Abstract
Purpose
To evaluate the validity of the cancer of the prostate risk assessment (CAPRA) score, a newly developed nomogram for preoperative prediction of recurrence after radical prostatectomy, in a single institution in Korea.
Materials and Methods
We retrospectively studied 115 men who had undergone radical prostatectomy as the first treatment for localized prostate cancer. The validity of the CAPRA score for the prediction of recurrence-free survival (RFS) and pathologic outcome was evaluated by using Kaplan-Meier analysis and a proportional hazards regression model. A seven-group model and a three-group model were used for the results.
Results
None of the variables of the CAPRA score was favorable compared with the previously reported data. The three-group model was significantly related with 3- and 5-year RFS (p<0.05), but the seven-group model was not. The concordance indices of the CAPRA score were 0.74 and 0.77. Of four components excluding the clinical T stage, three independently predicted RFS (age, Gleason sum, and percentage of positive biopsies). The CAPRA score was significantly related to the margin status, extracapsular extension, and seminal vesicle invasion in both the seven- and three-group models. In the three-group model, pathologic outcomes were more strongly related, especially a higher risk of seminal vesicle invasion.
Conclusions
The CAPRA score showed high accuracy for predicting RFS. In particular, the three-group model was more useful for predicting RFS and pathologic outcomes. Therefore, the CAPRA score may be a useful prediction model for risk stratification and may help clinicians to develop localized prostate cancer treatment.
Keywords: Nomograms, Prostatectomy, Prostatic neoplasms, Recurrence
INTRODUCTION
Prostate cancer is a significant cause of death among men in Europe and the United States [1,2]. In the United States, prostate cancer has the second highest disease mortality rate [2]. In Korea, the prevalence of prostate cancer quadrupled between 2002 and 2008 [3]. The incidence increased to 24.8 per 100,000 men in 2009, with a two-fold increase over that in 2008 [4].
Prostatectomy in patients with localized prostate cancer leads to a reduction in overall mortality [5,6]. However, all available treatments, including prostatectomy, may significantly impact the patient's quality of life [7]. Therefore, clinicians must attempt to determine at the time of diagnosis which patients might do well with active surveillance, who should receive immediate local treatment, and who will require aggressive multimodal therapy [8]. In recent years, several nomograms have been developed to assist clinicians in the prediction of patient outcome following these different treatments. However, most are limited by their complex nature or poor predictive value [9].
In 2005, Cooperberg et al. [8] derived the cancer of the prostate risk assessment (CAPRA) score to preoperatively predict biochemical recurrence-free survival (RFS) after radical prostatectomy in patients with localized prostate cancer. Its high accuracy was verified through analysis using SEARCH data with multicenter enrollment [10]. In the present study, we evaluated the validity of the CAPRA score at a single institution in Korea.
MATERIALS AND METHODS
Under institutional review board supervision at Inje University Busan Paik Hospital, patients who underwent prostatectomy without adjuvant therapy as the first-line treatment for localized prostate cancer were retrospectively analyzed from January 2008 to June 2013. A total of 115 patients met all inclusion criteria to calculate the CAPRA score and were included in the study group. Tables 1, 2 show all variables and an overview of the CAPRA score. The same variable definitions as those in the original CAPRA score design were used in this study [8]. Biochemical recurrence after radical prostatectomy was defined as two consecutive prostate-specific antigen (PSA) values of ≥0.2 ng/mL at any time postoperatively or any additional treatment more than 6 months after prostatectomy. The patients were divided into two study groups: the seven-group model (each CAPRA score sum) and the three-group model (low, intermediate, and high risk).
TABLE 1.
