Abstract
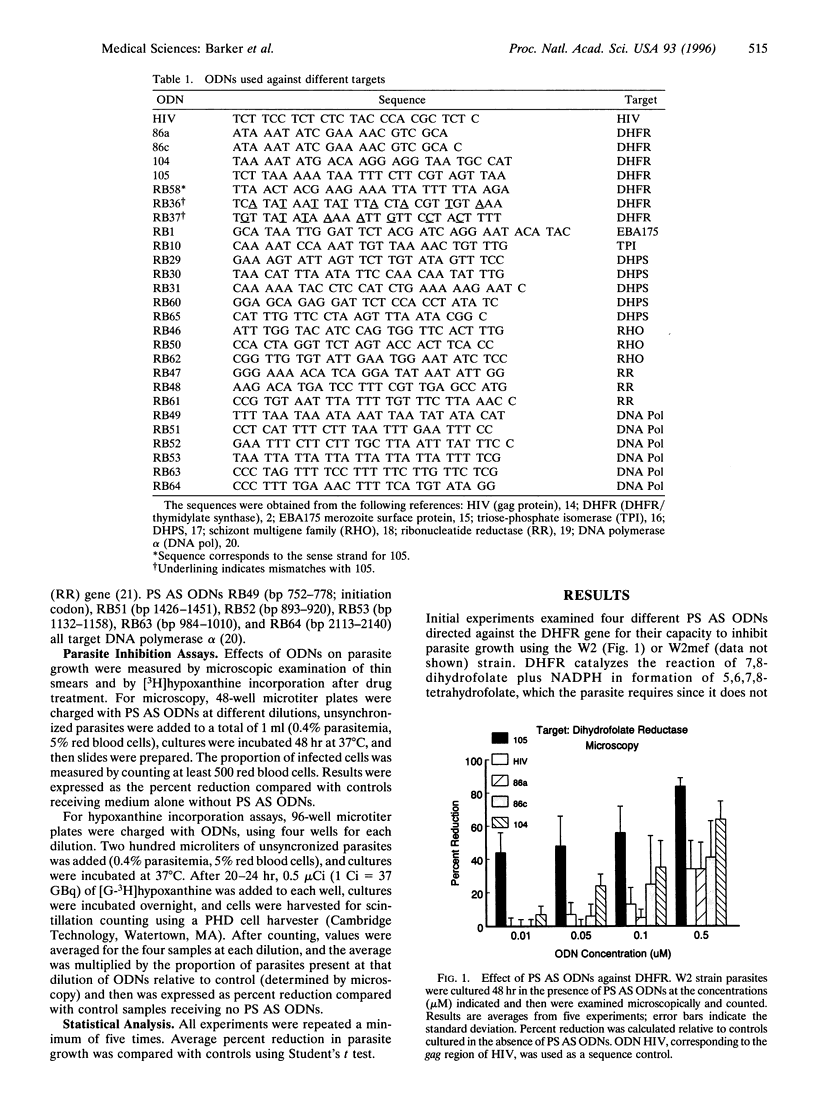
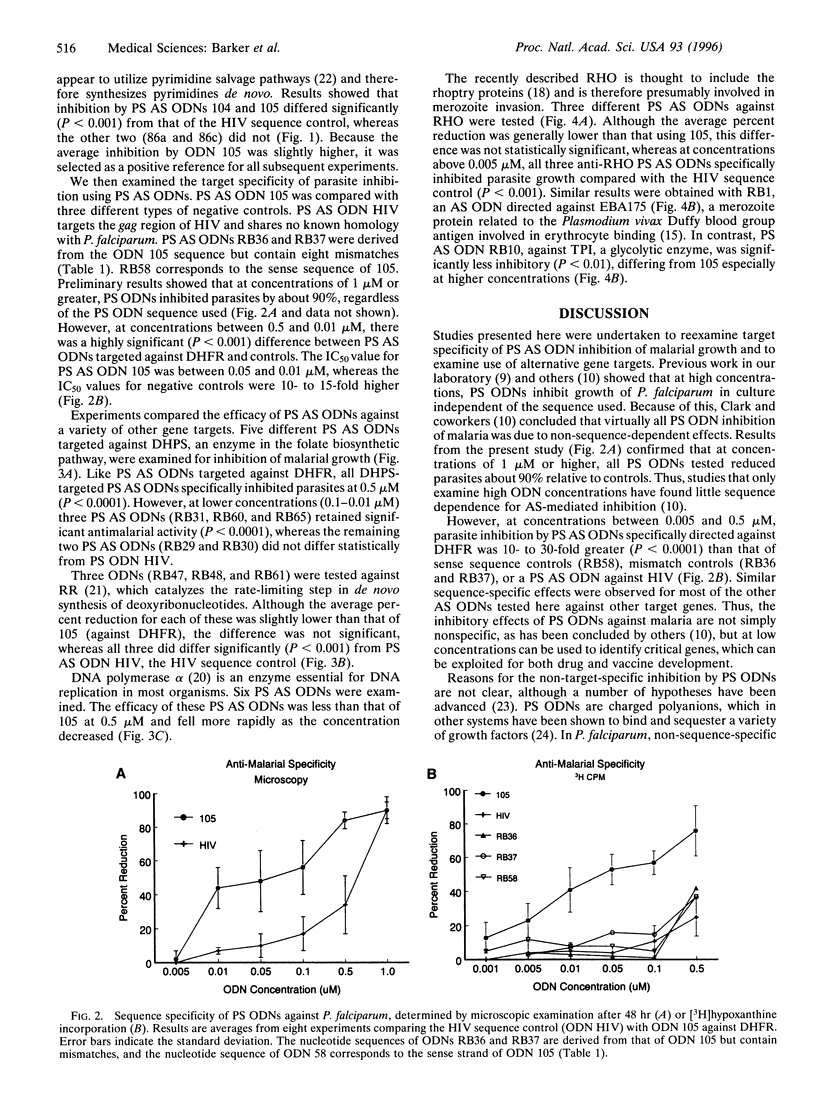
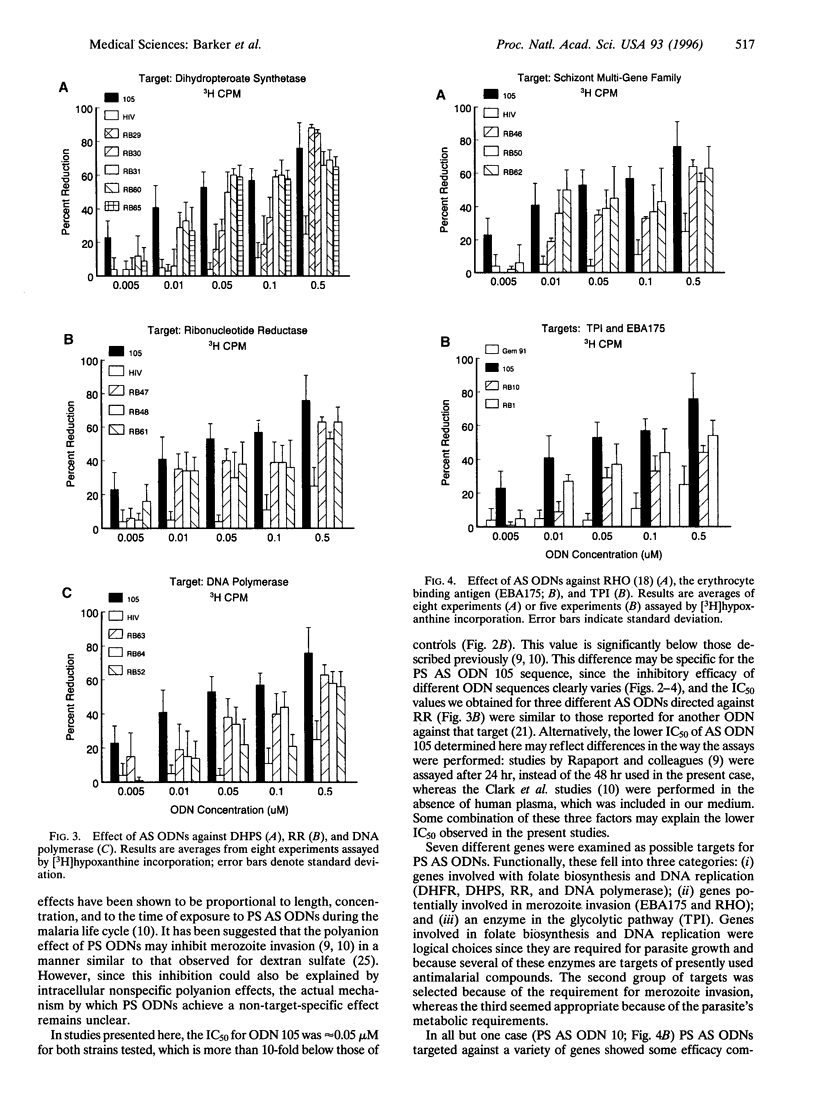
We studied inhibition of growth of the malaria parasite Plasmodium falciparum in in vitro culture using antisense (AS) oligodeoxynucleotides (ODNs) against different target genes. W2 and W2mef strains of drug-resistant parasites were exposed to AS ODNs over 48 hr, and growth was determined by microscopic examination and [3H]hypoxanthine incorporation. At ODN concentrations of 1 microM, phosphorothioate (PS) ODNs inhibited growth in a target-independent manner. However, between 0.5 and 0.005 microM, ODNs against dihydrofolate reductase, dihydropteroate synthetase, ribonucleotide reductase, the schizont multigene family, and erythrocyte binding antigen EBA175 significantly inhibited growth compared with a PS AS ODN against human immunodeficiency virus, two AS ODNs containing eight mismatches, or the sense strand controls (P < 0.0001). The IC50 was approximately 0.05 microM, whereas that for non-sequence-specific controls was 15-fold higher. PS AS ODNs against DNA polymerase alpha showed less activity than that for other targets, whereas a single AS ODN against triose-phosphate isomerase did not differ significantly from controls. We conclude that at concentrations below 0.5 microM, PS AS ODNs targeted against several malarial genes significantly inhibit growth of drug-resistant parasites in a nucleotide sequence-dependent manner. This technology represents an alternative method for identifying malarial genes as potential drug targets.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agrawal S. Antisense oligonucleotides as antiviral agents. Trends Biotechnol. 1992 May;10(5):152–158. doi: 10.1016/0167-7799(92)90203-8. [DOI] [PubMed] [Google Scholar]
