Abstract
Purpose
To assess the safety and efficacy of combining oncolytic adenovirus-mediated cytotoxic gene therapy (OAMCGT) with intensity modulated radiation therapy (IMRT) in intermediate-risk prostate cancer.
Methods and Materials
Forty-four men with intermediate-risk prostate cancer were randomly assigned to receive either OAMCGT plus IMRT (arm 1; n=21) or IMRT only (arm 2; n=23). The primary phase 2 endpoint was acute (≤90 days’) toxicity. Secondary endpoints included quality of life (QOL), prostate biopsy (12-core) positivity at 2 years, freedom from biochemical/clinical failure (FFF), freedom from metastases, and survival.
Results
Men in arm 1 exhibited a greater incidence of low-grade influenza-like symptoms, transaminitis, neutropenia, and thrombocytopenia than men in arm 2. There were no significant differences in gastrointestinal or genitourinary events or QOL between the two arms. Two-year prostate biopsies were obtained from 37 men (84%). Thirty-three percent of men in arm 1 were biopsy-positive versus 58% in arm 2, representing a 42% relative reduction in biopsy positivity in the investigational arm (P=.13). There was a 60% relative reduction in biopsy positivity in the investigational arm in men with <50% positive biopsy cores at baseline (P=.07). To date, 1 patient in each arm exhibited biochemical failure (arm 1, 4.8%; arm 2, 4.3%). No patient developed hormone-refractory or metastatic disease, and none has died from prostate cancer.
Conclusions
Combining OAMCGT with IMRT does not exacerbate the most common side effects of prostate radiation therapy and suggests a clinically meaningful reduction in positive biopsy results at 2 years in men with intermediate-risk prostate cancer.
Introduction
Prostate cancer is the leading cancer diagnosis in men in the United States. As most new cases are diagnosed when the disease is localized, external beam radiation therapy is an accepted treatment option. Prior to the advent of conformal techniques, a dose of ~70 Gy was considered standard. It is clear that this radiation dose is suboptimal for ≥50% of men who develop biochemical progression within 10 years (1), an event that often triggers the implementation of salvage therapies and puts these men at risk for clinical progression. Several studies have evaluated the merit of escalating the radiation dose as a means to improve outcome (2–6). Results from prospective randomized trials demonstrate that a modest 10% to 15% increase in the radiation dose improves biochemical disease-free survival in some men, and possibly all, prognostic risk groups. Despite these higher radiation doses, ≥25% of men still develop disease progression within 10 years. The fact that roughly the same proportion of men have residual cancer in their prostate 2 years after treatment suggests that many of these failures occur within the radiation field, and radiation doses >80 Gy are necessary to sterilize the prostate (7). Should the radiation dose be escalated further and risk the development of increased complications, or should we explore biological strategies to improve the effectiveness of radiation therapy without increasing the physical dose?
We have developed a multimodal, biological approach to improve the effectiveness of radiation therapy (8, 9). Our therapeutic platform uses an oncolytic adenovirus to deliver a pair of cytotoxic genes to the tumor. The oncolytic adenovirus itself generates an antitumor effect by replicating in and destroying cancer cells (oncolytic viral therapy). The therapeutic effect of the adenovirus is enhanced by invoking two cytotoxic gene systems (cytosine deaminase [CD]/5-fluorocytosine [5-FC] and herpes simplex virus thymidine kinase [HSV-1 TK]/valganciclovir [vGCV]) which render malignant cells sensitive to specific pharmacological agents (cytotoxic gene therapy) and sensitize them to ionizing radiation (radiosensitization). We have examined the toxicity of this multimodal approach in 4 phase 1 trials of prostate cancer (10–15). These studies demonstrated that the approach is safe and suggestive signs of efficacy emerged. Based on encouraging phase 1 results, we opened prospective, randomized, phase 2/3 trials in newly diagnosed cases of intermediate-risk prostate cancer. We report here the phase 2 results, focusing on toxicity, quality of life (QOL), and prostate biopsy findings at 2 years.
Methods and Materials
Study design
The study was designed as a seamless, adaptive, multisite, prospective, randomized, controlled phase 2/3 trial (16). The phase 2/3 study was designed to enroll 280 patients (140 in each arm) and have 3 interim investigations with a power of 80%. The first interim investigation would occur when the first 21 patients in arm 1 were randomized for toxicity assessment (phase 2 component). At that point, an interim safety analysis would be conducted with review by the Data and Safety Monitoring Board (DSMB). If there were no safety concerns, the trial could continue into phase 2, which was granted. Patients were randomly assigned to undergo gene therapy plus intensity modulated radiation therapy (IMRT; arm 1) or IMRT alone (arm 2). Patients were divided into 3 groups based on their Gleason score, prostate-specific antigen (PSA) level, and percent positive biopsy cores at baseline (Table 1). The primary phase 2 end point was acute (≤90 days’) toxicity. Secondary end points included QOL, prostate biopsy (12-core) positivity at 2 years, freedom from biochemical/clinical failure (FFF) using the nadir plus 2 ng/mL definition (17), freedom from metastases, and survival. The trial was conducted in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1975, as revised in 2000, approved by the institutional review boards (IRB), and monitored by an independent DSMB.
