Abstract
Purpose
In the treatment of interstitial cystitis, intravesical hyaluronic acid application may be suggested as a treatment option. In this randomized prospective study, the authors aimed to identify whether instilling the hyaluronic acid with electromotive drug administration (EMDA) would increase the tissue uptake and improve the efficacy.
Materials and Methods
The data of 31 patients who had been diagnosed with bladder pain syndrome/interstitial cystitis (BPS/IC) between 2004 and 2005 were examined. The patients were randomized to two groups: patients in group A received hyaluronic acid directly with a catheter and patients in group B received hyaluronic acid with EMDA. The patients were followed for 24 months and the two groups were compared at certain time intervals. The primary end points of the study were visual analogue scale (VAS) score, global response assessment, and micturition frequency in 24 hours.
Results
There were 6 males and 25 females. The two groups were similar in baseline parameters. The decrease in VAS score and the micturition frequency in 24 hours were significantly lower with EMDA at months 6 and 12. The difference between the two groups was not significant at months 1 and 24. Also, treatment with EMDA, positive KCl test, and pretreatment voiding frequency >17 were associated with higher response rates.
Conclusions
Hyaluronic acid installation is an effective glycosaminoglycan substitution therapy in patients with BPS/IC. Instillation of hyaluronic acid via EMDA can improve the efficacy of the treatment; however, lack of long-term efficacy is the major problem with this glycosaminoglycan substitution therapy.
Keywords: Hyaluronic acid, Interstitial cystitis, Painful bladder syndrome
INTRODUCTION
Bladder pain syndrome/interstitial cystitis (BPS/IC) is a clinical diagnosis primarily based on symptoms of urinary urgency, frequency, and suprapubic or pelvic pain. The prevalence of BPS/IC ranges from 10 to up to 510 cases per 100,000 population [1,2]. BPS/IC is frequently a diagnosis of exclusion. The etiology of BPS/IC is so far unknown, with several theories under discussion, including an autoimmune response, mast cell activation, neuropathic changes, occult infection, toxic substances in the urine, and a primary defect in the glycosaminoglycan (GAG) layer of the bladder mucosa [1,3,4,5]. Prominent among the theories is that BPS/IC may be related to a primary defect of the GAG layer of the bladder urothelium [6]. The major classes of GAGs include hyaluronic acid, heparin sulfate, heparin, chondroitin 4-sulfate and chondroitin 6-sulfate, dermatan sulfate, and keratan sulfate.
Hyaluronic acid contributes an important proportion of the GAGs of the bladder surface and is considered a good candidate for GAG substitution in patients with BPS/IC. GAG substitution therapy with hyaluronic acid has been shown to benefit patients by relieving the distressing symptoms of pain, urinary frequency, urgency, nocturia, and hematuria [7]. For intravesical hyaluronic acid therapy, several uncontrolled studies using 40 mg dissolved in 40 mL of normal saline solution weekly for 4 to 6 weeks and then monthly thereafter have reported response rates varying from 71% to 30% [1,7,8] in BPS/IC as well as in other chronic inflammatory bladder diseases such as radiation and recurrent bacterial cystitis [9,10]. However, like the other treatment options for patients with BPS/IC, intravesical hyaluronic acid therapy has no long-term efficacy and its benefits on BPS/IC symptoms decrease in 24 weeks [7]. On the other hand, up to 70% of patients treated with intravesical hyaluronic acid have no response to this GAG substitution therapy [1,7,8].
Several studies have evaluated the bladder distension with electromotive drug administration (EMDA) in patients with BPS/IC, and the findings of these studies suggest that office distention with EMDA is a viable alternative for select IC patients [11,12,13,14]. Furthermore, in a study by Di Stasi et al. [15], results in patients with T1 bladder cancer improved in patients administered bacille Calmette-Guérin (BCG) and mitomycin via EMDA compared with the group administered BCG alone. Electromotive mitomycin increases tissue uptake compared with passive diffusion [16]. Given that instillation of hyaluronic acid via EMDA may increase tissue uptake and improve efficacy, we designed the present randomized prospective study to evaluate whether intravesical hyaluronic acid installation with EMDA would improve the response rate and long-term efficacy of the therapy.