- Basco L. K., Eldin de Pécoulas P., Wilson C. M., Le Bras J., Mazabraud A. Point mutations in the dihydrofolate reductase-thymidylate synthase gene and pyrimethamine and cycloguanil resistance in Plasmodium falciparum. Mol Biochem Parasitol. 1995 Jan;69(1):135–138. doi: 10.1016/0166-6851(94)00207-4. [DOI] [PubMed] [Google Scholar]
- Beuria M. K., Das M. K. Dextran sulfate induced suppression of Plasmodium berghei parasitaemia. Indian J Exp Biol. 1991 Mar;29(3):284–285. [PubMed] [Google Scholar]
- Brooks D. R., Wang P., Read M., Watkins W. M., Sims P. F., Hyde J. E. Sequence variation of the hydroxymethyldihydropterin pyrophosphokinase: dihydropteroate synthase gene in lines of the human malaria parasite, Plasmodium falciparum, with differing resistance to sulfadoxine. Eur J Biochem. 1994 Sep 1;224(2):397–405. doi: 10.1111/j.1432-1033.1994.00397.x. [DOI] [PubMed] [Google Scholar]
- Bzik D. J., Li W. B., Horii T., Inselburg J. Molecular cloning and sequence analysis of the Plasmodium falciparum dihydrofolate reductase-thymidylate synthase gene. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8360–8364. doi: 10.1073/pnas.84.23.8360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabantchik Z. L. Altered membrane transport of malaria-infected erythrocytes: a possible pharmacologic target. Blood. 1989 Oct;74(5):1464–1471. [PubMed] [Google Scholar]
- Carcy B., Bonnefoy S., Guillotte M., Le Scanf C., Grellier P., Schrevel J., Fandeur T., Mercereau-Puijalon O. A large multigene family expressed during the erythrocytic schizogony of Plasmodium falciparum. Mol Biochem Parasitol. 1994 Dec;68(2):221–233. doi: 10.1016/0166-6851(94)90167-8. [DOI] [PubMed] [Google Scholar]
- Chakrabarti D., Schuster S. M., Chakrabarti R. Cloning and characterization of subunit genes of ribonucleotide reductase, a cell-cycle-regulated enzyme, from Plasmodium falciparum. Proc Natl Acad Sci U S A. 1993 Dec 15;90(24):12020–12024. doi: 10.1073/pnas.90.24.12020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark D. L., Chrisey L. A., Campbell J. R., Davidson E. A. Non-sequence-specific antimalarial activity of oligodeoxynucleotides. Mol Biochem Parasitol. 1994 Jan;63(1):129–134. doi: 10.1016/0166-6851(94)90015-9. [DOI] [PubMed] [Google Scholar]
