Abstract
Neopterin is synthesized by macrophages upon stimulation with γ-interferon, and high neopterin production is associated with cellular immune activation and increased production of reactive oxygen species (oxidant stress), but the clinical utility of urine neopterin levels in patients with heart failure (HF) have not been explored. 53 ambulatory patients with chronic systolic HF (left ventricular [LV] ejection fraction ≤40%) underwent comprehensive echocardiographic evaluation and cardiopulmonary exercise testing. Urine neopterin levels were quantified by liquid chromatography with tandem mass spectrometric analyses and corrected to urine creatinine (Cr) levels. In our study cohort, median urine neopterin level was 60 [interquartile range 40–86] μmol/mol Cr. There were modest correlations between urine neopterin levels and abnormalities in cardiac structure and function by echocardiography: LV ejection fraction (r= −0.33, p=0.017), indexed LV end-diastolic volume (r=0.31, p=0.029), indexed LV end-systolic volume (r=0.32, p=0.024), and E/septal Ea (r=0.28, p=0.041). Although there was no significant correlation between urine neopterin and maximal oxygen uptake (peak VO2: r= −0.25, p=0.07), there was a modest correlation between urine neopterin and maximal ventilation/carbon dioxide production ratio (VE/VCO2 max: r=0.38, p=0.005). In conclusion, increase in urine neopterin levels track with disease severity in patients with chronic systolic heart failure.
Keywords: Neopterin, heart failure, echocardiography, cardiopulmonary exercise testing
INTRODUCTIION
Neopterin is a pteridine that is produced as a catabolic product of guanosine triphosphate metabolism1. It is synthesized and released by activated monocytes or macrophages upon stimulation with gamma-interferon. Increased neopterin levels indicate endogenous formation of gamma-interferon produced by activated T cells (Th1 or cytotoxic T cells) and suggest cell-mediated immune activation2. Neopterin is produced together with tetrahydrobiopterin, which is a cofactor for oxidative cleavage of ether lipids3 and formation of nitric oxide from arginine4, 5. High neopterin level is associated with increased production of reactive oxygen species6, suggesting oxidative stress elicited by the immune system. Previous studies have reported that serum neopterin levels may be elevated in patients with coronary and peripheral artery diseases7, 8, correlate with the extent of disease8 and complex (vulnerable) plaques9, and also predict major adverse cardiovascular events in patients with chronic coronary artery disease10, 11, acute coronary syndromes12, 13, or severe peripheral artery disease7, 14. In addition, it has been shown that patients with congestive heart failure (HF) had higher blood neopterin levels7, 15, which correlated with the severity of HF with advanced signs and symptoms7, 16, and independently predicted future cardiac events for chronic HF patients17, 18. For acute decompensated HF, higher serum neopterin levels predict increased risk of developing renal dysfunction19. All these studies utilized circulating neopterin, while neopterin has long been isolated in the urine20 and the value and reproducibility of urine neopterin level in HF patients has not been determined. Utilizing mass spectrometry techniques, this study is to explore the application of urine neopterin in chronic systolic HF patients and its relationship with severity of HF.
METHODS
Fifty-three consecutively consented ambulatory patients with stable systolic HF that were prospectively enrolled to participate in a blood draw, urine collection, echocardiography and cardiopulmonary exercise testing. To participate, patents had to have a clinical diagnosis of chronic (>6 months’ duration) HF with left ventricular (LV) ejection fraction ≤40% by echocardiography in euvolemic state at the time of enrollment. We excluded subjects with a major cardiovascular event (myocardial infarction, unstable angina, stroke, transient ischemic attack, pulmonary embolism) within 30 days of enrollment. Other exclusion criteria including a history of significant chronic obstructive pulmonary disease, major surgery, hospitalization or emergency room visits for HF exacerbation or use of inotropic agents within the month preceding enrollment. This study was approved by the Cleveland Clinic Institutional Review Board, and all subjects provided written informed consent.
