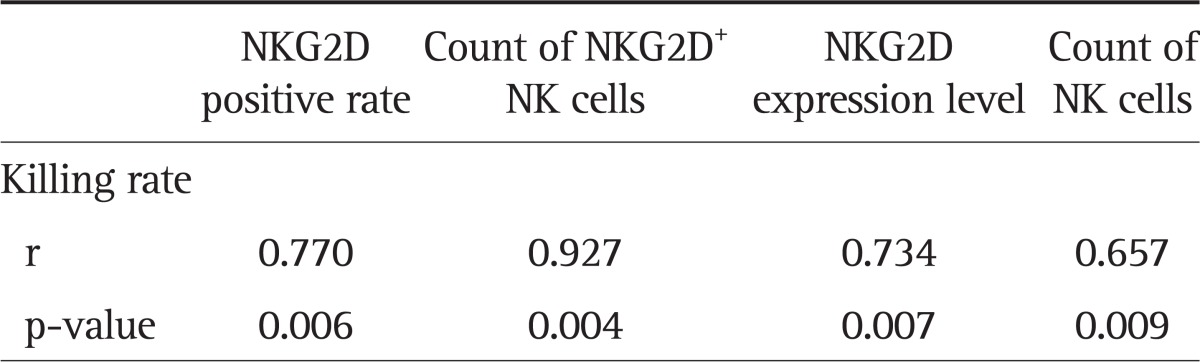
The referenced correlations are linear.
NK, natural killer; NKG2D, natural killer group 2D.
Table 7.
Correlations between the Child-Pugh Scores in the Hepatitis B Cirrhosis Group and the Killing Rate of NK Cells or NKG2D Expression of NK Cells
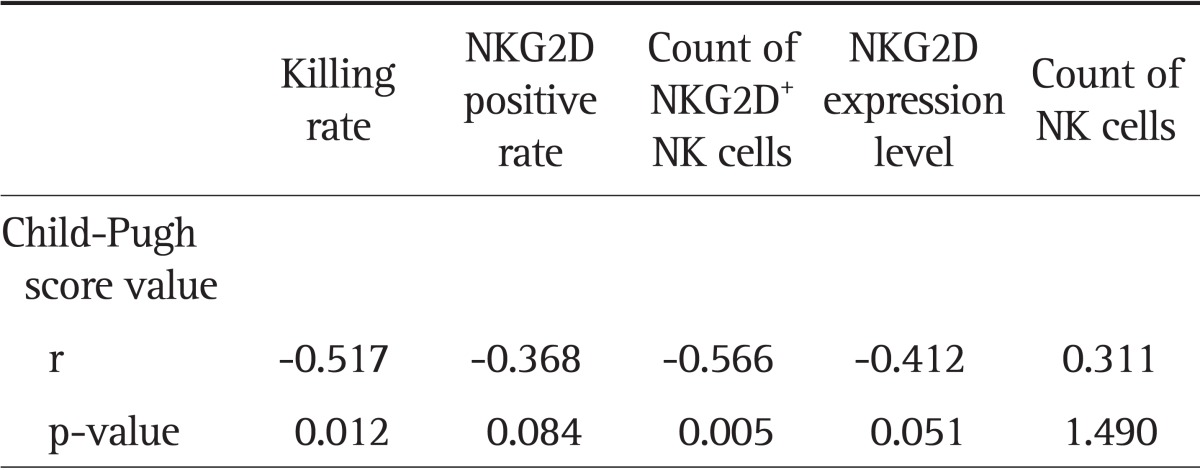
The referenced correlations are linear.
NK, natural killer; NKG2D, natural killer group 2D.
Table 8.
