Abstract
Background
Identification of poor prognostic factors in papillary thyroid carcinoma (PTC) patients is important for the patients' care and follow-up. We can sometimes see small tumor clusters without desmoplasia and no evidence of lymphatic emboli around the main tumor mass of PTC. We termed this form of tumor clustering, 'tumor sprouting,' and determined whether these tumors correlate with lymphovascular invasion, lymph node metastasis, and recurrence.
Methods
We analyzed a total of 204 cases of papillary thyroid macrocarcinoma. Number, size and distance from the main tumor of the tumor sprouting were observed and analyzed with clinicopathologic characteristics.
Results
Tumor sprouting was observed in 101 patients. Presence of tumor sprouting was significantly associated with positive resection margin (p=.002), lymphovascular invasion (p=.001), lymph node metastasis (p<.001), and recurrence (p=.004). Univariate analysis of recurrence-free survival revealed that tumor multiplicity (p=.037), positive resection margin (p=.007), lymphovascular invasion (p=.004), lymph node metastasis (p<.001), and tumor sprouting (p=.004) were poor prognostic factors. In multivariate analysis, positive resection margin was an independent poor prognostic factor of recurrence.
Conclusions
In conclusion, tumor sprouting is significantly correlated with lymph node metastasis and recurrence. Evaluation of tumor sprouting in PTC patients could be helpful in predicting tumor recurrence or lymph node metastasis.
Keywords: Tumor sprouting; Thyroid cancer, papillary; Lymphatic invasion; Lymph node metastasis; Recurrence
Papillary thyroid carcinoma (PTC) is the most frequent type of the thyroid malignancy.1 The prognosis of PTC is generally favorable, but more than 10% of patients are at risk of recurrence in long-term follow-up.2,3,4 To avoid unnecessary treatment, identifying PTC patients with a high risk of recurrence and/or metastasis is becoming more important given a significant increase in the incidence of PTC.5 Some study results have shown that clinicopathologic factors including extrathyroidal extension, patient age, tumor size, sex, multifocality, lymphatic invasion, and desmoplastic reaction are associated with poor prognosis of PTC.6,7,8,9,10,11,12,13,14
In many malignant epithelial tumors, lymphovascular invasion and lymph node metastasis are known to be independent poor prognostic factors, and lymphovascular invasion of tumor cells is considered to precede lymph node metastasis.15,16,17 In contrast to follicular carcinoma, PTCs are known to spread through lymphatic vessels rather than vascular channels and commonly metastasize to lymph nodes.18,19 Some previous studies have revealed that lymphatic invasion of PTC is associated with lymph node metastasis and/or poor prognosis.20,21,22 The wide range of prevalence of lymphatic invasion in PTC patients may be due to an unclear definition of lymphatic invasion.20,21,22
On hematoxylin and eosin (H&E) stained slides of tumor sections, tumor cell clusters without desmoplastic reactions around the main PTC mass are frequently observed. These tumor clusters are not connected to the main mass and sometimes show radial arrangement around the main mass. The tumor clusters may resemble lymphatic tumor emboli, but these cancer cell clusters lack evidence of lymphatic invasion such as endothelialization and thrombus formation, and are surrounded by lymphatic spaces. We hypothesized that these tumor cell clusters would be either a tentacular expansion of the main tumor mass or lymphatic emboli and the presence of these clusters will show an association with lymph node metastasis and recurrence of PTC patients. We named these cancer cell clusters "tumor sprouting" and, in this study, evaluated the correlation between tumor sproutings and clinicopathologic factors.
MATERIALS AND METHODS
Patients and clinical data
A total of 204 histologically-proven PTC patients who underwent surgical resection of the thyroid between May 2008 and December 2010 at the Korea University Guro Hospital were included in this study. Papillary microcarcinoma (tumor size ≤1 cm) patients were not included in this study. All included cases were classical type papillary carcinoma. All patients underwent standard hemithyroidectomy or total thyroidectomy with or without lymph node dissection. Lymph node dissection including central and/or lateral neck dissection was performed in 107 patients (52%). These patients were followed-up regularly (median follow-up, 35 months; range, 1 to 54 months) and evaluated for recurrence and distant metastasis by ultrasonography or computed tomography. When tumor recurrence was suspected, fine needle aspiration was performed for pathologic confirmation. Seventeen patients (8%) recurred including one patient with local recurrence and distant metastasis to the lung, and no patient has died during the follow-up period. Patients who have not undergone lymph node dissection (n=97) were regarded as the lymph node negative group, because these patients had no palpable or enlarged lymph node at their first follow-up visit. This study was approved by the Institutional Review Board of the Korea University Guro Hospital (KUGH11131-002).