- Elford B. C., Cowan G. M., Ferguson D. J. Parasite-regulated membrane transport processes and metabolic control in malaria-infected erythrocytes. Biochem J. 1995 Jun 1;308(Pt 2):361–374. doi: 10.1042/bj3080361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay F., Bustos D. G., Diquet B., Rojas Rivero L., Litaudon M., Pichet C., Danis M., Gentilini M. Cross-resistance between mefloquine and halofantrine. Lancet. 1990 Nov 17;336(8725):1262–1262. doi: 10.1016/0140-6736(90)92884-k. [DOI] [PubMed] [Google Scholar]
- Kutner S., Breuer W. V., Ginsburg H., Aley S. B., Cabantchik Z. I. Characterization of permeation pathways in the plasma membrane of human erythrocytes infected with early stages of Plasmodium falciparum: association with parasite development. J Cell Physiol. 1985 Dec;125(3):521–527. doi: 10.1002/jcp.1041250323. [DOI] [PubMed] [Google Scholar]
- Leonetti J. P., Mechti N., Degols G., Gagnor C., Lebleu B. Intracellular distribution of microinjected antisense oligonucleotides. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2702–2706. doi: 10.1073/pnas.88.7.2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisziewicz J., Sun D., Weichold F. F., Thierry A. R., Lusso P., Tang J., Gallo R. C., Agrawal S. Antisense oligodeoxynucleotide phosphorothioate complementary to Gag mRNA blocks replication of human immunodeficiency virus type 1 in human peripheral blood cells. Proc Natl Acad Sci U S A. 1994 Aug 16;91(17):7942–7946. doi: 10.1073/pnas.91.17.7942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metelev V., Agrawal S. Ion-exchange high-performance liquid chromatography analysis of oligodeoxyribonucleotide phosphorothioates. Anal Biochem. 1992 Feb 1;200(2):342–346. doi: 10.1016/0003-2697(92)90476-n. [DOI] [PubMed] [Google Scholar]