MATERIALS AND METHODS
Within the time period of 2004-2005, 31 patients who had been diagnosed with BPS/IC were examined. Inclusion criteria were as follows: all patients fulfilled the National Institute of Diabetes and Digestive and Kidney Diseases criteria for BPS/IC, no other treatments were allowed during the study, and patients had been evaluated for urinary tract infection and it had been ruled out. Exclusion criteria were as follows: any other medication for IC during the study period, neurogenic bladder, history of pelvic surgery or trauma to the pelvic region, presence of active urinary tract infection, frequency of urination less than 8 times/d, presence of bladder or lower ureteral calculi, presence of benign or malignant bladder tumors, presence of active genital herpes infection, or presence of chemical or radiation cystitis. Patients were randomized to 1 of 2 treatment groups. In group A, patients were treated with 40-mg intravesical hyaluronic acid (Cythyal, BioScience GmbH, Pontiac, MI, USA) administered via a hydrophilic 12-Fr Foley catheter and were instructed to retain the installation volume for at least 60 minutes. In group B, 40-mg hyaluronic acid (Cythyal) was instilled in the bladder with EMDA. Patients were catheterized with a 16-Fr (CE-DAS ORUGENICS/Ag 9701, Mirandola, MO, Italy) catheter (special catheter for EMDA with a spiral silver electrode in the first part), and 40-mg hyaluronic acid in 40-mL saline was instilled into the bladder by gravity. Two patch electrodes were placed in the suprapubic region by using an ample amount of contact gel to avoid burns. The electrodes were taped tightly in place to achieve good contact and to prevent dispersion of current over the abdomen. EMDA was then performed by using the current generator with the following parameters: polarity positive, rise rate 30-60 mA/s, peak current 60 mA, pulsed output, and treatment time 25 minutes. In both groups, instillations were performed weekly in the first month and then monthly after 2 months. The study protocol was approved by the ethical committee of our hospital.
All patients gave written informed consent. Urine analysis was performed for all patients and active urinary tract infection was ruled out. All patients followed the same diet controls (no caffeine, alcohol, or beverages that might acidify the urine, such as cranberry juice or orange juice) during their treatment and follow-up. Potassium sensitivity test performed in all patients before treatment. Cystoscopy with hydrodistention was performed before treatment in all patients and the presence of Hunner ulcers was noted. Baseline voiding symptoms were recorded in 2-day voiding diaries, a visual analogue scale (VAS) was used to assess worst pain, and IC symptom and problem indexes were used to assess IC. At visits at 1, 6, 12, and 24 months, the patients returned 2-day voiding diaries, marked the VAS score, filled out the symptom and problem questionnaires, and gave the global response assessment (GRA). The GRA was defined as follows: 1, worse; 2, no change; 3, slightly better; 4, moderately better; 5, much better; and 6, completely cured. Participants who reported categories 4 to 6 were considered treatment responders. The parameters used for assessment of treatment response were based on the study by Propert et al. [17]. The physicians who assessed the treatment response were blinded to the patients' means of application of hyaluronic acid.
The primary end points of the study were VAS score, GRA, and micturition frequency in 24 hours. Secondary end points were mean voided volume, number of nocturia episodes, and IC symptom and problem scores.
Randomization was performed by the block randomization method and NCSS software (NCSS, Kaysville, UT, USA) was used. Sample size was estimated as 25 with a power of 0.80 and an effect size of 50% (≥3 points of decrease in VAS score). Baseline factors were compared with the t-test and differences between treatment outcomes were calculated with the Student t-test. The paired-samples t-test was used to compare treatment results of a single group in certain time intervals. Proportions of responders were calculated with the Fisher exact test with p<0.05 considered significant. Multivariable Cox proportional hazards regression was performed to determine any factor predictive of treatment response. Statistical analysis was performed with SPSS ver. 15 (SPSS Inc., Chicago, IL, USA).
RESULTS
Of the 31 patients enrolled, 25 patients were female and 6 patients were male. There were 15 (3 males and 12 females) patients in group A, and 16 (3 males and 13 females) patients in group B. There were no significant differences in baseline characteristics between the groups (Table 1). During cystoscopy, Hunner ulcers were observed in 3 and 4 patients in groups A and B, respectively. Oral pentosan polysulfate had been prescribed to 6 patients in group A and 9 patients in group B previously and those patients did not respond to pentosan polysulfate. Also, 8 patients in group A and 8 patients in group B had a history of intravesical heparin therapy. Hydrodistention had not been used in any patient.