Correlations between the Child-Pugh Scores in the Liver Cancer Group and the Killing Rate of NK Cells or NKG2D Expression of NK Cells
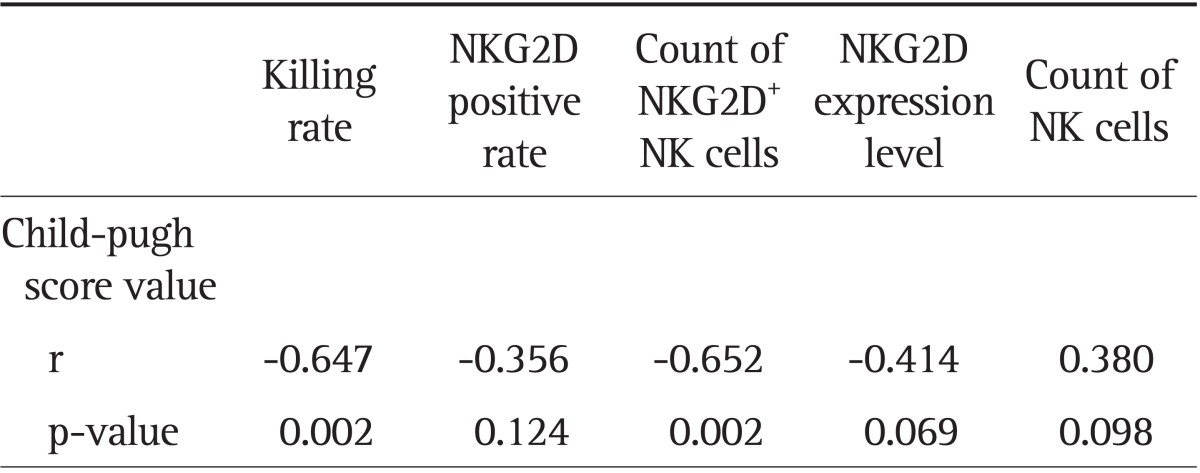
The referenced correlations are linear.
NK, natural killer; NKG2D, natural killer group 2D.
DISCUSSION
1. The relationship of NK cell cytotoxicity with NK cell NKG2D expression
NKG2D binding to its ligand on the surface of target cells activates the signaling through adapter protein DAP10/DAP12. The activated NK cells then acquire the ability to attack target cells. NKG2D activation determines the body's antitumor immunity to a large extent.3,6,7 This study showed that the number of NK cells, the positive rate of NKG2D, the NKG2D expression level, and the killing rate of NK cells in patients with chronic hepatitis B were significantly lower than those in the healthy group. This may illustrate that with the chronic infection of HBV, the number and the killing rate of NK cells decline, as well as the cellular immune function. With the progression of disease, these indices in the hepatitis B cirrhosis group decreased further, indicating that along with the development of liver disease, the cellular immune function in these patients decreased. In the liver cancer group, all results of above the indices dropped to the lowest levels, implying that the decline in the expression of NKG2D may play an important role in the occurrence and development of hepatic carcinoma. It is well known that HBV infection is a major cause of primary hepatocellular carcinoma. In this study, the 20 liver cancer cases were all positive for HBV, suggesting that hepatocellular carcinoma may be due to the long-term HBV infection with gradual reduction in cellular immune function. In the wake of reduction in NK cell number and declining in NK cell function, the hepatoma cells survived immunosurveillance, and may lead to the development of liver cancer. Further research is needed to prove whether improving the NKG2D expression in the patients with chronic hepatitis B infection can delay or completely suppress the development of hepatoma.
Groh et al.8 reported that NKG2D of NK cells played an important role in killing K562, HepGa, and other tumor cells. A large number of NKG2D ligands could be detected on the surface of tumor cells including hepatoma cells, which helped the NK cells to exert their antitumor effects. The results of this study showed that the killing rate of NK cells exhibited a significantly positive correlation with the counts of NK cells, the NKG2D expression level, and, especially, the number of NK cells expressing NKG2D molecules (the correlation coefficient was 0.927). Therefore, increasing the NK cell surface NKG2D expression in patients with liver cancer and other tumors may significantly enhance the NK cell killing effect and the antitumor immunity.