A comprehensive two-dimensional echocardiography was performed with a commercially available system (Vingmed, System Seven, General Electric, Piscataway, NJ) by a single American Society of Echocardiography registered research sonographer. Images were acquired by a dedicated research sonographer in the left lateral decubitus position with a phased-array transducer in the standard parasternal and apical views. Standard two-dimensional and Doppler data, triggered to the QRS complex, were digitally stored in a cine-loop format and analyzed in a blinded fashion from assay results. Subjects then underwent a symptom-limited cardiopulmonary stress testing using the modified Naughton protocol, and results were recorded on a Vmax Encore Metabolic Cart (CareFusion, San Diego, California), and peak oxygen consumption, and ventilator efficiency (assessed by ratio of maximal ventilation/carbon dioxide production) were measured according to clinical standards21.
Urine samples were collected at spot collection at midstream and in morning fasting condition, and centrifuged at 1500g for 10 min at 4°C. Samples were then stored in aliquots at −80°C and thawed on ice before analy sis. Since neopterin is slightly sensitive to direct sun light irradiation, samples were protected from light during transport and storage. Urine aliquots (200μL) are mixed with 200ng/ml rhamnopterin (internal standard) and then centrifuged at 12,000 rpm for 15min at 4°C. The supernatant was collected and an aliquot is injected onto a high-performance liquid chromatography (HPLC) column and neopterin levels were quantified by mass spectrometry using Waters Quattro Ultima (Micromass) connected to a Waters Alliance 2690 HPLC system. Urine pterins were separated on a 2×150mm C18, ODS (2), 5μm, 150Å column (Phenomenex, Torrance, CA) and resolved using a discontinuous gradient with 0.2% formic acid (solvent A) and 0.2% formic acid in methanol (solvent B). The gradient used was as follows: The column was first equilibrated with 100% solvent A for 2 minutes after the injection; a linear gradient was then run to 50% solvent B over the next 6 minutes and held for 4 minutes. Then the solvent was changed to 100% solvent A in a linear fashion over 0.5 minutes, held at 100% solvent A for 7.5 minutes. Flow rate was kept at 200μL/min. Analyses were performed online using liquid chromatography tandem mass spectrometry in the positive ion mode with multiple reaction monitoring using unique parent→daughter ion transition (254→206) and retention time for neoperin. Cone potential and collision energy are optimized for neopterin and the standard curve was generated with rhamnopterin as internal standard. Intra-assay and inter-assay coefficients of variance were <10%. Urine creatinine (Cr, for correction of urine excretion) and B-type natriuretic peptide levels were measured by the Architect ci8200 platform (Abbott Laboratories, Abbott Park, IL).
Continuous variables were summarized as mean ± standard deviation if normally distributed, or as median [interquartile range] if non-normally distributed. Normality was assessed by the Shapiro-Wilk W test. Differences in normally distributed continuous variables across clinical categories were assessed using the Student’s t test, while differences in non-normally distributed variables were assessed using the Wilcoxon rank-sum test. Univariate Spearman’s correlation analysis was used to determine the correlation between urine neopterin levels and echocardiographic and cardiopulmonary testing indices. All p-values reported were from two-sided tests and p-value <0.05 was considered statistically significant. Statistical analyses were performed using JMP 9.0.0 (SAS Institute, Cary, NC).
RESULTS
Table 1 illustrates the baseline clinical characteristics of our study population. The mean and median urine neopterin levels were 65±36 μmol/mol Cr and 60 [interquartile range 40–86] μmol/mol Cr, respectively. Urine neopterin levels directly correlated with age (r= 0.31, p=0.024), but did not differ according to gender, ethnicity, ischemic etiology, New York Heart Association functional class, history of hypertension or diabetes mellitus, or any medication use (p>0.10 for all). Interestingly, the correlation between B-type natriuretic peptide and neopterin did not reach statistical significance (r=0.243, p=0.086).
Table 1.