Table 1.
Patient baseline characteristics
| Characteristic | Arm 1 (n=21) | Arm 2 (n=23) | P value |
|---|---|---|---|
| Mean (range) age (years) | 68.0 (55–78) | 65.2 (51–79) | .24 |
| No. of patients (%) | |||
| White | 15 (71%) | 16 (70%) | .53 |
| African-American | 5 (24%) | 7 (30%) | |
| Other | 1 (5%) | 0 (0%) | |
| Clinical stage | |||
| T1 | 19 (90%) | 17 (74%) | .16 |
| T2 | 2 (10%) | 6 (26%) | |
| Gleason score | |||
| 6 | 3 (14%) | 4 (17%) | .78 |
| 7 | 18 (86%) | 19 (83%) | |
| Median (range) | 5.6 (1.5–13.0) | 5.6 (0.7–17.1) | .92 |
| PSA (ng/mL) | |||
| Stratification group* | |||
| 1 | 1 (5%) | 1 (4%) | > .99 |
| 2 | 15 (71%) | 17 (74%) | |
| 3 | 5 (24%) | 5 (22%) | |
| % of positive biopsy cores | |||
| <50% | 15 (71%) | 17 (74%) | .85 |
| ≥50% | 6 (29%) | 6 (26%) | |
| EPIC | |||
| Urinary incontinence | 90.9 | 96.4 | .11 |
| Urinary irritation/obstruction | 88.0 | 90.5 | .59 |
| Bowel | 91.4 | 95.5 | .12 |
| Sexual | 48.4 | 63.6 | .06 |
| Hormonal | 90.9 | 91.4 | .67 |
| Overall satisfaction | 81.6 | 73.8 | .36 |
| EQ-5D | |||
| Mobility | 1.2 | 1.1 | .19 |
| Self care | 1.0 | 1.0 | > .99 |
| Usual activities | 1.1 | 1.1 | .94 |
| Pain and discomfort | 1.5 | 1.4 | .87 |
| Anxiety and Depression | 1.2 | 1.2 | .68 |
| Overall Index | 0.9 | 0.9 | .72 |
| Overall health state | 82.5 | 78.7 | .71 |
| Median (range) PSA follow-up (years) | 4.1 (2.2–5.3) | 3.9 (1.2–5.4) | .55 |
Abbreviations: EPIC = Expanded Prostate Cancer Index Composite; EQ-5D = quality of life health status instrument; PSA = prostate-specific antigen.
Group definitions: group 1 = Gleason score of 5/6, PSA <10 ng/mL, and ≥50% positive biopsy cores; group 2 = Gleason score of 5/6 and PSA 10–20 ng/mL or Gleason score of 7 and PSA 0–20 ng/mL, and < 50% positive biopsy cores; group 3 = Gleason score of 5/6 and PSA 10–20 ng/mL or Gleason score of 7 and PSA 0–20 ng/mL, and ≥50% positive biopsy cores.
Eligibility criteria
Eligible patients had newly diagnosed, clinically localized, intermediate-risk prostate cancer, defined as clinical stage T1/T2 and Gleason score of 7 or PSA concentration of 10 to 20 ng/mL. Shortly after the trial opened, the eligibility criteria were expanded to include men with Gleason 5/6 and PSA of <10 ng/mL and ≥50% positive biopsy cores because these patients tend to respond biochemically like intermediate-risk patients (18). Patients were required to have biopsy core-proven adenocarcinoma of the prostate within 180 days of registration, a Karnofsky performance status ≥70, creatinine clearance ≥50 mL/min/m2, platelet count ≥100,000/μL, absolute neutrophil count ≥1000/μL, hemoglobin level ≥10.0 g/dL, bilirubin level ≤1.5 mg/dL, and SGOT/AST and SGPT/ALT ≤2.5 times the upper limit of normal. Patients with the following characteristics were excluded from the study: prostate volume >120 cc; positive lymph nodes or evidence of metastatic disease; previous invasive malignancy, except for nonmelanoma skin cancer within the previous 5 years; prognosis for survival <5 years; previous treatment for prostate cancer, including radiation therapy, prostatectomy, cryosurgery, bilateral orchiectomy, androgen suppression therapy, or chemotherapy; unstable angina that required hospitalization or transmural myocardial infarction within the previous 6 months; acute infection that required specific therapy within the previous 72 hours; chronic obstructive pulmonary disease or other respiratory illness that required hospitalization within the previous 3 months; history of inflammatory bowel disease; positive serological test for human immunodeficiency virus infection or hepatitis; liver disease, including hepatitis; immunosuppressive therapy, including systemic corticosteroids; impaired immunity or susceptibility to viral infections; known allergy to any product used in the protocol; serious medical or psychiatric illness or concomitant medication that might interfere with the patient’s ability to tolerate or complete the trial.