The characteristics of the present data set compared with the CaPSURE data, German data, and Japanese data on the basis of the CAPRA scoring system
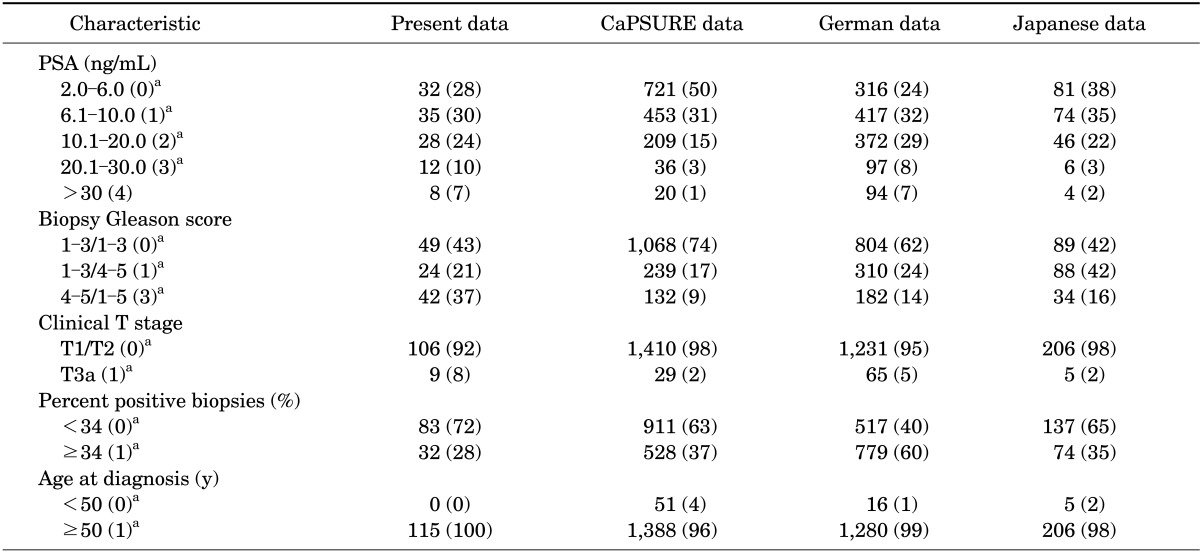
Values are presented as number (%).
CAPRA, cancer of the prostate risk assessment; PSA, prostate-specific antigen.
a:CAPRA points.
TABLE 2.
Results of Cox regression analysis about each variable
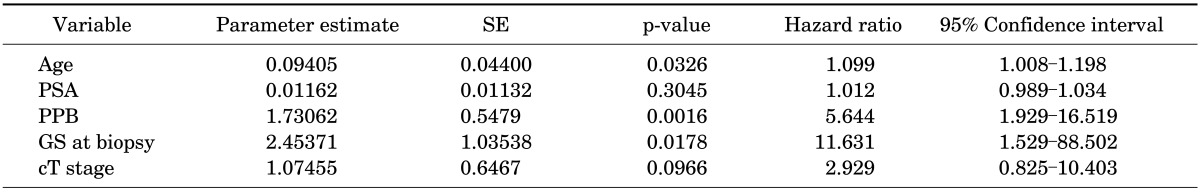
SE, standard error; PSA, prostate-specific antigen; PPB, percentage of positive biopsies; GS, Gleason score; cT stage, clinical T stage through preoperative work-up.
The 3- and 5-year RFS rates were evaluated with Kaplan-Meier analysis for probabilities. The concordance index for the CAPRA scoring system was calculated with logistic regression and Cox regression to determine the predicted survival probabilities. Logistic regression analyses were performed, and odds ratios (ORs) were calculated for the occurrence of positive surgical margins, extracapsular extension, and seminal vesicle invasion. SAS 9.3 (SAS Institute Inc., Cary, NC, USA) and R ver. 3.01 (R Foundation for Statistical Computing, Vienna, Austria) were used for the statistical analyses, and p<0.05 was considered statistically significant.
RESULTS
The mean age of the patients was 66.4 (standard deviation [SD], 6.5) years. The mean preoperative PSA level was 12.7 (SD, 13.2) ng/mL, and 15 patients (13%) developed recurrence at a median of 13 (SD, 12.1) months. The patient characteristics are listed in Tables 1, 2 and are compared with those from the CaPSURE dataset, German data, and Japan data. The number of patients with a high preoperative PSA level (>10 ng/mL) was similar to that of the German multicenter data (41.7%). However, the present study showed higher biopsy Gleason scores than those in the German multicenter data.