Baseline characteristics of study population (n=53).
| Demographics | Value |
|---|---|
| Age (years) | 53 ± 13 |
| Male | 40 (75%) |
| African American | 9 (17%) |
|
| |
| Heart failure history | |
| NYHA class II or III | 45 (92%) |
| Ischemic etiology | 16 (33%) |
|
| |
| Medications | |
| Angiotensin converting enzyme inhibitors or angiotensin-receptor blockers | 45 (92%) |
| Beta-blockers | 48 (91%) |
| Spironolactone | 21 (43%) |
| Loop diuretics | 31 (63%) |
|
| |
| Echocardiographic indices | |
| Left ventricular ejection fraction (%) | 31 ± 8 |
| Left ventricular end-diastolic volume, indexed (mL/m2) | 101 ± 44 |
| Ratio of mitral inflow E wave to tissue Doppler septal Ea wave | 16 ± 10 |
|
| |
| Cardiopulmonary exercise testing | |
| Ventilatory equivalent ratio for oxygen and carbon dioxide | 34 ± 6 |
| Maximal oxygen uptake (mL/kg/min) | 19 ± 6 |
|
| |
| Laboratory data | |
| B-type natriuretic peptide (pg/mL) | 79 [23–227] |
| Urine neopterin (μmol/mol creatinine) | 60 [40–86] |
Table 2 and Figure 1 present the relationships between urine neopterin levels and echocardiographic indices of cardiac structure and performance. In our study cohort, higher urine neopterin levels directly correlated with greater indexed LV end-diastolic volume (r=0.31, p=0.029) and indexed LV end-systolic volume (r=0.32, p=0.024). Higher urine neopterin levels also were associated with worse LV systolic dysfunction (LV ejection fraction: r= −0.33, p=0.017; Figure 1), LV diastolic dysfunction (ratio of mitral inflow E wave to tissue Doppler septal Ea wave: r=0.28, p=0.041; Figure 1), and higher right ventricular systolic pressure (right ventricular systolic pressure: r=0.36, p=0.033; Figure 1).
Table 2.
Correlations between Urine Neopterin and Echocardiographic and Cardiopulmonary Exercise Testing Indices.
| Variable | Spearman’s r | p-value |
|---|---|---|
| Left ventricular ejection fraction (%) | −0.33 | 0.017 |
| Left ventricular end-diastolic dimension, indexed (mL/m2) | 0.31 | 0.029 |
| Left ventricular end-systolic dimension, indexed (mL/m2) | 0.32 | 0.024 |
| Ratio of mitral inflow E wave to tissue Doppler septal Ea wave | 0.28 | 0.041 |
| Right ventricular systolic pressure (mmHg) | 0.36 | 0.033 |
| Ventilatory equivalent ratio for oxygen and carbon dioxide | 0.38 | 0.005 |
| Maximal oxygen uptake (mL/kg/min) | −0.25 | 0.069 |
Figure 1.
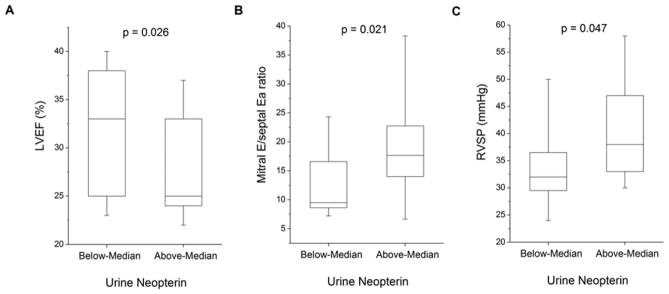
Comparison of echocardiographic indices across median urine neopterin level (60 μmol/mol Cr).
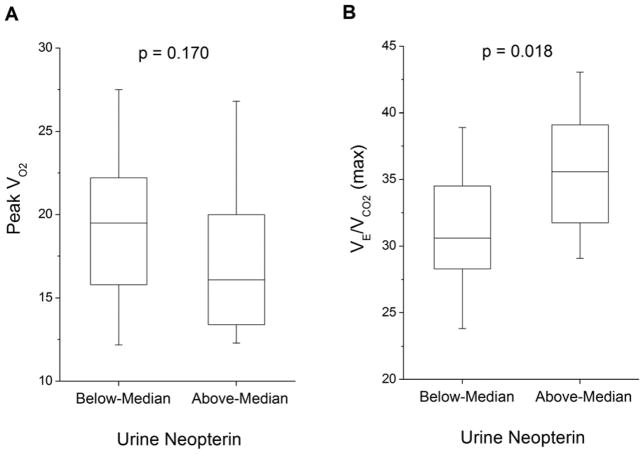
Table 2 and Figure 2 show the relationships between urine neopterin levels and cardiopulmonary testing indices. Urine neopterin levels directly correlated with maximal ventilation/carbon dioxide production ratio (r=0.38, p=0.005, Figure 2). While patients with urine neopterin levels above median showed greater degree of exercise ventilatory inefficiency compared to those below median neopterin levels, there was no significant difference in peak oxygen consumption (VO2) levels between above- and below-median neopterin groups (p=0.170; Figure 2).