TABLE 1.
Baseline characteristics of the two groups
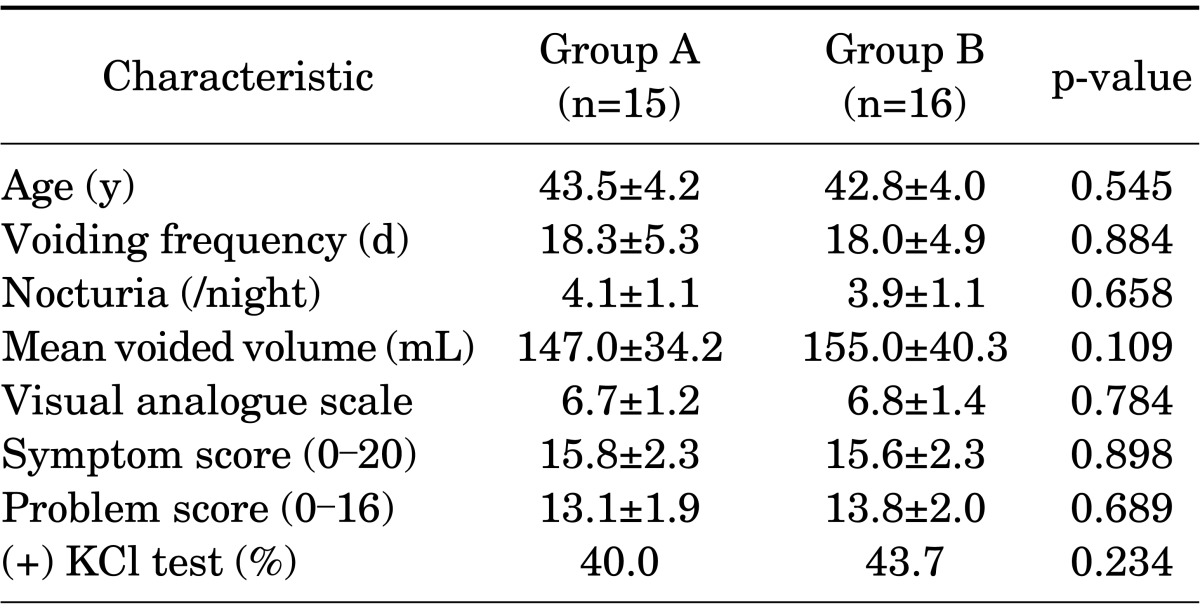
Values are presented as mean±standard deviation.
Group A, direct instillation; group B, instillation via electromotive drug administration.
All of the enrolled patients completed the study period. There were no withdrawals and no patients were lost to follow-up. None of the patients experienced any serious adverse events and no patients refused treatment related to side effects.
Treatment responses in both groups were compared at certain time intervals and are summarized in Table 2. Both treatment arms were successful at months 6 and 12 compared with baseline in all parameters, but group B was found to have statistically better results in those given time intervals. However, both groups were found to have no statistically significant improvement at month 1 compared with baseline. At month 24, voiding frequency and VAS were the only parameters that were significantly improved compared with baseline in both groups, and there were no statistically significant differences between the groups. The results of the multivariable Cox proportional hazards regression showed that treatment with EMDA, positive KCl test result, and voiding frequency >17 were associated with higher response rates. The other parameters involved in the multivariate analysis were not found to be associated with higher response rates. The results of the multivariate analysis are summarized in Table 3.
TABLE 2.
Treatment response in both groups at certain time intervals
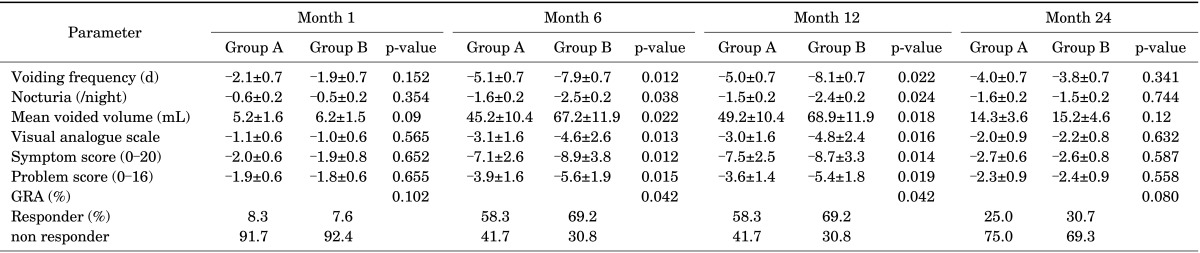
Values are presented as mean±standard deviation.