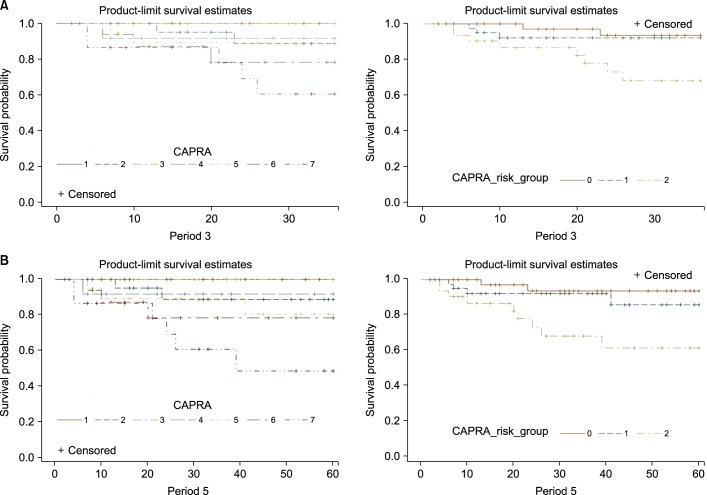
Fig. 1 shows the 3- and 5-year RFS rates represented by survival curves. These rates decreased as the CAPRA score increased, but the trend was not statistically significant. However, in the three-group model, the 3- and 5-year RFS rates significantly decreased as the risk increased (p=0.011). For patients with low (score of 0-2), intermediate (score of 3-5), and high risk (score of 6-10), the 3- and 5-year RFS rates were 93.5%/93.5%, 92.2%/85.6%, and 68.1%/61.3%, respectively. The concordance indices of the CAPRA score for the 3- and 5-year RFS rates were 0.74 and 0.77, respectively.
FIG. 1.
Kaplan-Meier survival curve and performance of the CAPRA score for 3- and 5-year RFS (7-group model and 3-group model). (A) 3-Year RFS about CAPRA score, 7-group model and 3-group model. (B) 5-Year RFS about CAPRA score, 7-group model and 3-group model. CAPRA, cancer of the prostate risk assessment; RFS, recurrence-free survival.
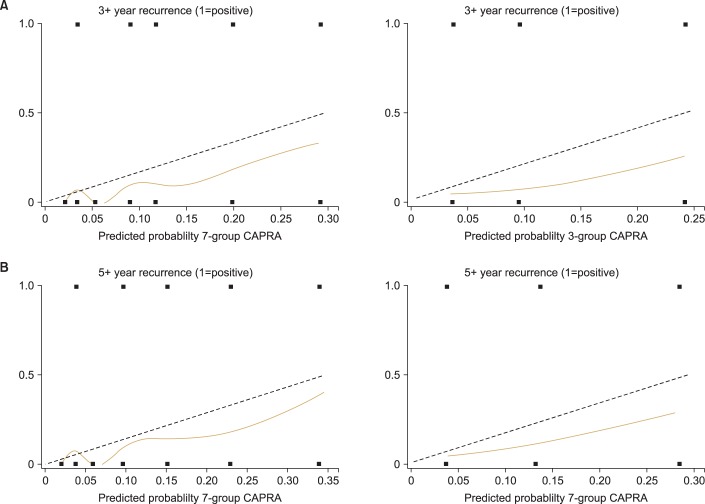
The CAPRA scores of both the three- and seven-group models predicted that the probability of recurrence generally increased with the actual probability of recurrence of the 3- and 5-year RFS. However, in all situations, the CAPRA score tended to underestimate the risk of recurrence (Fig. 2).
FIG. 2.
Performance of the CAPRA score for predicting biochemical recurrence (7-group model and 3-group model). (A) Predicting 3-year biochemical recurrence, 7-group model and 3-group model. (B) Predicting 5-year biochemical recurrence, 7-group model and 3-group model. Broken line shows prediction about ideal nomogram. Below it means underprediction; above it means overprediction. CAPRA, cancer of the prostate risk assessment.
With the exception of the preoperative serum PSA level and clinical T stage, most predictor variables for the CAPRA score (i.e., age, Gleason sum, and percentage of positive biopsies) were related to RFS (Table 2). The strongest predictor variable was the Gleason sum with a hazard ratio of 11.6 (p=0.018), and the most reliable variable was the percentage of positive biopsies with a hazard ratio of 5.6 (p=0.002).
We found significantly higher rates of positive surgical margins, extracapsular extension, and seminal vesicle invasion coinciding with higher CAPRA scores or risks in the three-group model (Table 3). Positive extracapsular extension occurred in 13.3% of patients with a CAPRA score of 0 to 1 and in 66.7% with a CAPRA score of 7 to 10 (p=0.003). In the three-group model, positive surgical margins occurred in 16.3% of patients in the low-risk group and in 45.2% in the high-risk group (p=0.008).
TABLE 3.