Treatment
Informed consent was obtained from all patients before study-specific procedures were initiated. Men in arm 1 received a single intraprostatic injection of 1 × 1012 viral particles (vp) of the Ad5-yCD/mutTKSR39rep-ADP adenovirus (13) on day 1. The injection was performed on an outpatient basis as described previously (10–15). The total injection volume was 3.0 mL. The adenovirus was distributed over the entire prostate; however, the dose distribution was skewed to sextants with cancer based on the baseline biopsy report (see Supplementary material Table S1). Two days later, men in arm 1 were given 5-FC (150 mg/kg/day, 4 times per day; Ancobon) and vGCV (1800 mg/kg/day, twice per day; Valcyte) orally for 2 weeks (week days only). Patient compliance was monitored by the study nurse. All patients received 40 × 2-Gy fractions (80 Gy) of IMRT. For arm 1, IMRT commenced with administration of 5-FC plus vGCV. Androgen suppression therapy (AST) was not allowed until biochemical recurrence was documented.
Assessments
Toxicity assessments were performed once a week during the 8-week treatment course. Toxicities were graded using the National Cancer Institute’s Common Terminology Criteria for Adverse Events version 3. Evaluations included complete blood counts, blood chemistries, and physician assessment. Any observations regarding radiation reactions were documented and graded by the treating physician. Gastrointestinal (GI) and genitourinary (GU) events were graded using Radiation Therapy Oncology Group (RTOG) acute morbidity scoring criteria. Patients were evaluated at scheduled follow-up visits at 1, 3, 6, 9, 12, 18, and 24 months and annually thereafter. Evaluations were identical to those during the 8-week treatment course with the addition of serum PSA measurement, digital rectal examination at 6, 12, 18, and 24 months, 12-core prostate biopsy results with pathology assessment at 24 months, and QOL assessments at 6, 12, 24, and 36 months. Results of biopsy samples obtained at 2 years were scored using a 2-tier system, positive or negative for adenocarcinoma, by two GU pathologists blinded to the study treatment. Both of the pathologists were required to agree on the diagnosis or the specimens would be reviewed by a third pathologist. Radiation treatment effects were documented but not used in the assessment of cancer.
Patient-reported outcomes were measured using 2 validated instruments. QOL was measured using the comprehensive Expanded Prostate Cancer Index Composite (EPIC) instrument (19, 20). Patient values/preferences were measured using the EuroQol EQ-5D instrument (21). Compliance with completing QOL forms was excellent (baseline, 100%/100%; 6 months, 100%/91%; 12 months, 100%/91%; 24 months, 90%/95%; 36 months, and 93%/88% for arms 1 and 2, respectively).
Statistical methods
Two-sample t-tests for continuous variables and χ2/Fishers exact tests for categorical variables were used to test for arm differences among baseline characteristics and adverse events occurring through day 90. Intent-to-treat design was applied in testing for the effects of the investigational therapy on 2-year prostate biopsy result outcomes. Generalized estimating equation methods were used to test for arm differences over time after normalizing individual patient’s data to their baseline EPIC and EQ-5D QOL scores. All P values are 2-sided.
Results
Patients and treatment
From January 2008 through July 2010, 44 patients were enrolled at 2 institutions. Patients were randomly assigned to arm 1 (gene therapy plus IMRT) or arm 2 (IMRT alone). Patient baseline characteristics of the 2 arms were well balanced (Table 1). Median follow-up of surviving patients’ PSA results from randomization was 4.0 years.
All men in arm 1 received the prescribed dose of adenovirus and 14 (67%) received the full prescribed dose of prodrug. Per protocol, 5-FC and vGCV were reduced to 50% to 75% of the prescribed dose in 6 patients owing to transient thrombocytopenia (n=2), neutropenia (n=2), and nausea/vomiting (n=2). One patient received a partial dose of prodrug owing to patient noncompliance. All 44 patients received the prescribed dose of IMRT.
Toxicity
Grade 3 or higher treatment-related and unexpected adverse events (at ≤90 days) are reported in Table 2. Adverse events deemed treatment-related were based on the findings of 4 previous phase 1 trials (10–15). Men in arm 1 exhibited a greater incidence of influenza-like symptoms, trans-aminitis, neutropenia, and thrombocytopenia than men in arm 2. These events were expected and attributable to the oncolytic adenovirus (influenza-like symptoms, trans-aminitis) and prodrug (neutropenia, thrombocytopenia). The majority of adverse events (98%) were grades 1 and 2 and transient. One patient in arm 1 developed deep vein thrombosis and pulmonary embolism 12 weeks after the adenovirus injection. While this patient was in the hospital, it was discovered that he also had community-acquired pneumonia and a right bundle block. These events were reviewed by the DSMB and judged to be unrelated to the investigational therapy. Each of the unexpected grade 3 events of hyperglycemia, hypermagnesemia, and hypophophatemia in arm 1 occurred in an isolated patient and were transient.
Table 2.
