Abstract
Purpose
We compared the efficacy, survival rate, and adverse events between bicalutamide 150-mg monotherapy and combined androgen blockade (CAB) in men with locally advanced prostate cancer.
Materials and Methods
From March 2003 to July 2012, we retrospectively included 74 patients who were treated for more than 3 months and were followed up for more than 6 months. 25 men were treated with bicalutamide 150-mg only (group 1) and 49 men received CAB (group 2). Serum prostate-specific antigen (PSA) change, survival rate, and adverse events were compared between the 2 groups.
Results
The PSA levels before and after treatment were 37.0±32.8 ng/mL and 9.5±27.0 ng/mL in group 1 (p<0.001) and 50.2±40.0 ng/mL and 20.0±35.8 ng/mL in group 2 (p<0.001). Mean survival rates were 78.9% in group 1 and 52.3% in group 2 (p=0.055). There were no statistically significant differences in adverse events between the 2 groups (p=0.304). The International Index of Erectile Function 5 (IIEF-5) score before treatment was 19.3±5.9 in group 1 and 18.3±5.8 in group 2 (p=0.487). The IIEF-5 score after treatment was 17.1±6.3 in group 1 and 14.0±6.1 in group 2, which was a statistically significant difference (p=0.036).
Conclusions
The PSA change, mean survival rate, and adverse events in patients with locally advanced prostate cancer treated with bicalutamide 150-mg and CAB did not differ significantly. However, sexual function was better in the bicalutamide 150-mg group. Therefore, bicalutamide 150-mg monotherapy could be considered as a treatment for locally advanced prostate cancer in patients concerned about sexual function.
Keywords: Bicalutamide, Prostatic neoplasms, Therapy
INTRODUCTION
Prostate cancer is the most common cancer in men in the world, and it is estimated that there were 913,000 new cases and 258,000 deaths from prostate cancer worldwide in 2008 [1]. The prevalence of prostate cancer in Korea quadrupled between 2002 and 2008, with the highest increased incidence rate in total forms of malignancy [2]. Furthermore, the prevalence rate of prostate cancer is rapidly increasing globally. However, the presence of clinically advanced disease (i.e., T3-4) decreased from 11.8% to 5.3% recently [3].
Many treatment modalities are available for locally advanced prostate cancer, such as radical prostatectomy, radiation therapy, hormone therapy, and combined treatment. Although many studies have been undertaken to determine which treatment is the most effective, there is no standard treatment as yet [4,5,6]. Two kinds of hormone treatment, combined androgen blockade (CAB) and bicalutamide monotherapy, have been used in locally advanced prostate cancer. Bicalutamide 150-mg monotherapy is an effective and potent well-tolerated nonsteroidal antiandrogen that is recommended for once daily dosing in locally advanced prostate cancer. There have been no reports in Korea in which patients with locally advanced prostate cancer were divided into two groups who received either bicalutamide monotherapy or CAB. In the present study, therefore, we aimed to estimate the effectiveness of bicalutamide monotherapy compared with CAB in men with locally advanced prostate cancer.
MATERIALS AND METHODS
1. Patients
Patients were 74 cases in whom locally advanced prostate cancer (clinical stage T3N0M0, T4N0M0) was diagnosed through transrectal ultrasound-guided prostate biopsy from March 2003 to July 2012. The patients were treated for more than 3 months, were followed up for more than 6 months, and were analyzed retrospectively. Of 74 patients, 25 (clinical stage T3, 15 cases; T4, 10 cases) were treated with bicalutamide 150-mg only (group 1) and 49 (clinical stage T3, 28 cases; T4, 21 cases) had CAB (group 2). We started hormone treatment with informed consent, after explaining the various treatment modalities to the patients. We explained the pros and cons of CAB and monotherapy, especially the side effects, to patients, and we did not divide the group according to specific criteria.