- Offensperger W. B., Offensperger S., Walter E., Teubner K., Igloi G., Blum H. E., Gerok W. In vivo inhibition of duck hepatitis B virus replication and gene expression by phosphorothioate modified antisense oligodeoxynucleotides. EMBO J. 1993 Mar;12(3):1257–1262. doi: 10.1002/j.1460-2075.1993.tb05767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmapriya A. A., Tang J., Agrawal S. Large-scale synthesis, purification, and analysis of oligodeoxynucleotide phosphorothioates. Antisense Res Dev. 1994 Fall;4(3):185–199. doi: 10.1089/ard.1994.4.185. [DOI] [PubMed] [Google Scholar]
- Ramazeilles C., Mishra R. K., Moreau S., Pascolo E., Toulmé J. J. Antisense phosphorothioate oligonucleotides: selective killing of the intracellular parasite Leishmania amazonensis. Proc Natl Acad Sci U S A. 1994 Aug 16;91(17):7859–7863. doi: 10.1073/pnas.91.17.7859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranie J., Kumar V. P., Balaram H. Cloning of the triosephosphate isomerase gene of Plasmodium falciparum and expression in Escherichia coli. Mol Biochem Parasitol. 1993 Oct;61(2):159–169. doi: 10.1016/0166-6851(93)90062-3. [DOI] [PubMed] [Google Scholar]
- Rapaport E., Misiura K., Agrawal S., Zamecnik P. Antimalarial activities of oligodeoxynucleotide phosphorothioates in chloroquine-resistant Plasmodium falciparum. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8577–8580. doi: 10.1073/pnas.89.18.8577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin H., Salem J. S., Li L. S., Yang F. D., Mama S., Wang Z. M., Fisher A., Hamann C. S., Cooperman B. S. Cloning, sequence determination, and regulation of the ribonucleotide reductase subunits from Plasmodium falciparum: a target for antimalarial therapy. Proc Natl Acad Sci U S A. 1993 Oct 15;90(20):9280–9284. doi: 10.1073/pnas.90.20.9280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim B. K., Orlandi P. A., Haynes J. D., Klotz F. W., Carter J. M., Camus D., Zegans M. E., Chulay J. D. Primary structure of the 175K Plasmodium falciparum erythrocyte binding antigen and identification of a peptide which elicits antibodies that inhibit malaria merozoite invasion. J Cell Biol. 1990 Nov;111(5 Pt 1):1877–1884. doi: 10.1083/jcb.111.5.