Treatment-related and unexpected grade ≥3 adverse events through day 90
| Event | Arm 1 (n=21)
|
Arm 2 (n=23)
|
P value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Grade
|
No. of events (%)
|
Grade
|
No. of events (%)
|
||||||||
| 1 | 2 | 3 | 4 | Total | 1 | 2 | 3 | 4 | Total | ||
| Blood/bone marrow | |||||||||||
| Anemia | 4 (19%) | 4 (19%) | 9 (39%) | 9 (39%) | .19 | ||||||
| Leukopenia | 6 (29%) | 4 (19%) | 10 (48%) | 5 (22%) | 1 (4%) | 6 (26%) | .19 | ||||
| Lymphopenia | 4 (19%) | 11 (52%) | 5 (24%) | 20 (95%) | 5 (22%) | 10 (43%) | 2 (9%) | 17 (74%) | .20 | ||
| Neutropenia | 4 (19%) | 2 (10%) | 1 (5%) | 7 (33%) | 1 (4%) | 1 (4%) | .03 | ||||
| Thrombocytopenia | 9 (43%) | 1 (5%) | 10 (48%) | 3 (13%) | 3 (13%) | .03 | |||||
| Cardiac | |||||||||||
| Right bundle block | 1 (5%) | 1 (5%) | 0 (0%) | .48 | |||||||
| Hypertension | 0 (0%) | 2 (9%) | 2 (9%) | .49 | |||||||
| Coagulation | |||||||||||
| PTT | 0 (0%) | 1 (4%) | 1 (4%) | >.99 | |||||||
| Constitutional | |||||||||||
| Fatigue | 12 (57%) | 5 (24%) | 17 (81%) | 14 (61%) | 1 (4%) | 1 (4%) | 16 (70%) | .20 | |||
| Gastrointestinal* | 11 (52%) | 1 (5%) | 12 (57%) | 11 (48%) | 2 (9%) | 13 (57%) | >.99 | ||||
| Genitourinary* | 6 (29%) | 8 (38%) | 1 (5%) | 15 (71%) | 11 (48%) | 5 (22%) | 2 (9%) | 18 (78%) | .53 | ||
| Urinary retention | 11 (52%) | 1 (5%) | 12 (57%) | 8 (35%) | 1 (4%) | 9 (39%) | .28 | ||||
| Metabolic/laboratory | |||||||||||
| ALT (SGPT) | 9 (43%) | 1 (5%) | 10 (48%) | 0 (0%) | <.01 | ||||||
| AST (SGOT) | 8 (38%) | 3 (14%) | 11 (52%) | 2 (9%) | 2 (9%) | <.01 | |||||
| CPK | 0 (0%) | 1 (4%) | 1 (4%) | >.99 | |||||||
| Hyperglycemia | 1 (5%) | 1 (5%) | 0 (0%) | .48 | |||||||
| Hypermagnesemia | 1 (5%) | 1 (5%) | 0 (0%) | .48 | |||||||
| Hypophosphatemia | 1 (5%) | 1 (5%) | 0 (0%) | .48 | |||||||
| Respiratory | |||||||||||
| Pneumonitis (NOS) | 1 (5%) | 1 (5%) | 0 (0%) | .48 | |||||||
| Syndromes | |||||||||||
| Influenza-like | 15 (71%) | 1 (5%) | 16 (76%) | 1 (4%) | 1 (4%) | <.01 | |||||
| Vascular | |||||||||||
| Deep vein thrombosis | 1 (5%) | 1 (5%) | 0 (0%) | .48 | |||||||
| Pulmonary embolism | 1 (5%) | 1 (5%) | 0 (0%) | .48 | |||||||
| Totals | 99 (66%) | 38 (25%) | 13 (9%) | 1 (1%) | 151 (100%) | 69 (70%) | 20 (20%) | 10 (10%) | 0 (0%) | 99 (100%) | |
Abbreviations: ALT = XXXX; AST = androgen suppression therapy; CPK = XXXX; PTT = XXXX; SGOT = XXXX; SGPT = XXXX.
Gastrointestinal (GI) and genitourinary (GU) events were graded using the RTOG acute morbidity scoring criteria. GI events include diarrhea, rectal bleeding, and rectal pain. GU events include urinary frequency/urgency and dysuria. Urinary retention is not included in the RTOG acute GU morbidity scoring criteria and therefore is listed separately.
GI and GU events are the most common side effects of prostate radiation therapy. Less than 10% of men in each arm exhibited acute (≤90 days) grade 2 or higher GI toxicity. Although acute grade 2 or higher GU events were more prevalent (arm 1 had 43%; arm 2 had 31%), no significant differences in acute GI or GU toxicity were noted between the 2 arms.