2. Methods
Serum prostate-specific antigen (PSA) change, survival rate, and adverse events including those on sexual function were compared between before and after treatment. Each patient underwent history taking, digital rectal exam (DRE), and routine laboratory tests including PSA measurement. Each patient had been diagnosed with prostate cancer through transrectal ultrasound-guided prostate biopsy, with differentiation measured according to the Gleason grading system. Bone scan, prostate magnetic resonance imaging (MRI), or abdominopelvic computed tomography was carried out for clinical staging. Clinical stage was determined on the basis of the 1999 American Joint Cancer Committee clinical staging system.
Group 1 patients took only bicalutamide 150-mg once per day, and group 2 patients received CAB with subcutaneous goserelin acetate 3.6 mg every 28 days and bicalutamide 50 mg once per day. Mean follow-up periods were 60.2±51.3 months in group 1 and 38.1±33.4 months in group 2.
To check treatment efficacy, serum PSA was measured before treatment and at 3-month intervals during treatment, and bone scan and prostate MRI were conducted when progression was suspected clinically. Adverse events were evaluated through history taking, physical exam, and serum chemistry tests such as liver enzyme levels. Sexual function was evaluated through the International Index of Erectile Function 5 (IIEF-5).
We treated patients with locally advanced prostate cancer with hormone therapy after informed consent was given for the treatment modality. We recommended the treatment modality randomly after full explanation of CAB and bicalutamide monotherapy.
3. Statistics
Patient characteristics were analyzed by use of independent t-tests, each group's follow-up period and PSA difference were analyzed by use of the Mann-Whitney U-test, survival rate was analyzed by the Kaplan-Meier method, PSA decline after treatment was analyzed by the Wilcoxon signed rank test, and comparison of the survival period of expired patients was analyzed by use of the Mann-Whitney U-test. IBM SPSS ver. 19.0 (IBM Co., Armonk, NY, USA) was used to perform all the statistical analyses and a p-value <0.05 was considered statistically significant.
RESULTS
The mean age of the patients was 75.7±5.8 years in group 1 and 72.3±8.2 years in group 2 (p=0.065). The mean Gleason score of the patients was 6.6±2.0 in group 1 and 7.2±1.8 in group 2, with no statistically significant difference between the groups (p=0.158). The PSA level before treatment was 37.0±32.8 ng/mL in group 1 and 50.2±40.0 ng/mL in group 2 (p=0.143) (Table 1). The follow-up PSA level after treatment was 2.0±4.6 ng/mL in group 1 and 5.7±12.4 ng/mL in group 2 (p=0.115), with no statistically significant difference between groups (Table 2).
TABLE 1.
Baseline clinical characteristics of each group
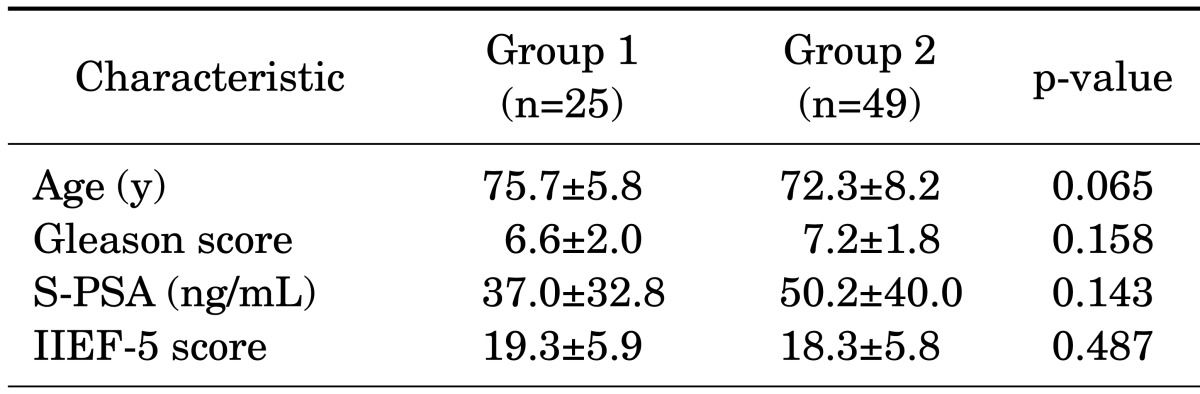
Values are presented as mean±standard deviation.