Group A, direct instillation; group B, instillation via electromotive drug administration; GRA, global response assessment.
TABLE 3.
Results of the multivariate analysis of treatment response
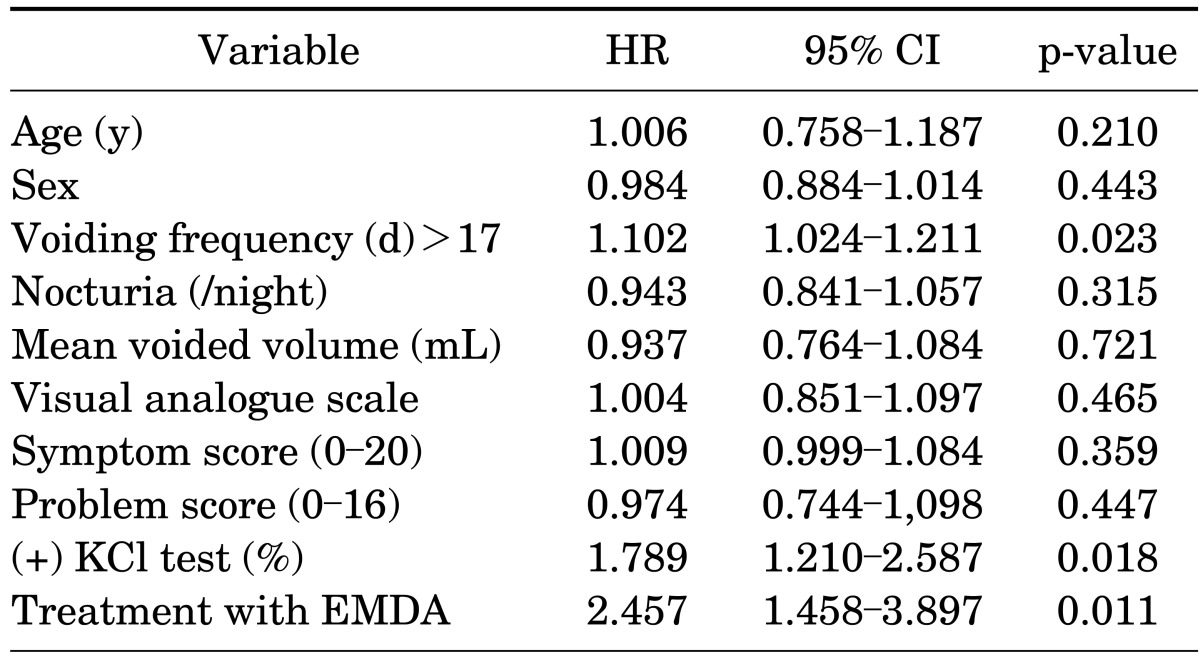
HR, hazard ratio; CI, confidence interval; EMDA, electromotive drug admin-istration.
DISCUSSION
Because of its uncertain cause and pathogenesis, there is currently no cure for BPS/IC and the goal of therapy is symptomatic relief. Improvement can be attained with several treatment options, including oral therapy (e.g., pentosan polysulfate sodium), intravesical therapy (e.g., GAG substitution therapy with specific agents), and even surgical interventions, although relapses are common. GAG substitution therapy with hyaluronic acid in patients with BPS/IC has been evaluated in previous studies. Explaining the mechanism of hyaluronic acid treatment in patients with BPS/IC as just the reconstitution of a morphologically defective GAG layer may not be exactly right. Hyaluronic acid has various biological activities including inhibiting the adherence of immune complexes to polymorphonuclear cells [18], marked inhibition of leukocyte migration and aggregation depending on viscosity [19], regulation of fibroblast and endothelial cell proliferation [20], enhancement of connective tissue healing [21], and creation of a barrier over membranes, thus regulating movement of solutes [22]. All of the biological activities listed above may contribute to the action of hyaluronic acid in interstitial cystitis.