Pathological outcomes by CAPRA score (7-group model and 3-group model)
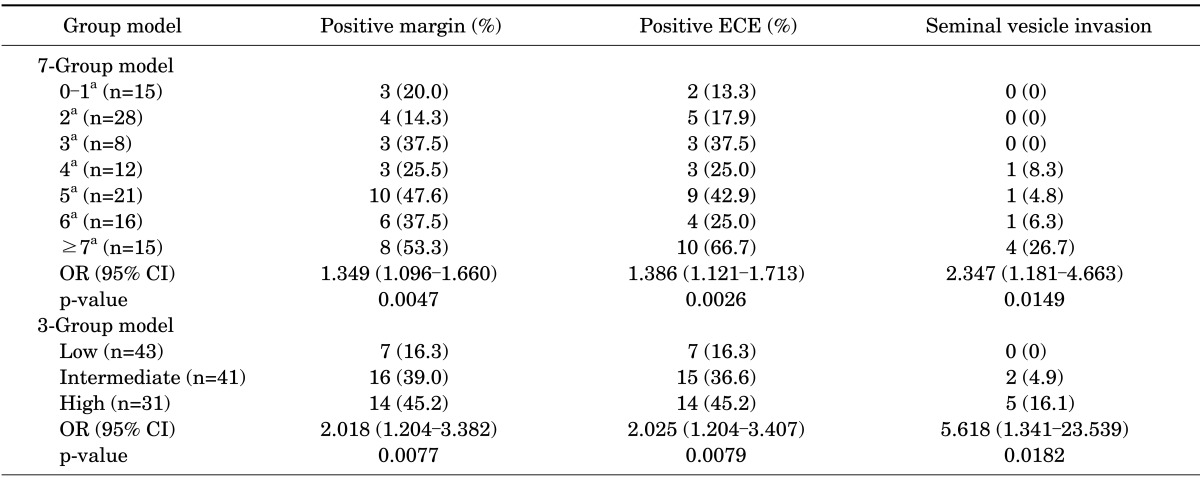
Values are presented as number (%) unless otherwise indicated.
CAPRA, cancer of the prostate risk assessment; ECE, extracapsular extension; OR, odds ratio.
a:CAPRA score.
DISCUSSION
Radical prostatectomy is performed in many institutions for the treatment of localized prostate cancer. Some patients experience biochemical recurrence after surgery followed by local recurrence and metastasis. These events result not only in an increased incidence of cancer-related morbidities, but also in a heightened level of anxiety for patients and clinicians. Therefore, well-established predictors of the risk of disease progression should help clinicians and patients to choose and assess the effectiveness of the various therapeutic options and should especially help with postoperative disease assessment. Furthermore, preoperative counseling might be easier for patients with prostate cancer.
Many nomograms for preoperative risk assessment have recently been introduced. The D'Amico risk classification and the Kattan nomogram have been widely used. In low-risk patients with prostate cancer, the D'Amico risk classification performs well; however, there is significant overlap between intermediate- and high-risk patients [11]. The Kattan nomogram was introduced with better integration of multiple risk factors, but it is difficult to apply without the paper forms of the instruments at hand. A hand-held computer version of the Kattan nomogram is also available. However, in low-risk patients in a community setting, the RFS is overestimated [12].
The CAPRA score originated from the CaPSURE database, so it performs well in community-based cohorts, comparable with the Kattan nomogram. In a previous study, the c-indices for the three systems were 0.63 (0.55-0.72) for the D'Amico classification, 0.65 (0.56-0.75) for the Kattan nomogram, and 0.66 (0.57-0.75) for the CAPRA score [8]. In a US multicenter survey using the SEARCH database, the c-index of the CAPRA score was 0.68 [10]. However, a higher prediction ability for recurrence was obtained in a German multicenter study (0.78 and 0.81) [13]. In Japan, the c-index of the CAPRA score was 0.755 [14]. The prediction ability of the 3-year RFS rate is variable among systematic reviews and meta-analyses. Furthermore, the CAPRA score is reasonably accurate for predicting metastasis (c-index=0.78) and cancer-specific mortality (c-index=0.80) [15].
The c-index for the 3- and 5-year RFS in the present study was 0.74 and 0.77, respectively. These rates are higher than those of the original development set and US multicenter survey, but lower than the German multicenter survey and previous systematic reviews. Loeb et al. [16] evaluated the CAPRA score in a single-surgeon radical prostatectomy series, similar to our circumstances; they calculated a c-index of 0.764 for 5-year RFS, similar to our result. The short follow-up period and the more censored data than in other large-scale studies were probably the reasons for the differences. In addition, the preoperative characteristics of the patients differed from those in other studies.