Group 1, monotherapy group; group 2, combined androgen blockade group; PSA, prostate-specific antigen; IIEF, International Index of Erectile Function.
TABLE 2.
Comparison of PSA changes in each group
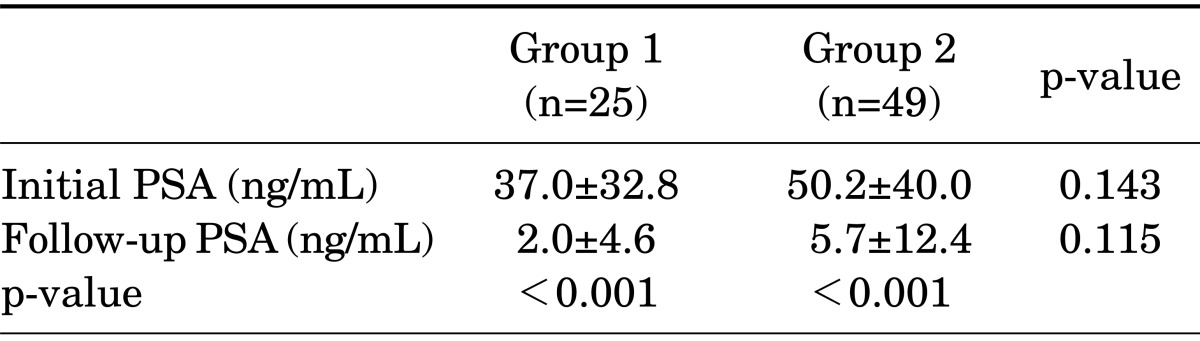
Values are presented as mean±standard deviation.
PSA, prostate-specific antigen; group 1, monotherapy group; group 2, combined androgen blockade group.
Both groups showed a statistically significant change in the PSA level from before to after treatment. The PSA level in group 1 was 37.0±32.8 ng/mL before treatment and 2.0±4.6 ng/mL after treatment (p<0.001). The corresponding values in group 2 were 50.2±40.0 ng/mL and 5.7±12.4 ng/mL, respectively (p<0.001) (Table 2).
The overall survival period was 29.3±19.3 months in group 1 and 62.5±42.1 months in group 2 (Fig. 1). As shown in Fig. 1, the survival rates of both groups decreased over time; however, there were no statistically significant differences between the 2 groups (p=0.119).
FIG. 1.
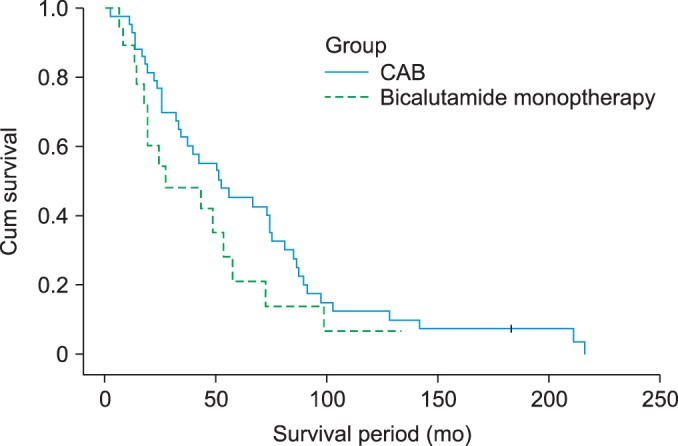
Cumulative survival of patients according to treatment assignment. Survival rates of both group decreased over time, however, they had no statistically significant difference (p=0.197). CAB, combined androgen blockade.