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein C. A., Krieg A. M. Problems in interpretation of data derived from in vitro and in vivo use of antisense oligodeoxynucleotides. Antisense Res Dev. 1994 Summer;4(2):67–69. doi: 10.1089/ard.1994.4.67. [DOI] [PubMed] [Google Scholar]
- Triglia T., Cowman A. F. Primary structure and expression of the dihydropteroate synthetase gene of Plasmodium falciparum. Proc Natl Acad Sci U S A. 1994 Jul 19;91(15):7149–7153. doi: 10.1073/pnas.91.15.7149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verspieren P., Cornelissen A. W., Thuong N. T., Hélène C., Toulmé J. J. An acridine-linked oligodeoxynucleotide targeted to the common 5' end of trypanosome mRNAs kills cultured parasites. Gene. 1987;61(3):307–315. doi: 10.1016/0378-1119(87)90194-6. [DOI] [PubMed] [Google Scholar]
- Volkman S. K., Wilson C. M., Wirth D. F. Stage-specific transcripts of the Plasmodium falciparum pfmdr 1 gene. Mol Biochem Parasitol. 1993 Feb;57(2):203–211. doi: 10.1016/0166-6851(93)90196-5. [DOI] [PubMed] [Google Scholar]
- Wellstein A., Zugmaier G., Califano J. A., 3rd, Kern F., Paik S., Lippman M. E. Tumor growth dependent on Kaposi's sarcoma-derived fibroblast growth factor inhibited by pentosan polysulfate. J Natl Cancer Inst. 1991 May 15;83(10):716–720. doi: 10.1093/jnci/83.10.716. [DOI] [PubMed] [Google Scholar]
- White J. H., Kilbey B. J., de Vries E., Goman M., Alano P., Cheesman S., McAleese S., Ridley R. G. The gene encoding DNA polymerase alpha from Plasmodium falciparum. Nucleic Acids Res. 1993 Aug 11;21(16):3643–3646. doi: 10.1093/nar/21.16.3643. [DOI] [PMC free article] [PubMed] [Google Scholar]