Reports in the literature of treatment results with long-term follow-up differ. In a study by Morales et al. [7], the complete or partial response rate of intravesical hyaluronic acid treatment for up to 1 year was 71%; however, beyond week 24 there was a moderate decrease in the response rate of the medication. In a Danish open and uncontrolled pilot study [1], 20 patients with BPS/IC received weekly bladder instillations of hyaluronic acid for 1 month and monthly instillations for an additional 2 months. The patients were then offered further monthly instillations, and all patients were evaluated after 3 years. In contrast with the Morales et al. [7]'s study, the Danish study showed long-term efficacy of intravesical hyaluronic acid therapy. Three years after the initiation of treatment, 65% of patients (13 of the 20 patients) still responded to treatment in terms of symptom remission and pain score reduction. However, the treatment schedules of these two studies were not the same. The patients in the Danish study received a longer duration of intravesical hyaluronic acid therapy (monthly for 3 years vs. 1 year). We think that the difference in these long-term efficacy results may be due to the longer duration of intravesical hyaluronic acid in the Danish study. Treatment results with long-term follow-up were also recently reported by Engelhardt et al. [23]. In that study, 24 of the 48 patients were evaluated after 5 years, and 20 of these patients reported decreased pain.
Several studies have reported that installation of the agent into the bladder with EMDA improves the treatment efficacy in patients with bladder cancer and BPS/IC. A study by Di Stasi et al. [24] compared mitomycin delivery between passive diffusion and electromotive administration in high-risk patients with superficial bladder cancer. Peak plasma concentrations of mitomycin were 5.5 times higher in patients who underwent electromotive delivery than in those who underwent passive diffusion, which suggests that the cells of the bladder wall were exposed to incremental concentrations of this magnitude. The median time to first recurrence of a tumor was nearly doubled with electromotive delivery compared with passive diffusion and was similar to BCG alone-a third comparator group. In another study by Di Stasi et al. [15], the investigators randomly assigned 212 patients with pathological T1 bladder cancer to BCG alone or BCG and electromotive mitomycin. They concluded that the BCG and electromotive mitomycin group had significantly fewer progressions and deaths from bladder cancer.
Performing EMDA in patients with BPS/IC has been evaluated in several studies [11,12,13,14]. Lidocaine and dexamethasone installation with EMDA followed by cystodistention in 21 women showed a 25% success rate up to 6 months after instillation [14]. Using a similar technique, Riedl et al. [25] noted complete resolution of bladder symptoms in 61% of patients (8 of 13). In a study by Rose et al. [13], bladder distention with two different anesthetic strategies was evaluated: simple instillation of alkalized lidocaine and EMDA of lidocaine. Those authors concluded that lidocaine EMDA is superior to alkalized lidocaine in that it allows for a greater distention of the bladder for a longer period of time. In another study by the same group of authors, comparison between bladder distension with lidocaine EMDA and distension with general or spinal anesthesia was made [11]. Distension achieved with EMDA was similar to distension with general or spinal anesthesia.
In the present study, there was significant improvement in both treatment arms at months 6 and 12 compared with baseline in terms of micturition frequency, mean voided volume, number of nocturia episodes, IC symptom and problem scores, VAS score, and GRA. Our findings are compatible with the literature [1,7]. Furthermore, this improvement in group B (hyaluronic acid instilled via EMDA) was significantly higher than that in group A (hyaluronic acid instilled via hydrophilic 12-Fr Foley catheter) for all parameters listed. This may mean that EMDA improves the efficacy of hyaluronic acid in patients with BPS/IC as a result of increasing the tissue uptake of hyaluronic acid compared with that after installation with a hydrophilic catheter. Additionally, Morales et al. [7] stated that the effectiveness of hyaluronic acid therapy decreased after 24 weeks. However, in our study group at 1 year, there was still significant improvement in all parameters with lower-dose hyaluronic acid than in the Morales et al. [7]'s study. This may relate to different groups of patients with different BPS/IC pathogenesis. At month 24, the effectiveness of the therapy was decreased, and voiding frequency and VAS were the only parameters that were significantly improved compared with baseline in both groups. At this point, the installation with EMDA group showed superiority to group A. The findings of the present study may suggest that EMDA improves hyaluronic acid efficacy; however, it does not increase the duration of efficacy.