Adverse events in group 1 were gynecomastia in 12 cases, breast pain in 9 cases, asthenia in 3 cases, anemia and diarrhea in 1 case, and constipation in 1 case, whereas those in group 2 were hot flashes in 25 cases, asthenia in 10 cases, anemia in 1 case, and diarrhea in 1 case. There were no statistically significant differences between the groups (p=0.304) (Table 3). There were no patient withdrawals owing to adverse events in either group. However, in group 1, three patients who complained of severe breast pain were treated with tamoxifen citrate. Other adverse events improved with conservative therapy.
TABLE 3.
Adverse events occurring in patients receiving randomized therapy in each group
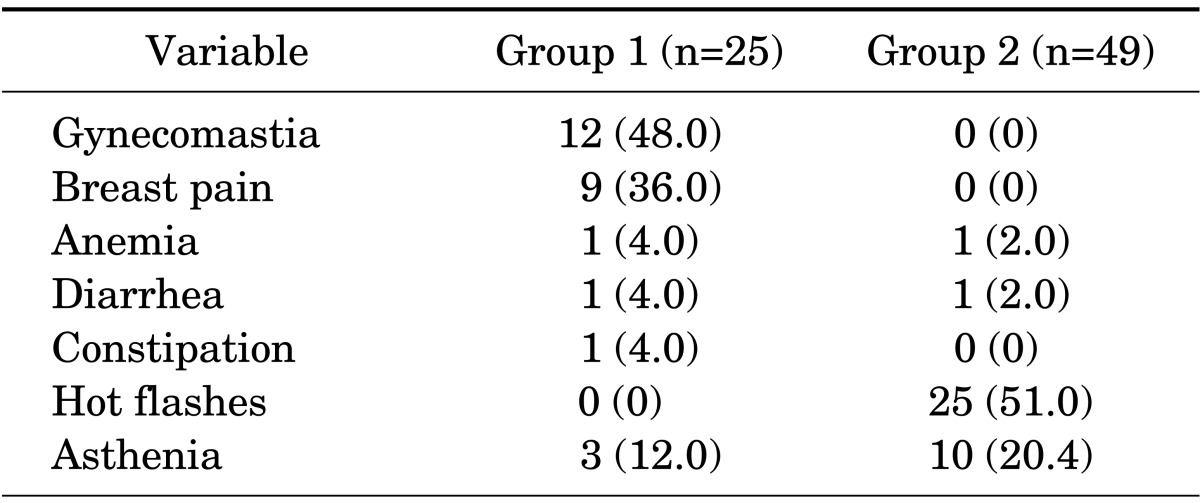
Values are presented as number (%). There were no statistically significant differences between groups 1 and 2 (p=0.304).
Group 1, monotherapy group; group 2, combined androgen blockade group.
Initial sexual function as evaluated by the IIEF-5 score was 19.3±5.9 in group 1 and 18.3±5.8 in group 2 (p=0.487). The follow-up IIEF-5 scores after treatment were 17.1±6.3 in group 1 and 14.0±6.1 in group 2 (p=0.036), with a statistically significant difference (Table 4). Both groups had statistically significant decreases in the IIEF-5 score after treatment: from 19.3±5.9 to 17.1±6.3 in group 1 (p=0.033) and from 18.3±5.8 to 14.0±6.1 in group 2 (p<0.001) (Table 4).
TABLE 4.