Figure 2.
Comparison of Peak VO2 (Figure 2A) and VE/VCO2 (max) (Figure 2B) across median urine neopterin level (60 μmol/mol Cr).
DISCUSSION
The key finding of our study is the association between urine neopterin levels and cardiac structural and functional abnormalities in patients with chronic systolic HF with relatively preserved renal function. We observed that urine neopterin was positively correlated with left ventricular enlargement as well as left ventricular systolic and diastolic dysfunction and exercise ventilation inefficiency, yet had no significant relationship with B-type natriuretic peptide levels or peak oxygen consumption. These findings are consistent with previous reports with serum neopterin22. Novel findings from our objective cardiopulmonary exercise testing data further supported the notion that urine neopterin reflects clinically meaningful and distinct metabolic defect related to activated cellular immune responses in chronic systolic HF.
Neopterin, 2-amino-4-hydroxy-6-(D-erythro-1′, 2′, 3′-trihydroxypropyl)-pteridine, belongs to the class of pteridines, which biosynthetically derives from guanosine triphosphate especially in macrophages in response to gamma-interferon stimulation7. Therefore, determinations of neopterin levels reflect the stage of activation of the cellular immune system and may provide insights in the pathogenesis and progression of various inflammatory and immune-mediated diseases7. Neopterin is biochemically inert and chemically stable in body fluids. It is consistently eliminated by the kidney as its primary excretion route, therefore changes of neopterin concentrations in serum are reflected by urine levels. Urinary test has much less protein interference than blood test. Urine sample preparation doesn’t need protein precipitation and supernatant drying so is much easier and faster. Also, urine samples can be collected non-invasively with much higher volume than blood samples. Previous studies have demonstrated that high neopterin levels in patients with HF were significantly correlated with gamma-interferon and soluble CD8 (predominantly expressed on the surface of cytotoxic T cells) levels7, as well as tumor necrosis factor-α in advanced chronic HF patients23. All of these supported the involvement of stimulated cellular immunity and inflammation in the development and progression of HF. The positive albeit modest correlation between neopterin levels and degree of systolic and diastolic dysfunction suggested over-activated cellular immunity and inflammation might also contribute to the disease progression of HF24. Although immune-modulators have been largely unsuccessful in improving cardiovascular outcomes of the broad HF patient population, it is conceivable that subsets of patients with detectable activated cellular immunity may still warrant reconsideration of an immune modulatory therapeutic approach.
Maximal ventilation/carbon dioxide production ratio slope has been shown to have important prognostic value for chronic HF patients21, and is a well-established criteria for consideration of advanced therapies. There are several known determinants of this measure, including increase in physiological dead space caused by alveolar hypoperfusion due to impaired endothelial vasodilatory capacity or neuroendocrine activation, as well as heightened ventilation during exercise associated with reduced cardiac output and thus to poor pulmonary perfusion in HF25. Another potential contributor is the abnormal chemoreflex and/or metaboreflex activity in HF25. Our novel findings regarding the direct correlation of urine neopterin and the slope of the ventilation to carbon dioxide production ratio is therefore quite interesting, as such underlying physiologic defects may be cause or consequence of activated cellular immune responses as reflected by elevated neopterin levels. Such exercise ventilation inefficiency has been associated with pulmonary hypertension and right heart failure26, also noted in the modest correlation between urine neopterin levels and estimated right ventricular systolic pressure by echocardiography in our study.