Quality of life
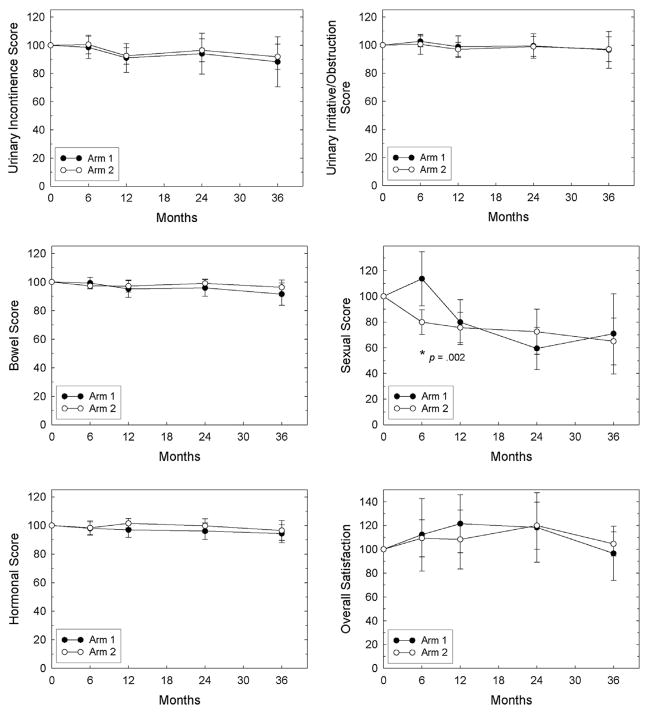
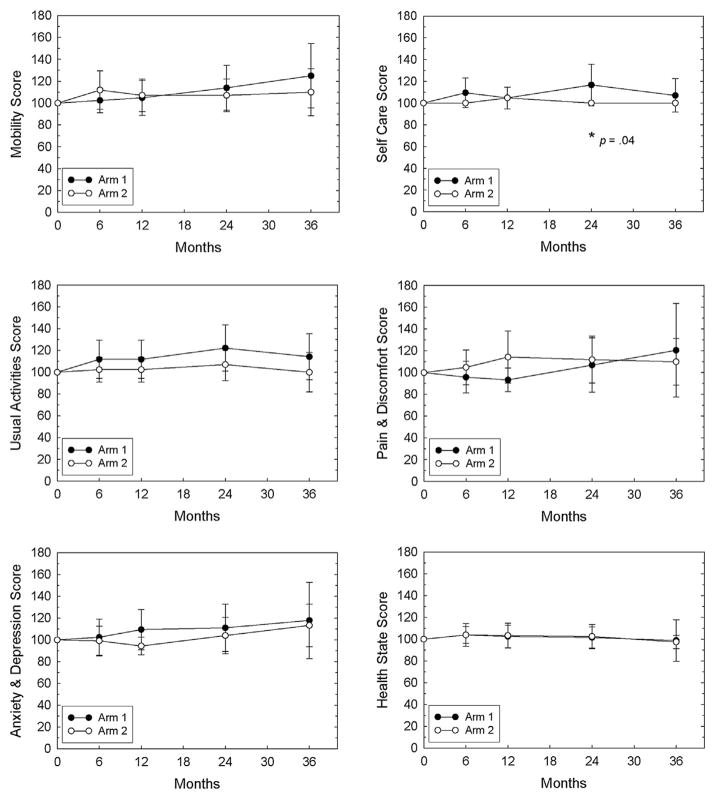
There were no significant differences between the treatment arms for any of the EPIC domains at any time point except for the sexual domain at 6 months, which was better in arm 1 than in arm 2 (Fig. 1). Similarly, there were no significant differences between the treatment arms for any of the EQ-5D dimensions at any time point except for self-care at 24 months, which was better in arm 1 than in arm 2 (Fig. 2). Over time, no significant changes in QOL were found in either arm except for the EPIC sexual domain in arm 1 (6 to 24 months) and overall satisfaction scores in both arms (24 to 36 months), both of which decreased with time.
Fig. 1.
EPIC scores. Mean ± 95% confidence interval values are plotted for each arm. Domain scores at 6, 12, 24, and 36 months were normalized to the patient’s own baseline value (defined as 100%) before the mean for each arm was determined. Mean raw scores for each domain at baseline are shown in Table 1. *Indicates significant differences between the arms, and the P value is shown. EPIC = Expanded Prostate Cancer Index Composite.
Fig. 2.
EQ-5D scores. Mean ± 95% confidence interval values are plotted for each arm. Domain scores at 6, 12, 24, and 36 months were normalized to the patient’s own baseline value (defined as 100%) before the mean for each arm was determined. Mean raw scores for each domain at baseline are shown in Table 1. *Indicates significant differences between the arms, and the P value is shown. EQ-5D = quality of life health status instrument.
Efficacy
Prostate biopsy outcome at ≥2 years after radiation therapy is highly prognostic for long-term outcome (22–24). Hence, short-term efficacy was assessed by a 12-core prostate biopsy analysis 2 years after completion of IMRT.
Two-year biopsy results were obtained from 37 men (84%). Of the 7 men who did not undergo the 2-year biopsy (3 in arm 1, 4 in arm 2), 2 expired before the biopsy could be obtained, 3 declined, 1 had concomitant medical issues, and 1 withdrew from the study before the 2-year end point. There was a 42% (P=.13) and 34% (P=.14) relative reduction in biopsy positivity results in arm 1 based on actual biopsies and intent to treat (ITT), respectively (Table 3). This type 1 error is within the accepted range for randomized phase 2 trials (25). There was 60% (P=.07) and 48% (P=.08) relative reduction in biopsy positivity results in arm 1 in men with <50% positive biopsy cores at baseline based on actual biopsies and ITT, respectively. Owing to the adenovirus injection algorithm that skewed the adenovirus dose distribution to the positive sextants (see Supplemental Table S1), men in arm 1 with <50% positive cores received 2.5 times the adenovirus dose/positive core than men with ≥50% positive cores (Table 4).
Table 3.