Comparison of changes in the IIEF-5 score in each group
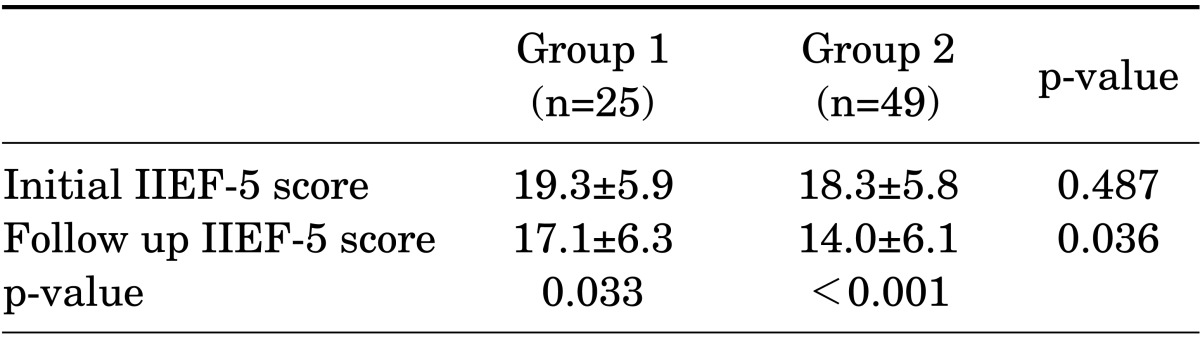
Values are presented as mean±standard deviation.
IIEF, International Index of Erectile Function; group 1, monotherapy group; group 2, combined androgen blockade group.
In each group, the difference in PSA between before and after treatment was 32.6±29.7 and 40.9±37.8, respectively (p=0.422), with no statistically significant difference. By contrast, the difference in IIEF-5 score was 2.2±6.0 and 4.3±4.4, respectively (p=0.043), which was statistically significantly different between the groups.
DISCUSSION
As a result of the aging of the population and western dietary habits among Koreans, the incidence of prostate cancer in Korea is growing rapidly. The definition of locally advanced prostate cancer is based on clinical examination, including the DRE, and clear evidence of spread outside of the prostate capsule (T3a), involvement of the seminal vesicles (T3b), or involvement of adjacent organs (T4) [3]. The widespread application of prostate cancer early-detection efforts and PSA screening has resulted in both an increased diagnosis of prostate cancers and earlier identification of these tumors. Despite the stage migration associated with PSA testing and the growing number of low-stage and organ-confined tumors, at least 10% of men with newly diagnosed prostate cancer have locally advanced prostate cancer. However, the proportion of patients presenting with locally advanced prostate cancer at diagnosis (clinical stage T3-4) has decreased in the past 20 years, largely as a result of widespread PSA screening. Despite this decrease, locally advanced prostate cancer remains a common clinical problem and management remains controversial [7].
Currently, no consensus exists regarding the optimal management of locally advanced prostate cancer. The current standard treatment for patients with locally advanced prostate cancer is radical prostatectomy or radiation therapy with concurrent and adjuvant androgen deprivation therapy [8].
The use of radical prostatectomy for the management of locally advanced prostate cancer has decreased because radical prostatectomy alone is an insufficient treatment modality in locally advanced prostate cancer. Many patients treated with radical prostatectomy despite improved surgeon skill with a robot require adjuvant treatment with radiation therapy or hormone therapy.
Radiation therapy is used with alternative methods other than radical prostatectomy to treat locally advanced prostate cancers. Over the past 15 years, the development of new radiotherapy techniques including intensity-modulated radiotherapy (IMRT) has allowed for acceptable morbidity in patients with localized prostate cancer. Although a plethora of studies describe the results of radiation therapy for clinical stage C or T3 disease, many of these studies were performed before the widespread use of serum PSA determinations for early detection and modern radiation techniques for treatment of locally advanced prostate cancer, such as three-dimensional conformal radiation therapy, IMRT, and irradiation of the whole pelvis in addition to the prostate [9]. The risk of lymph node metastasis and low overall survival rate for radiation therapy alone for the treatment of locally advanced prostate cancer requires systemic treatment including androgen deprivation therapy. Radiation therapy is used with androgen deprivation therapy in locally advanced prostate cancer.