2. The impact of liver function in cirrhosis and liver cancer patients on NK cell NKG2D expression and its killer function
How could the liver function affect NK cell NKG2D expression and its killer function in patients with liver diseases remains an unanswered question. The present study showed that if we did not classify the liver function using Child-Pugh classification, only NKG2D expression level showed statistically significant difference between the liver cancer and the cirrhotic groups (p<0.05), while NK cell killing rate, NKG2D positive expression rate, NKG2D+ NK cell counts were not significantly different (p>0.05). But when liver function was classified based on the same Child-Pugh scoring, NK cell NKG2D expression and killing function in liver cancer patients were lower than those in the cirrhotic patients, and the p-values for NKG2D+ NK number, NKG2D expression levels, and NK cell killing rate were all less than 0.05 (except for NKG2D positive rate at p>0.05). However, this may be due to the small sample size. Further verifications with larger sample size are needed. NK cell NKG2D expression and killer function in Child C patients were also reduced when compared with Child B patients both in the cirrhotic and the liver cancer groups (p<0.05). Further study showed that Child-Pugh score in both liver cancer and hepatitis B cirrhosis groups was negatively related to the NK cell killing rate, the number of NKG2D+ NK cells, the NKG2D expression rate, the NKG2D expression level in NK cells, and the number of NK cells. These suggested that the worse the liver function, the lower the NKG2D expression level of NK cells, as well as the lower the NK cell activity. Thus improving liver function may help to improve the cellular immunity in patients with chronic liver diseases and to ease the disease condition. Although the change in NK cell subpopulations could affect the cytotoxic activity of NK cells and NKG2D expression, the results of this study suggested that liver function had no significant effect on the distribution of NK cell subpopulations.
In summary, NK cell activity and NKG2D expression in liver cancer patients are reduced significantly, as well as the NK cell subset distribution. The NK cell activity is related to NKG2D expression and NK cell subpopulations. The liver function can affect the NK cell receptor NKG2D expression and NK cell killing activity, but has no significant effect on the distribution of NK cell subsets. This study provides new insights and theoretical basis regarding NK cells in tumor immunity and immune regulation. Further studies are needed to improve the NK cell surface NKG2D expression in patients with liver cancer and other tumors. It may open a new avenue for the clinical application of NK cells.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Natarajan K, Dimasi N, Wang J, Mariuzza RA, Margulies DH. Structure and function of natural killer cell receptors: multiple molecular solutions to self, nonself discrimination. Annu Rev Immunol. 2002;20:853–885. doi: 10.1146/annurev.immunol.20.100301.064812. [DOI] [PubMed] [Google Scholar]
- 2.Coudert JD, Held W. The role of the NKG2D receptor for tumor immunity. Semin Cancer Biol. 2006;16:333–343. doi: 10.1016/j.semcancer.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 3.Pende D, Cantoni C, Rivera P, et al. Role of NKG2D in tumor cell lysis mediated by human NK cells: cooperation with natural cytotoxicity receptors and capability of recognizing tumors of nonepithelial origin. Eur J Immunol. 2001;31:1076–1086. doi: 10.1002/1521-4141(200104)31:4<1076::aid-immu1076>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 4.Lu Z, Zhong N. The internal medicine. 7th ed. Beijing: People's Medical Publishing House; 2008. [Google Scholar]
- 5.European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of chronic hepatitis B. J Hepatol. 2009;50:227–242. doi: 10.1016/j.jhep.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Conejo-Garcia JR, Benencia F, Courreges MC, et al. Letal, A tumor-associated NKG2D immunoreceptor ligand, induces activation and expansion of effector immune cells. Cancer Biol Ther. 2003;2:446–451. doi: 10.4161/cbt.2.4.479. [DOI] [PubMed] [Google Scholar]
- 7.Bauer S, Groh V, Wu J, et al. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. 1999;285:727–729. [PubMed] [Google Scholar]
- 8.Groh V, Wu J, Yee C, Spies T. Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature. 2002;419:734–738. doi: 10.1038/nature01112. [DOI] [PubMed] [Google Scholar]