Two-year prostate biopsy results
| Procedure | Patients with positive biopsy results*
|
P value | |
|---|---|---|---|
| Arm 1 no. of patients/total no. of patients (%) | Arm 2 no. of patients/total no. of patients (%) | ||
| All patients | |||
| Biopsied | 6/18 (33%) | 11/19 (58%) | .13 |
| ITT† | 9/21 (43%) | 15/23 (65%) | .14 |
| Stratification group‡ | |||
| 1 | |||
| Biopsied | 0/1 (0%) | 1/1 (100%) | >.99 |
| ITT | 0/1 (0%) | 1/1 (100%) | >.99 |
| 2 | |||
| Biopsied | 3/13 (23%) | 8/14 (57%) | .07 |
| ITT | 5/15 (33%) | 11/17 (65%) | .08 |
| 3 | |||
| Biopsied | 3/4 (75%) | 2/4 (50%) | .47 |
| ITT | 4/5 (80%) | 3/5 (60%) | .49 |
| % Positive biopsy cores | |||
| < 50% | |||
| Biopsied | 3/13 (23%) | 8/14 (57%) | .07 |
| ITT | 5/15 (33%) | 11/17 (65%) | .08 |
| ≥ 50% | |||
| Biopsied | 3/5 (60%) | 3/5 (60%) | >.99 |
| ITT | 4/6 (67%) | 4/6 (67%) | >.99 |
Number of patients with a positive 2-year biopsy over the total number of patients with percentage in parentheses.
Intent-to-treat (ITT) includes all patients, whether or not a 2-year biopsy was performed. Those patients who did not have biopsies were given a positive score.
See Table 1 legend.
Table 4.
Relationship between adenovirus dose per positive core and reduction in biopsy positivity at 2 years for men in arm 1
| % Positive cores at baseline | Mean adenovirus dose per positive core | Relative adenovirus dose per positive core | Relative reduction in biopsy positivity |
|---|---|---|---|
| <50% | 3.2 × 1011 virus particles | 1.00 | 60% |
| ≥50% | 1.3 × 1011 virus particles | 0.40 | 0% |
To date, 1 patient in each arm has exhibited biochemical failure (arm 1 had 4.8%; arm 2 had 4.3%). These events occurred 14.5 months (arm 1) and 13.8 months (arm 2) after randomization. No patient developed hormone-refractory or metastatic disease, and none died from prostate cancer.
Discussion
There are 2 ways to improve the effectiveness of radiation therapy: increase the prescribed radiation dose by physical means (“turn up the dial”) or increase the intrinsic effectiveness of the radiation dose by biological/chemical means (“turn up the gain”). Not only is radiation dose escalation logical, it is a clinically proven strategy that has earned its place in the management of localized prostate cancer (2–5). The major concern with dose escalation is that it comes at the price of increased risk of developing GI and GU complications owing to the close proximity of the rectum and urinary bladder. Hence, as with all therapies, the benefits must be weighed carefully against the risks.
As a possible alternative (complementary) to dose escalation, we have developed a strategy to increase the effectiveness of radiation therapy by biological/chemical means. Our premise is that the therapeutic gain achieved by biological/chemical enhancement may be greater than that which is possible with physical means. Administering 80 Gy to the prostate in the presence of a biological/chemical modifier that results in a sensitization enhancement ratio of 1.3 would be equivalent to more than 100 Gy. We demonstrated previously that implementation of the CD/5-FC and HSV-1 TK/vGCV enzyme/prodrug systems results in sensitization enhancement ratios ranging from 1.4 to 2.4 in vitro (26–28). We now demonstrate that combining OAMCGT with contemporary dose IMRT results in a 42% relative reduction in biopsy positivity at 2 years in men with intermediate-risk prostate cancer and a 60% relative reduction in men with <50% positive biopsy cores. These results were achieved without exacerbation of the most common complications of prostate radiation therapy or diminishing the patient’s QOL. We consider these results are encouraging for prostate biopsy outcome at ≥2 years and are strong predictors of long-term outcome (22–24). We acknowledge the limitations of the prostate biopsy end point such as sampling error and subjectivity of the biopsy interpretation (29). That is why the protocol called for a ≥12-core 2-year biopsy analysis, and concordance on the diagnosis was required from 2 GU pathologists blinded to the study treatment. Indeed, prostate biopsy analysis at ≥2 years is the earliest end point available with which to assess efficacy in the setting of a prostate cancer clinical trial.