The relatively weak relations between neopterin and standard prognostic markers in HF such as B-type natriuretic peptide and peak oxygen consumption may deserve some discussion. Although in general natriuretic peptides and neopterin track with changes in myocardial function22, 27, dissociation in serum neopterin or inflammatory marker levels and B-type natriuretic peptide has been previously observed following improvements in hemodynamics following pacing therapy28. Our relatively stable ambulatory patient population with a relatively low range of natriuretic peptide levels may also dampen such association. In contrast, peak oxygen consumption may also have more variability due to the dependency of adequate exertional effort.
There are several important study limitations that are worth noting. This is a relatively stable group of ambulatory patients with chronic systolic HF, therefore no long-term outcomes were available. Notably, the overall urine neopterin levels were relatively low, which implied that activated cellular immunity are not as robust, despite the fact that their cardiopulmonary exercise test results indicate that they have mild to moderate impairment. While attempt is made to conduct all the urine collection in early morning, there are slight variations in the timing of spot urine collection which may affect the quantification of urine neopterin. We also do not have direct quantification of degree of activated cellular immunity. Nevertheless, further studies are warranted to determine the clinical significance of urine neopterin measurements, particularly in providing distinct clinical insights and/or incremental prognostic value in the setting of HF.
Acknowledgments
Funding Support: This research was supported by National Institutes of Health grants RO1 HL103931, P01HL076491, P01HL098055, R01HL103931, and the Cleveland Clinic Clinical Research Unit of the Cleveland Clinic/Case Western Reserve University CTSA UL1TR 000439.
Footnotes
Conflict of Interest
All other authors have no relationships to disclose.
Disclosure: Dr. Hazen reports being listed as co-inventor on pending and issued patents held by the Cleveland Clinic relating to cardiovascular diagnostics and therapeutics. Dr. Hazen reports having been paid as a consultant or speaker for the following companies: Abbott Diagnostics, Cleveland Heart Lab, Esperion, Lilly, Liposcience Inc., Merck & Co., Inc., and Pfizer Inc. Dr. Hazen reports receiving research funds from Abbott, Cleveland Heart Lab, Liposcience Inc., and Pfizer Inc. Dr. Hazen reports having the right to receive royalty payments for inventions or discoveries related to cardiovascular diagnostics or therapeutics from the companies shown below: Abbott Laboratories, Inc., Cleveland Heart Lab., Esperion, Frantz Biomarkers, LLC, Liposcience Inc., and Siemens.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brown GM. The biosynthesis of pteridines. Adv Enzymol Relat Areas Mol Biol. 1971;35:35–77. doi: 10.1002/9780470122808.ch2. [DOI] [PubMed] [Google Scholar]
- 2.Fuchs D, Weiss G, Reibnegger G, Wachter H. The role of neopterin as a monitor of cellular immune activation in transplantation, inflammatory, infectious, and malignant diseases. Crit Rev Clin Lab Sci. 1992;29:307–341. doi: 10.3109/10408369209114604. [DOI] [PubMed] [Google Scholar]
- 3.Tietz A, Lindberg M, Kennedy EP. A new pteridine-requiring enzyme system for the oxidation of glyceryl ethers. J Biol Chem. 1964;239:4081–4090. [PubMed] [Google Scholar]