In the present study, the proportion of patients with a high preoperative PSA level (>10 ng/mL) was higher than that in the CaPSURE database when the CAPRA score was first introduced (41.7% vs. 19%), but similar to that in the German data. Furthermore, the proportion of patients with a primary Gleason score of 4 was higher than that in the CaPSURE and German multicenter data (36.5% vs. 9% vs. 14%, respectively) [8,10]. Generally, lower-risk patients with prostate cancer have been included in US studies. This difference may have also caused different results.
In this study, the only significant relationship was seen in the three-group model between the 3- and 5-year RFS and increasing risk; otherwise, in the seven-group model, the trend of RFS was the same as that in the three-group model, but there was no statistical significance. In both the seven- and three-group models, the CAPRA score predicted probabilities of recurrence that were generally higher than the actual probabilities of recurrence. This is similar to the findings of the German multicenter study. In the German study, the probabilities showed a slight underestimation of the risk of recurrence within the medium-probability range of recurrence and an overestimation of the risk of recurrence within the high-probability range of recurrence [13]. However, our study showed a general underestimation of the risk of recurrence in both the three- and seven-group models for the 3- and 5-year RFS.
Pathologic outcomes were not available for the development set of the CaPSURE study, but the SEARCH study analyzed the prediction ability of the CAPRA score for pathologic outcomes [8,10]. It showed a statistically significant relationship between pathologic outcomes (i.e., positive surgical margin status, extracapsular extension, seminal vesicle involvement, and lymph node involvement) and an increasing CAPRA score. Seminal vesicle involvement was particularly striking, with a near doubling of risk (OR, 1.80; 95% confidence interval [CI], 1.6-2.02) [10]. Our results were the same: the CAPRA score showed an increasing probability in terms of the margin status, extracapsular extension, and seminal vesicle invasion. In addition, in the seven-group model analysis, seminal vesicle invasion was the most predictable result with an increasing CAPRA score (OR, 2.35; 95% CI, 1.2-4.7). Only three patients were diagnosed with lymph node invasion as a pathologic outcome in the present study. In the three-group model, the three pathologic outcomes were also significantly related to increasing risk, and seminal vesicle invasion was also the most predictable (OR, 5.62; 95% CI, 1.34-23.5). This validity of the CAPRA score was also proven by the German multicenter study, and no organ confined with advanced disease was the most predictable (OR, 2.11; 95% CI, 1.9-2.3) [13].
There were some limitations to the current study. First, few patients were studied compared with other large cohorts (i.e., CaPSURE, SEARCH); therefore, this study is not representative of all Korean prostate cancer patients. However, it is the first study in Korea on a new method for predicting prognoses and results after radical prostatectomy. Second, because of the short follow-up period, more detailed results regarding RFS could not be obtained.
CONCLUSIONS
Clinicians must decide on treatments after considering the individual traits of patients, such as socioeconomic factors, preferences regarding quality of life, and even genetic factors. Thus, it must be understood that the CAPRA score, like other recurrence-prediction instruments, cannot replace clinical decision-making in patients with localized prostate cancer.
Nevertheless, the CAPRA score showed good predictive accuracy for RFS at our institution. This instrument might help clinicians and patients in planning and counseling at the time of a localized prostate cancer diagnosis, especially when prostatectomy is planned. In addition, characteristics such as convenient use, the scoring system (seven-group model), and the ease of risk stratification (three-group model) may support its widespread use. However, additional assessments of its validity are needed in Korea, which is experiencing an increasing incidence of prostate cancer, through multi-institutional studies involving large cohorts.
Footnotes
The authors have nothing to disclose.