One important consideration when designing this study was how to distribute the adenovirus throughout the prostate. We considered 3 strategies: (1) deposit the adenovirus uniformly, (2) deposit the adenovirus only in sextants known to harbor cancer, or (3) deposit the adenovirus in all sextants but skew the dose distribution to sextants known to harbor cancer. Because all men received IMRT and the entire prostate was included in the target volume, we chose the third option and applied the OAMCGT as a therapeutic “boost.” Owing to the skewed adenovirus distribution, men with <50% positive biopsy cores received 2.5 times the adenovirus dose per positive core than men with ≥50% positive cores. We hypothesize that this is the underlying reason why men with <50% positive biopsy cores showed a 60% relative reduction in biopsy positivity, whereas men with ≥50% positive cores showed no effect. Although it is likely that the OAMCGT helped reduce the prostatic tumor burden in men with ≥50% positive cores, we speculate that the adenovirus dose per tumor volume was insufficient to eradicate the cancer in these men, causing them to score positively in the 2-year biopsy assessment owing to their residual disease. Moving forward, it would be logical to escalate the adenovirus dose beyond 1 × 1012 vp with the hope of benefitting men with more advanced disease (ie, intermediate risk with ≥50% positive biopsy cores and high-risk disease). Our results suggest that an escalation of 2.5-fold or greater in the adenovirus dose, from 1.3 × 1011 vp to 3.2 × 1011 vp/positive core (Table 4) might be sufficient to move a significant fraction of intermediate-risk men with ≥50% positive cores from the biopsy-positive to biopsy-negative category. Moreover, we administered 5 × 1012 vp to men with high-risk disease in another prostate cancer radiation therapy trial and found that the combined treatment was well tolerated (15). This adenovirus dose corresponds to ≥4.2 × 1011 vp/positive core (assuming 12 cores), thereby exceeding our proposed therapeutic threshold. Hence, escalating the adenovirus dose above 1 × 1012 vp safely is feasible.
One issue that remains is how OAMCGT compares with other biological therapies used in the treatment of prostate cancer. AST is often combined with prostate radiation therapy, and it improves all measures of outcome including survival in men with intermediate- to high-risk disease (30–34). A logical question to ask is why even consider OAMCGT, why not just treat intermediate-risk patients with ≥4 months of AST and IMRT? First, all studies that demonstrated a benefit of combining AST with prostate radiation therapy used radiation doses ≤70 Gy. The benefit, if any, of combining AST with contemporary dose (~80-Gy) prostate radiation therapy has not been demonstrated and is currently under investigation (RTOG 08-15) in men with intermediate-risk prostate cancer. Second, although 4 months of AST was associated with minimal morbidity in RTOG 94-08, other prospective studies have found that combining AST (mean duration of 5 months) with prostate radiation therapy is associated with worse outcomes across multiple QOL domains (20).
Conclusions
We report here that combining OAMCGT with contemporary dose IMRT does not diminish the patient’s QOL relative to IMRT alone. Finally, we find it encouraging that the 42% relative reduction in 2-year biopsy positivity reported here closely resembles the effect of 4 months of AST reported in RTOG 94-08 (41% relative reduction in intermediate-risk group). Considering that 29% of the men in arm 1 of this study received a suboptimal adenovirus dose (≥50% positive biopsy cores), it is possible that a higher dose of OAMCGT may provide a greater local benefit than 4 months of AST when combined with prostate radiation therapy. Importantly, our results were obtained using a contemporary radiation dose of 80 Gy. Whether this improvement in local tumor control translates into better biochemical/clinical control or, more importantly, survival will require longer follow up. The fact that OAMCGT has been shown to significantly lengthen PSA doubling time and generate long-lasting effects suggests that it may induce secondary effects such as development of antitumor immunity (12). If this is true, its therapeutic reach may not be limited to the local tumor but may extend to disseminated disease as well.
Supplementary Material
Summary.
A randomized phase 2 trial was conducted to examine the potential of oncolytic adenovirus-mediated cytotoxic gene therapy (OAMCGT) in improving the outcome of intensity modulated radiation therapy (IMRT) in intermediate-risk prostate cancer. Men who received OAMCGT and IMRT showed a 42% reduction in biopsy positivity at 2 years relative to men who received IMRT only. The addition of OAMCGT did not exacerbate the most common side effects of prostate radiation therapy or diminish the patients’ quality of life.
Footnotes
Conflict of interest: none.
Supplementary material for this article can be found at www.redjournal.org.
References
- 1.Hanks G, Hanlon A, Epstein B, et al. Dose response in prostate cancer with 8–12 years follow-up. Int J Radiat Oncol Biol Phys. 2002;54:427–435. doi: 10.1016/s0360-3016(02)02954-1. [DOI] [PubMed] [Google Scholar]
- 2.Kuban D, Tucker S, Dong L, et al. Long-term results of the MD Anderson randomized dose-escalation trial for prostate cancer. Int J Radiat Oncol Biol Phys. 2008;70:67–74. doi: 10.1016/j.ijrobp.2007.06.054. [DOI] [PubMed] [Google Scholar]
- 3.Zietman A, Bae K, Slater J, et al. Randomized trial comparing conventional-dose with high-dose conformal radiation therapy in early-stage adenocarcinoma of the prostate: long-term results from proton radiation oncology group/American college of radiology 95-09. J Clin Oncol. 2010;28:1106–1111. doi: 10.1200/JCO.2009.25.8475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dearnaley D, Sydes M, Graham J, et al. Escalated dose versus standard dose conformal radiotherapy in prostate cancer: first results from the MRC RT01 randomized controlled trial. Lancet Oncol. 2007;8:475–487. doi: 10.1016/S1470-2045(07)70143-2. [DOI] [PubMed] [Google Scholar]
- 5.Al-Mamgani A, van Putten W, Heemsbergen W, et al. Update of Dutch multicenter dose-escalation trial of radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2008;72:980–988. doi: 10.1016/j.ijrobp.2008.02.073. [DOI] [PubMed] [Google Scholar]
- 6.Zelefsky M, Yamada Y, Fuks Z, et al. Long-term results of conformal radiotherapy for prostate cancer: impact of dose escalation on biochemical tumor control and distant metastases-free survival outcomes. Int J Radiat Oncol Biol Phys. 2008;71:1028–1033. doi: 10.1016/j.ijrobp.2007.11.066. [DOI] [PubMed] [Google Scholar]
- 7.Eade T, Hanlon A, Horwitz E, et al. What dose of external beam radiation therapy is high enough for prostate cancer? Int J Radiat Oncol Biol Phys. 2007;68:682–689. doi: 10.1016/j.ijrobp.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.XXXX.