Men with locally advanced prostate cancer can expect to receive treatment for many years and the impact of treatment on quality of life is an important consideration in this setting, particularly when choosing between treatment options that confer similar survival benefits, such as castration and bicalutamide monotherapy [10]. To minimize adverse impacts on quality of life, antiandrogen monotherapy alone has been proposed as an alternative to orchiectomy or luteinizing hormone-releasing hormone agonists. Iversen et al. [11] reported on the effects of 150-mg of bicalutamide in men with localized or locally advanced prostate cancer, most of whom were untreated initially (81%). At a median follow-up of 5.3 years, survival in those with locally advanced disease was improved with bicalutamide compared with placebo (hazard ratio [HR], 0.68). Overall, the risk of disease progression was reduced by 43%, with the greatest benefit in locally advanced tumors (HR, 0.4). The combined analysis of the three Early Prostate Cancer bicalutamide trials (n=8,113) confirmed improved progression-free survival in the bicalutamide group [12]. Overall survival was not significantly different between the treatment and placebo arms in each trial, but men with locally advanced disease receiving bicalutamide alone appeared to have improved survival [12]. In our experience, bicalutamide monotherapy in locally advanced prostate cancer had a survival benefit comparable to that of CAB.
Bicalutamide monotherapy is thought to be an appropriate substitute for CAB in the treatment of locally advanced prostate cancer. For such reasons, much attention has been paid to nonsteroidal antiandrogen therapy alone, but research on effects, survival rates, adverse events, and sexual function compared with CAB is not sufficient in Korea. Thus, the present study compared the treatment effects, survival rates, adverse events, and sexual function of a bicalutamide 150-mg monotherapy group and a CAB group with treatment spanning 10 years. CAB uses bicalutamide 50 mg, but for the bicalutamide monotherapy, there were controversies on the dosage. It is known that the PSA response to bicalutamide is dose-related. In other research, 150- to 200-mg bicalutamide therapy alone reached a state at which there was no further increase in response [13]. Since then, bicalutamide monotherapy with 150-mg daily has been studied. Iversen et al. [14] reported that in previously untreated patients with M0 prostate cancer, bicalutamide 150-mg monotherapy is equivalent to castration in terms of survival rate at a median follow-up of 4 years, with 31% of cases resulting in death, and represents a well-tolerated alternative to either surgical or medical castration. Also, bicalutamide 150-mg offers quality of life advantages over both methods of castration with respect to sexual interest and physical capacity [14].
The next factor to consider is the adverse events. With respect to adverse events and improvements in the PSA level, bicalutamide 150-mg monotherapy and CAB showed no significant differences [15]. Koh et al. [13] reported that the response to treatment was excellent in terms of the decrease in PSA and adverse events after bicalutamide monotherapy. As seen in the results above, the two groups showed no significant differences in our study. In previous studies about bone mineral density, however, experimental results suggested that nonsteroidal antiandrogens do not affect bone mineral density [16]. The major differences between bicalutamide and castration were observed for the expected pharmacological effects of castration (hot flushes, 39%-44%) and bicalutamide (breast pain, 37%-39%; gynecomastia, 35%-39%), which were reported at levels of incidence consistent with those reported for bicalutamide 50-mg monotherapy compared with castration [17]. Gynecomastia is generally produced by a rise in circulating estradiol levels in prostate cancer patients, which occurs as an effect of peripheral aromatization of testosterone, the levels of which are usually increased by antiandrogen monotherapy. In our study, the rates of gynecomastia and breast pain were 48% and 36%, respectively. Severe breast pain was treated with tamoxifen. Considering adverse events, nonsteroidal antiandrogen monotherapy could be as effective as castration, antagonizing both the testicular and the adrenal androgens at the receptor level without any deleterious effects on sexual potency or libido [10].
Quality of life is a major consideration in prostate cancer treatment. Iversen et al. [18] compared the quality of life between a bicalutamide monotherapy group and a castration group of patients with locally advanced prostate cancer; the bicalutamide monotherapy group showed better results for both sexual function and physical activity. In other research, there were statistically significant benefits in patients treated with bicalutamide relative to those treated with castration in the quality of life domains of sexual interest (p=0.029) and physical capacity (assesses activities such as walking, dressing, bathing, shopping, climbing stairs, sports, and bending; p=0.046) after 12 months of treatment [19].