The number of patients in the study was low, and the findings should be confirmed in multicenter trails with a higher number of patients. Lack of a control group is a considerable limitation of the study; however, other studies of hyaluronic acid therapy have shown favorable findings in patients with BPS/IC [1,25]. On the other hand, we think that it is not ethical to treat this group of patients with placebo for a long time period. Plasma concentrations of hyaluronic acid could be determined in both groups; however, there were studies showing that EMDA increases the plasma concentration of the agent, so we did not perform this measurement.
CONCLUSIONS
Hyaluronic acid installation is an effective GAG substitution therapy in patients with BPS/IC. Instilling hyaluronic acid via EMDA can improve the efficacy of the treatment; however, lack of long-term efficacy is the major problem of this GAG substitution therapy.
Footnotes
The authors have nothing to disclose.
References
- 1.Kallestrup EB, Jorgensen SS, Nordling J, Hald T. Treatment of interstitial cystitis with Cystistat: a hyaluronic acid product. Scand J Urol Nephrol. 2005;39:143–147. doi: 10.1080/00365590410015876-1. [DOI] [PubMed] [Google Scholar]
- 2.Temml C, Wehrberger C, Riedl C, Ponholzer A, Marszalek M, Madersbacher S. Prevalence and correlates for interstitial cystitis symptoms in women participating in a health screening project. Eur Urol. 2007;51:803–808. doi: 10.1016/j.eururo.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 3.Gupta SK, Pidcock L, Parr NJ. The potassium sensitivity test: a predictor of treatment response in interstitial cystitis. BJU Int. 2005;96:1063–1066. doi: 10.1111/j.1464-410X.2005.05776.x. [DOI] [PubMed] [Google Scholar]
- 4.Parsons CL, Stein PC, Bidair M, Lebow D. Abnormal sensitivity to intravesical potassium in interstitial cystitis and radiation cystitis. Neurourol Urodyn. 1994;13:515–520. doi: 10.1002/nau.1930130503. [DOI] [PubMed] [Google Scholar]
- 5.Moldwin RM, Sant GR. Interstitial cystitis: a pathophysiology and treatment update. Clin Obstet Gynecol. 2002;45:259–272. doi: 10.1097/00003081-200203000-00027. [DOI] [PubMed] [Google Scholar]
- 6.Parsons CL. The therapeutic role of sulfated polysaccharides in the urinary bladder. Urol Clin North Am. 1994;21:93–100. [PubMed] [Google Scholar]
- 7.Morales A, Emerson L, Nickel JC, Lundie M. Intravesical hyaluronic acid in the treatment of refractory interstitial cystitis. J Urol. 1996;156:45–48. [PubMed] [Google Scholar]
- 8.Porru D, Campus G, Tudino D, Valdes E, Vespa A, Scarpa RM, et al. Results of treatment of refractory interstitial cystitis with intravesical hyaluronic acid. Urol Int. 1997;59:26–29. doi: 10.1159/000283012. [DOI] [PubMed] [Google Scholar]
- 9.Constantinides C, Manousakas T, Nikolopoulos P, Stanitsas A, Haritopoulos K, Giannopoulos A. Prevention of recurrent bacterial cystitis by intravesical administration of hyaluronic acid: a pilot study. BJU Int. 2004;93:1262–1266. doi: 10.1111/j.1464-410X.2004.04850.x. [DOI] [PubMed] [Google Scholar]
- 10.Manas A, Glaria L, Pena C, Sotoca A, Lanzos E, Fernandez C, et al. Prevention of urinary tract infections in palliative radiation for vertebral metastasis and spinal compression: a pilot study in 71 patients. Int J Radiat Oncol Biol Phys. 2006;64:935–940. doi: 10.1016/j.ijrobp.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 11.Rose AE, Azevedo KJ, Payne CK. Office bladder distention with electromotive drug administration (EMDA) is equivalent to distention under general anesthesia (GA) BMC Urol. 2005;5:14. doi: 10.1186/1471-2490-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Henry R, Patterson L, Avery N, Tanzola R, Tod D, Hunter D, et al. Absorption of alkalized intravesical lidocaine in normal and inflamed bladders: a simple method for improving bladder anesthesia. J Urol. 2001;165(6 Pt 1):1900–1903. doi: 10.1097/00005392-200106000-00014. [DOI] [PubMed] [Google Scholar]