References
- 1.Bray F, Lortet-Tieulent J, Ferlay J, Forman D, Auvinen A. Prostate cancer incidence and mortality trends in 37 European countries: an overview. Eur J Cancer. 2010;46:3040–3052. doi: 10.1016/j.ejca.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 3.Statistics Korea [Internet] Daejeon: Statistics Korea; [2012 Mar 10]. Available from: http://kostat.go.kr/portal/english/index.action. [Google Scholar]
- 4.Jung KW, Park S, Kong HJ, Won YJ, Lee JY, Seo HG, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2009. Cancer Res Treat. 2012;44:11–24. doi: 10.4143/crt.2012.44.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tewari A, Raman JD, Chang P, Rao S, Divine G, Menon M. Long-term survival probability in men with clinically localized prostate cancer treated either conservatively or with definitive treatment (radiotherapy or radical prostatectomy) Urology. 2006;68:1268–1274. doi: 10.1016/j.urology.2006.08.1059. [DOI] [PubMed] [Google Scholar]
- 6.Wong YN, Mitra N, Hudes G, Localio R, Schwartz JS, Wan F, et al. Survival associated with treatment vs observation of localized prostate cancer in elderly men. JAMA. 2006;296:2683–2693. doi: 10.1001/jama.296.22.2683. [DOI] [PubMed] [Google Scholar]
- 7.Wei JT, Dunn RL, Sandler HM, McLaughlin PW, Montie JE, Litwin MS, et al. Comprehensive comparison of health-related quality of life after contemporary therapies for localized prostate cancer. J Clin Oncol. 2002;20:557–566. doi: 10.1200/JCO.2002.20.2.557. [DOI] [PubMed] [Google Scholar]
- 8.Cooperberg MR, Pasta DJ, Elkin EP, Litwin MS, Latini DM, Du Chane J, et al. The University of California, San Francisco Cancer of the Prostate Risk Assessment score: a straightforward and reliable preoperative predictor of disease recurrence after radical prostatectomy. J Urol. 2005;173:1938–1942. doi: 10.1097/01.ju.0000158155.33890.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cooperberg MR, Broering JM, Carroll PR. Risk assessment for prostate cancer metastasis and mortality at the time of diagnosis. J Natl Cancer Inst. 2009;101:878–887. doi: 10.1093/jnci/djp122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cooperberg MR, Freedland SJ, Pasta DJ, Elkin EP, Presti JC, Jr, Amling CL, et al. Multiinstitutional validation of the UCSF cancer of the prostate risk assessment for prediction of recurrence after radical prostatectomy. Cancer. 2006;107:2384–2391. doi: 10.1002/cncr.22262. [DOI] [PubMed] [Google Scholar]
- 11.Mitchell JA, Cooperberg MR, Elkin EP, Lubeck DP, Mehta SS, Kane CJ, et al. Ability of 2 pretreatment risk assessment methods to predict prostate cancer recurrence after radical prostatectomy: data from CaPSURE. J Urol. 2005;173:1126–1131. doi: 10.1097/01.ju.0000155535.25971.de. [DOI] [PubMed] [Google Scholar]
- 12.Greene KL, Meng MV, Elkin EP, Cooperberg MR, Pasta DJ, Kattan MW, et al. Validation of the Kattan preoperative nomogram for prostate cancer recurrence using a community based cohort: results from cancer of the prostate strategic urological research endeavor (capsure) J Urol. 2004;171(6 Pt 1):2255–2259. doi: 10.1097/01.ju.0000127733.01845.57. [DOI] [PubMed] [Google Scholar]
- 13.May M, Knoll N, Siegsmund M, Fahlenkamp D, Vogler H, Hoschke B, et al. Validity of the CAPRA score to predict biochemical recurrence-free survival after radical prostatectomy. Results from a european multicenter survey of 1,296 patients. J Urol. 2007;178:1957–1962. doi: 10.1016/j.juro.2007.07.043. [DOI] [PubMed] [Google Scholar]
- 14.Ishizaki F, Hoque MA, Nishiyama T, Kawasaki T, Kasahara T, Hara N, et al. External validation of the UCSF-CAPRA (University of California, San Francisco, Cancer of the Prostate Risk Assessment) in Japanese patients receiving radical prostatectomy. Jpn J Clin Oncol. 2011;41:1259–1264. doi: 10.1093/jjco/hyr136. [DOI] [PubMed] [Google Scholar]
- 15.Meurs P, Galvin R, Fanning DM, Fahey T. Prognostic value of the CAPRA clinical prediction rule: a systematic review and meta-analysis. BJU Int. 2013;111:427–436. doi: 10.1111/j.1464-410X.2012.11400.x. [DOI] [PubMed] [Google Scholar]
- 16.Loeb S, Carvalhal GF, Kan D, Desai A, Catalona WJ. External validation of the cancer of the prostate risk assessment (CAPRA) score in a single-surgeon radical prostatectomy series. Urol Oncol. 2012;30:584–589. doi: 10.1016/j.urolonc.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]