In each group in our study, the IIEF-5 score showed a statistically significant difference. Thus, bicalutamide monotherapy is recommended for locally advanced prostate cancer patients who wish to maintain sexual interest.
Because bicalutamide monotherapy did not show any significant differences in survival rate, adverse events, or quality of life, it could be considered as one of the treatments for locally advanced prostate cancer. However, because there are some reports saying that CAB can quickly improve patients with a large tumor that presses against the spinal cord, patients with ureteral obstruction, and patients with severe cancer symptoms, CAB should be prioritized in some cases [20]. Recently, Iversen et al. [21] reported that bicalutamide 150-mg improved progression-free survival in patients with locally advanced prostate cancer but not in patients with localized disease. In our results, bicalutamide monotherapy 150-mg daily showed no statistically significant difference in mean survival period, PSA change, or adverse events compared with CAB in locally advanced prostate cancer. Also, sexual function was better in the bicalutamide 150-mg group. Thus, bicalutamide 150-mg might represent an alternative for patients with locally advanced prostate cancer considering androgen-deprivation therapy.
CONCLUSIONS
Because bicalutamide monotherapy 150-mg daily had no statistically significant differences in mean survival period, PSA change, or adverse events compared with CAB, it can be considered for locally advanced prostate cancer with respect to adverse events. However, the treatment decision should be made according to the patient's preference, age, underlying disease, and concern about adverse events, particularly with respect to sexual interest.
The limitation of this study is that the data were collected retrospectively. In the future, a prospective study with a large enough sample is needed.
Footnotes
The authors have nothing to disclose.
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Statistics Korea [Internet] Daejeon: Statistics Korea; [2012 Mar 10]. Available from: http://kostat.go.kr/portal/english/index.action. [Google Scholar]
- 3.Meng MV, Carroll PR. Treatment of locally advanced prostate cancer. In: Wein AJ, Kavoussi LR, Novick AC, Partin AW, Peters CA, editors. Campbell-Walsh urology. 10th ed. Philadelphia: Saunders; 2012. pp. 2903–2920. [Google Scholar]
- 4.Ischia J, Gleave M. Radical prostatectomy in high-risk prostate cancer. Int J Urol. 2013;20:290–300. doi: 10.1111/iju.12069. [DOI] [PubMed] [Google Scholar]
- 5.Bolla M, Van Tienhoven G, Warde P, Dubois JB, Mirimanoff RO, Storme G, et al. External irradiation with or without long-term androgen suppression for prostate cancer with high metastatic risk: 10-year results of an EORTC randomised study. Lancet Oncol. 2010;11:1066–1073. doi: 10.1016/S1470-2045(10)70223-0. [DOI] [PubMed] [Google Scholar]
- 6.Warde P, Mason M, Ding K, Kirkbride P, Brundage M, Cowan R, et al. Combined androgen deprivation therapy and radiation therapy for locally advanced prostate cancer: a randomised, phase 3 trial. Lancet. 2011;378:2104–2111. doi: 10.1016/S0140-6736(11)61095-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooperberg MR, Moul JW, Carroll PR. The changing face of prostate cancer. J Clin Oncol. 2005;23:8146–8151. doi: 10.1200/JCO.2005.02.9751. [DOI] [PubMed] [Google Scholar]