- 4.Kwon NS, Nathan CF, Stuehr DJ. Reduced biopterin as a cofactor in the generation of nitrogen oxides by murine macrophages. J Biol Chem. 1989;264:20496–20501. [PubMed] [Google Scholar]
- 5.Tayeh MA, Marletta MA. Macrophage oxidation of l-arginine to nitric oxide, nitrite, and nitrate. Tetrahydrobiopterin is required as a cofactor. J Biol Chem. 1989;264:19654–19658. [PubMed] [Google Scholar]
- 6.Fuchs D, Avanzas P, Arroyo-Espliguero R, Jenny M, Consuegra-Sanchez L, Kaski JC. The role of neopterin in atherogenesis and cardiovascular risk assessment. Curr Med Chem. 2009;16:4644–4653. doi: 10.2174/092986709789878247. [DOI] [PubMed] [Google Scholar]
- 7.De Rosa S, Cirillo P, Pacileo M, Petrillo G, D’Ascoli GL, Maresca F, Ziviello F, Chiariello M. Neopterin: From forgotten biomarker to leading actor in cardiovascular pathophysiology. Curr Vasc Pharmacol. 2011;9:188–199. doi: 10.2174/157016111794519372. [DOI] [PubMed] [Google Scholar]
- 8.Erren M, Reinecke H, Junker R, Fobker M, Schulte H, Schurek JO, Kropf J, Kerber S, Breithardt G, Assmann G, Cullen P. Systemic inflammatory parameters in patients with atherosclerosis of the coronary and peripheral arteries. Arterioscler Thromb Vasc Biol. 1999;19:2355–2363. doi: 10.1161/01.atv.19.10.2355. [DOI] [PubMed] [Google Scholar]
- 9.Avanzas P, Arroyo-Espliguero R, Cosin-Sales J, Aldama G, Pizzi C, Quiles J, Kaski JC. Markers of inflammation and multiple complex stenoses (pancoronary plaque vulnerability) in patients with non-st segment elevation acute coronary syndromes. Heart. 2004;90:847–852. doi: 10.1136/hrt.2003.015826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Avanzas P, Arroyo-Espliguero R, Quiles J, Roy D, Kaski JC. Elevated serum neopterin predicts future adverse cardiac events in patients with chronic stable angina pectoris. Eur Heart J. 2005;26:457–463. doi: 10.1093/eurheartj/ehi111. [DOI] [PubMed] [Google Scholar]
- 11.Zouridakis E, Avanzas P, Arroyo-Espliguero R, Fredericks S, Kaski JC. Markers of inflammation and rapid coronary artery disease progression in patients with stable angina pectoris. Circulation. 2004;110:1747–1753. doi: 10.1161/01.CIR.0000142664.18739.92. [DOI] [PubMed] [Google Scholar]
- 12.Dominguez-Rodriguez A, Abreu-Gonzalez P, Garcia-Gonzalez M. Usefulness of neopterin levels and left ventricular function for risk assessment in survivors of acute myocardial infarction. Int J Cardiol. 2006;111:318–320. doi: 10.1016/j.ijcard.2005.11.024. [DOI] [PubMed] [Google Scholar]
- 13.Nazer B, Ray KK, Sloan S, Scirica B, Morrow DA, Cannon CP, Braunwald E. Prognostic utility of neopterin and risk of heart failure hospitalization after an acute coronary syndrome. Eur Heart J. 2011;32:1390–1397. doi: 10.1093/eurheartj/ehr032. [DOI] [PubMed] [Google Scholar]
- 14.Barani J, Mattiasson I, Lindblad B, Gottsater A. Cardiac function, inflammatory mediators and mortality in critical limb ischemia. Angiology. 2006;57:437–444. doi: 10.1177/0003319706290743. [DOI] [PubMed] [Google Scholar]
- 15.Fuchs D, Samsonov M, Tilz GP, Reibnegger G, Belenkov JN, Nassonov EL, Wachter H. Stimulated cellular immune system in patients with congestive heart failure. Eur J Clin Chem Clin Biochem. 1993;31:111–114. doi: 10.1515/cclm.1993.31.3.111. [DOI] [PubMed] [Google Scholar]
- 16.Aukrust P, Ueland T, Muller F, Andreassen AK, Nordoy I, Aas H, Kjekshus J, Simonsen S, Froland SS, Gullestad L. Elevated circulating levels of c-c chemokines in patients with congestive heart failure. Circulation. 1998;97:1136–1143. doi: 10.1161/01.cir.97.12.1136. [DOI] [PubMed] [Google Scholar]