- 13.Rose AE, Payne CK, Azevedo K. Pilot study of the feasibility of in-office bladder distention using electromotive drug adminstration (EMDA) Neurourol Urodyn. 2005;24:254–260. doi: 10.1002/nau.20106. [DOI] [PubMed] [Google Scholar]
- 14.Rosamilia A, Dwyer PL, Gibson J. Electromotive drug administration of lidocaine and dexamethasone followed by cystodistension in women with interstitial cystitis. Int Urogynecol J Pelvic Floor Dysfunct. 1997;8:142–145. doi: 10.1007/BF02764846. [DOI] [PubMed] [Google Scholar]
- 15.Di Stasi SM, Giannantoni A, Giurioli A, Valenti M, Zampa G, Storti L, et al. Sequential BCG and electromotive mitomycin versus BCG alone for high-risk superficial bladder cancer: a randomised controlled trial. Lancet Oncol. 2006;7:43–51. doi: 10.1016/S1470-2045(05)70472-1. [DOI] [PubMed] [Google Scholar]
- 16.Di Stasi SM, Vespasiani G, Giannantoni A, Massoud R, Dolci S, Micali F. Electromotive delivery of mitomycin C into human bladder wall. Cancer Res. 1997;57:875–880. [PubMed] [Google Scholar]
- 17.Propert KJ, Mayer RD, Wang Y, Sant GR, Hanno PM, Peters KM, et al. Responsiveness of symptom scales for interstitial cystitis. Urology. 2006;67:55–59. doi: 10.1016/j.urology.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 18.Sato H, Takahashi T, Ide H, Fukushima T, Tabata M, Sekine F, et al. Antioxidant activity of synovial fluid, hyaluronic acid, and two subcomponents of hyaluronic acid. Synovial fluid scavenging effect is enhanced in rheumatoid arthritis patients. Arthritis Rheum. 1988;31:63–71. doi: 10.1002/art.1780310110. [DOI] [PubMed] [Google Scholar]
- 19.Balasz EA, Denlinger JL. The role of hyaluronic acid in arthritis and its therapeutic use. In: Peyron JG, editor. Osteoarthritis: current clinical and fundamental problems. Paris: Geigy; 1984. pp. 165–174. [Google Scholar]
- 20.Goldberg RL, Toole BP. Hyaluronate inhibition of cell proliferation. Arthritis Rheum. 1987;30:769–778. doi: 10.1002/art.1780300707. [DOI] [PubMed] [Google Scholar]
- 21.Abatangelo G, Martelli M, Vecchia P. Healing of hyaluronic acid-enriched wounds: histological observations. J Surg Res. 1983;35:410–416. doi: 10.1016/0022-4804(83)90030-6. [DOI] [PubMed] [Google Scholar]
- 22.Hadler NM, Napier MA. Structure of hyaluronic acid in synovial fluid and its influence on the movement of solutes. Semin Arthritis Rheum. 1977;7:141–152. doi: 10.1016/0049-0172(77)90020-8. [DOI] [PubMed] [Google Scholar]
- 23.Engelhardt PF, Morakis N, Daha LK, Esterbauer B, Riedl CR. Long-term results of in-travesical hyaluronan therapy in bladder pain syndrome/interstitial cystitis. Int Urogynecol J. 2011;22:401–405. doi: 10.1007/s00192-010-1294-y. [DOI] [PubMed] [Google Scholar]
- 24.Di Stasi SM, Giannantoni A, Stephen RL, Capelli G, Navarra P, Massoud R, et al. Intravesical electromotive mitomycin C versus passive transport mitomycin C for high risk superficial bladder cancer: a prospective randomized study. J Urol. 2003;170:777–782. doi: 10.1097/01.ju.0000080568.91703.18. [DOI] [PubMed] [Google Scholar]
- 25.Riedl CR, Knoll M, Plas E, Pfluger H. Electromotive drug administration and hydrodis-tention for the treatment of interstitial cystitis. J Endourol. 1998;12:269–272. doi: 10.1089/end.1998.12.269. [DOI] [PubMed] [Google Scholar]