- 8.Sridharan S, Dal Pra A, Catton C, Bristow RG, Warde P. Locally advanced prostate cancer: current controversies and optimisation opportunities. Clin Oncol (R Coll Radiol) 2013;25:499–505. doi: 10.1016/j.clon.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 9.Kupelian P. External beam radiation therapy: role of androgen deprivation. World J Urol. 2003;21:190–199. doi: 10.1007/s00345-003-0355-y. [DOI] [PubMed] [Google Scholar]
- 10.Abrahamsson PA. Treatment of locally advanced prostate cancer: a new role for antiandrogen monotherapy? Eur Urol. 2001;39(Suppl 1):22–28. doi: 10.1159/000052546. [DOI] [PubMed] [Google Scholar]
- 11.Iversen P, Johansson JE, Lodding P, Lukkarinen O, Lundmo P, Klarskov P, et al. Bicalutamide (150 mg) versus placebo as immediate therapy alone or as adjuvant to therapy with curative intent for early nonmetastatic prostate cancer: 5.3-year median follow-up from the Scandinavian Prostate Cancer Group Study Number 6. J Urol. 2004;172(5 Pt 1):1871–1876. doi: 10.1097/01.ju.0000139719.99825.54. [DOI] [PubMed] [Google Scholar]
- 12.Wirth MP, See WA, McLeod DG, Iversen P, Morris T, Carroll K, et al. Bicalutamide 150 mg in addition to standard care in patients with localized or locally advanced prostate cancer: results from the second analysis of the early prostate cancer program at median followup of 5.4 years. J Urol. 2004;172(5 Pt 1):1865–1870. doi: 10.1097/01.ju.0000140159.94703.80. [DOI] [PubMed] [Google Scholar]
- 13.Koh JS, Lee CB, Suh HJ, Lee YB, Cho DH, Lee JY. Efficacy of bicalutamide monotherapy in locally advanced prostate cancer. Korean J Urol. 2004;45:108–113. [Google Scholar]
- 14.Iversen P, Tyrrell CJ, Kaisary AV, Anderson JB, Baert L, Tammela T, et al. Casodex (bicalutamide) 150 mg monotherapy compared with castration in patients with previously untreated nonmetastatic prostate cancer: results from two multicenter randomized trials at a median follow-up of 4 years. Urology. 1998;51:389–396. doi: 10.1016/s0090-4295(98)00004-1. [DOI] [PubMed] [Google Scholar]
- 15.Kim DG, Chang HJ, Lee KS. The therapeutic effect of monotherapy and combined therapy for androgen blockade in patients with metastatic prostate cancer. Korean J Urol. 2003;44:12–16. [Google Scholar]
- 16.Broulik PD, Starka L. Effect of antiandrogens casodex and epitestosterone on bone composition in mice. Bone. 1997;20:473–475. doi: 10.1016/s8756-3282(97)00012-4. [DOI] [PubMed] [Google Scholar]
- 17.Tyrrell CJ, Kaisary AV, Iversen P, Anderson JB, Baert L, Tammela T, et al. A randomised comparison of 'Casodex' (bicalutamide) 150 mg monotherapy versus castration in the treatment of metastatic and locally advanced prostate cancer. Eur Urol. 1998;33:447–456. doi: 10.1159/000019634. [DOI] [PubMed] [Google Scholar]
- 18.Iversen P, Tyrrell CJ, Kaisary AV, Anderson JB, Van Poppel H, Tammela TL, et al. Bicalutamide monotherapy compared with castration in patients with nonmetastatic locally advanced prostate cancer: 6.3 years of followup. J Urol. 2000;164:1579–1582. [PubMed] [Google Scholar]
- 19.Iversen P. Quality of life issues relating to endocrine treatment options. Eur Urol. 1999;36(Suppl 2):20–26. doi: 10.1159/000052339. [DOI] [PubMed] [Google Scholar]
- 20.Eisenberger MA, Blumenstein BA, Crawford ED, Miller G, McLeod DG, Loehrer PJ, et al. Bilateral orchiectomy with or without flutamide for metastatic prostate cancer. N Engl J Med. 1998;339:1036–1042. doi: 10.1056/NEJM199810083391504. [DOI] [PubMed] [Google Scholar]
- 21.Iversen P, McLeod DG, See WA, Morris T, Armstrong J, Wirth MP, et al. Antiandrogen monotherapy in patients with localized or locally advanced prostate cancer: final results from the bicalutamide Early Prostate Cancer programme at a median follow-up of 9.7 years. BJU Int. 2010;105:1074–1081. doi: 10.1111/j.1464-410X.2010.09319.x. [DOI] [PubMed] [Google Scholar]