- 17.Sasaki T, Takeishi Y, Suzuki S, Niizeki T, Kitahara T, Katoh S, Ishino M, Shishido T, Watanabe T, Kubota I. High serum level of neopterin is a risk factor of patients with heart failure. Int J Cardiol. 2010;145:318. doi: 10.1016/j.ijcard.2009.11.042. [DOI] [PubMed] [Google Scholar]
- 18.Wietlicka-Kokoszanek I, Jablecka A, Smolarek I, Bogdanski P, Chmara E, Korzeniowska K, Kazmierczak M, Kazmierczak E, Musialik K. Neopterin as a prognostic marker in patients with chronic heart failure. Med Sci Monit. 2010;16:CR232–237. [PubMed] [Google Scholar]
- 19.Dominguez-Rodriguez A, Abreu-Gonzalez P, Juarez-Prera RA, Arroyo-Ucar E, Hernandez-Garcia C, Tome MC, Blanco-Palacios GE, Kaski JC. Usefulness of serum neopterin levels in acute decompensated heart failure to predict renal dysfunction. Biomarkers. 2012;17:134–139. doi: 10.3109/1354750X.2011.643486. [DOI] [PubMed] [Google Scholar]
- 20.Sakurai A, Goto M. Neopterin: Isolation from human urine. J Biochem. 1967;61:142–145. doi: 10.1093/oxfordjournals.jbchem.a128513. [DOI] [PubMed] [Google Scholar]
- 21.Balady GJ, Arena R, Sietsema K, Myers J, Coke L, Fletcher GF, Forman D, Franklin B, Guazzi M, Gulati M, Keteyian SJ, Lavie CJ, Macko R, Mancini D, Milani RV. Clinician’s guide to cardiopulmonary exercise testing in adults: A scientific statement from the american heart association. Circulation. 2010;122:191–225. doi: 10.1161/CIR.0b013e3181e52e69. [DOI] [PubMed] [Google Scholar]
- 22.Caruso R, De Chiara B, Campolo J, Verde A, Musca F, Belli O, Parolini M, Cozzi L, Moreo A, Frigerio M, Parodi O. Neopterin levels are independently associated with cardiac remodeling in patients with chronic heart failure. Clin Biochem. 2013;46:94–98. doi: 10.1016/j.clinbiochem.2012.10.022. [DOI] [PubMed] [Google Scholar]
- 23.Wiedermann CJ, Beimpold H, Herold M, Knapp E, Braunsteiner H. Increased levels of serum neopterin and decreased production of neutrophil superoxide anions in chronic heart failure with elevated levels of tumor necrosis factor-alpha. J Am Coll Cardiol. 1993;22:1897–1901. doi: 10.1016/0735-1097(93)90776-w. [DOI] [PubMed] [Google Scholar]
- 24.Vaduganathan M, Greene SJ, Butler J, Sabbah HN, Shantsila E, Lip GY, Gheorghiade M. The immunological axis in heart failure: Importance of the leukocyte differential. Heart Fail Rev. 2013;18:835–845. doi: 10.1007/s10741-012-9352-9. [DOI] [PubMed] [Google Scholar]
- 25.Tumminello G, Guazzi M, Lancellotti P, Pierard LA. Exercise ventilation inefficiency in heart failure: Pathophysiological and clinical significance. Eur Heart J. 2007;28:673–678. doi: 10.1093/eurheartj/ehl404. [DOI] [PubMed] [Google Scholar]
- 26.Clark AL, Swan JW, Laney R, Connelly M, Somerville J, Coats AJ. The role of right and left ventricular function in the ventilatory response to exercise in chronic heart failure. Circulation. 1994;89:2062–2069. doi: 10.1161/01.cir.89.5.2062. [DOI] [PubMed] [Google Scholar]
- 27.Rubaj A, Rucinski P, Oleszczak K, Trojnar MK, Wojcik M, Wysokinski A, Kutarski A. Inflammatory activation following interruption of long-term cardiac resynchronization therapy. Heart Vessels. 2013;28:583–588. doi: 10.1007/s00380-012-0285-y. [DOI] [PubMed] [Google Scholar]
- 28.Rubaj A, Rucinski P, Kutarski A, Dabrowska-Kugacka A, Oleszczak K, Zimon B, Trojnar M, Zapolski T, Drozd J, Tarkowski A, Wysokinski A. Cardiac hemodynamics and proinflammatory cytokines during biatrial and right atrial appendage pacing in patients with interatrial block. J Interv Card Electrophysiol. 2013;37:147–154. doi: 10.1007/s10840-013-9792-8. [DOI] [PMC free article] [PubMed] [Google Scholar]