- 9.XXXX.
- 10.XXXX.
- 11.XXXX.
- 12.XXXX.
- 13.XXXX.
- 14.XXXX.
- 15.XXXX.
- 16.XXXX.
- 17.Roach M, Hanks G, Thames H, et al. Defining biochemical failure following radiotherapy with or without hormone therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006;65:965–974. doi: 10.1016/j.ijrobp.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 18.Spalding A, Diagnault S, Sandler H, et al. Percent positive biopsy cores as a prognostic factor for prostate cancer treated with external beam radiation. Urology. 2007;69:936–940. doi: 10.1016/j.urology.2007.01.066. [DOI] [PubMed] [Google Scholar]
- 19.Wei J, Dunn R, Litwin M, et al. Development and validation of the expanded prostate cancer index composite (EPIC) for comprehensive assessment of health-related quality of life in men with prostate cancer. Urology. 2000;56:899–905. doi: 10.1016/s0090-4295(00)00858-x. [DOI] [PubMed] [Google Scholar]
- 20.Sanda M, Dunn R, Michalski J, et al. Quality of life and satisfaction with outcome among prostate cancer survivors. N Engl J Med. 2008;358:1250–1261. doi: 10.1056/NEJMoa074311. [DOI] [PubMed] [Google Scholar]
- 21.Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med. 2001;33:337–343. doi: 10.3109/07853890109002087. [DOI] [PubMed] [Google Scholar]
- 22.Kuban D, el-Mahdi A, Schellhammer P. The significance of post-irradiation prostate biopsy with long-term follow-up. Int J Radiat Oncol Biol Phys. 1992;24:409–414. doi: 10.1016/0360-3016(92)91053-p. [DOI] [PubMed] [Google Scholar]
- 23.Zelefsky M, Reuter V, Fuks Z, et al. Influence of local tumor control on distant metastases and cancer related mortality after external beam radiotherapy for prostate cancer. J Urol. 2008;179:1368–1373. doi: 10.1016/j.juro.2007.11.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crook J, Malone S, Perry G, et al. Twenty-four-month postradiation prostate biopsies are strongly predictive of 7-year disease-free survival: results from a Canadian randomized trial. Cancer. 2009;115:673–679. doi: 10.1002/cncr.24020. [DOI] [PubMed] [Google Scholar]
- 25.Cannistra S. Phase II trials in Journal of Clinical Oncology. J Clin Oncol. 2009;27:3073–3076. doi: 10.1200/JCO.2009.23.1811. [DOI] [PubMed] [Google Scholar]
- 26.XXXX.
- 27.XXXX.
- 28.XXXX.
- 29.Crook J, Malone S, Perry G, et al. Postradiotherapy prostate biopsies: what do they really mean? Results for 498 patients. Int J Radiat Oncol Biol Phys. 2000;48:355–367. doi: 10.1016/s0360-3016(00)00637-4. [DOI] [PubMed] [Google Scholar]
- 30.Bolla M, Collette L, Blank L, et al. Long-term results with immediate androgen suppression and external irradiation in subjects with locally advanced prostate cancer (an EORTC study): a phase III randomised trial. Lancet. 2002;360:103–106. doi: 10.1016/s0140-6736(02)09408-4. [DOI] [PubMed] [Google Scholar]
- 31.Pilepich M, Winter K, Lawton C, et al. Androgen suppression adjuvant to definitive radiotherapy in prostate carcinoma- long-term results of phase III RTOG 85-31. Int J Radiat Oncol Biol Phys. 2005;61:1285–1290. doi: 10.1016/j.ijrobp.2004.08.047. [DOI] [PubMed] [Google Scholar]
- 32.Horwitz E, Bae K, Hanks G, et al. Ten-year follow-up of Radiation Therapy Oncology Group protocol 92-02: a phase III trial of the duration of elective androgen deprivation in locally advanced prostate cancer. J Clin Oncol. 2008;26:2497–2504. doi: 10.1200/JCO.2007.14.9021. [DOI] [PubMed] [Google Scholar]
- 33.D’Amico A, Chen M, Renshaw A, et al. Androgen suppression and radiation vs radiation alone for prostate cancer: a randomized trial. JAMA. 2008;299:289–295. doi: 10.1001/jama.299.3.289. [DOI] [PubMed] [Google Scholar]
- 34.Jones C, Hunt D, McGowan D, et al. Radiotherapy and short-term androgen deprivation for localized prostate cancer. N Engl J Med. 2011;365:107–118. doi: 10.1056/NEJMoa1012348. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.