Clinicopathologic data and histopathologic review
Clinicopathological data including age, sex, type of surgery, distant metastasis, recurrence, and follow-up data were obtained from medical records. H&E stained glass slides were reviewed by two pathologists (E.L and B.-H.K). Tumor size, multifocality, desmoplastic reaction, extrathyroidal extension, tumor margin status, lymph node metastasis, and lymphovascular invasion were determined by slide review.23 The presence of separate tumor clusters without a connection to the main tumor mass was regarded as a multifocal tumor when the long diameter of the tumor cluster was larger than 0.3 cm or a desmoplastic reaction was observed around the tumor cluster. Cases showing one or more desmoplastic area(s), observed at 100× magnification, were classified as the desmoplasia-positive group. Lymphovascular invasion was defined as a tumor cell cluster located within the endothelialized space with or without fibrin thrombus.24
We defined "tumor sprouting" as a separate tumor cluster lacking desmoplastic reaction, with a distance from the main tumor border or desmoplasia of more than 0.1 cm, and the size of the tumor cluster not more than 0.3 cm. We evaluated the number and maximum size of tumor sprouting and the maximum distance from the main tumor mass. Tumor sprouting evaluation was performed using one representative tumor section slide per patient that contained the largest cross section of the main mass. Immunohistochemical staining of D2-40 (1:40, Clone D2-40, Signet Laboratories, Dedham, MA, USA), cytokeratin 19 (1:250, RCK108, Dako, Glostrup, Denmark) and high molecular weight cytokeratin (HMW-CK; 1:300, 34βE12, Dako) were performed on all representative tumor sections to aid in finding tumor sprouting and lymphatic invasion (Figs. 1, 2).
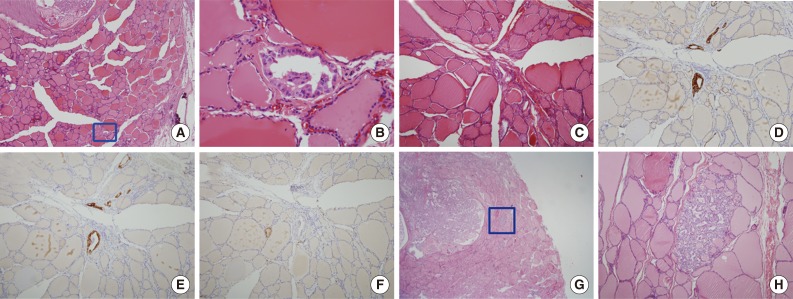
Fig. 1.
Morphologic features of tumor sproutings. Tumor sprouting is present in the lower portion of the figure (blue box) separated from the main tumor in the upper portion (A) and a magnified view of blue-boxed area shows tumor sprouting. Tumor sprouting exhibits typical nuclear features of papillary thyroid carcinoma, but lacks evidence of lymphatic emboli such as an endothelialized tumor cluster or endothelialized lymphatic spaces (B). Another example of tumor sprouting. Tumor sprouting is easily found with cytokeratin 19 (CK19) or high molecular weight cytokeratin (HMW-CK) staining. Immunoreactivity with D2-40 is not observed (C, hematoxylin and eosin staining; D, CK19 staining; E, HMW-CK staining; F, D2-40 staining). (G) Another example of tumor sprouting (blue box). Tumor sproutings are observed around the main tumor mass. (H) High magnification view of tumor sprouting.
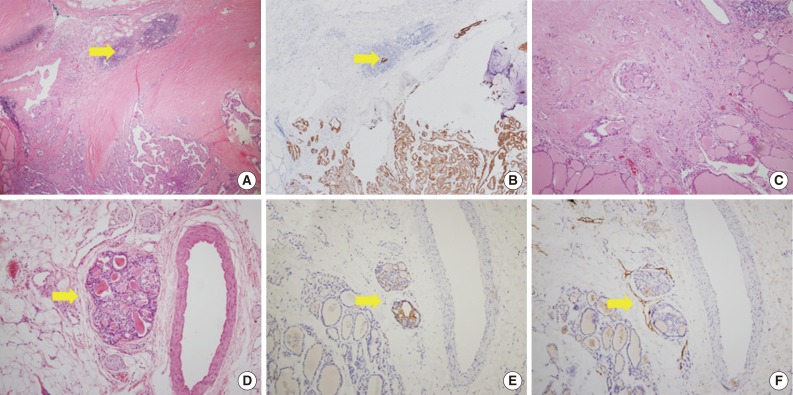
Fig. 2.
Microscopic figures of separate tumor clusters excluded from tumor sprouting and figures of lymphovascular tumor emboli. Tumor clusters too close to the tumor border or desmoplastic area (arrows) are excluded from tumor sproutings (A, hematoxylin and eosin [H&E] staining; B, cytokeratin 19 [CK19] staining). (C) Tumor clusters with desmoplastic reactions are also excluded. Lymphovascular tumor emboli (arrows) showing endothelialized lymphatic spaces with D2-40 staining (D, H&E staining; E, CK19 staining; F, D2-40 staining).
Statistical analysis
IBM SPSS ver. 20 (IBM Inc., Armonk, NY, USA) was used for statistical analysis. To evaluate the correlation between tumor sprouting and clinicopathologic factors, we performed Pearson's chi-square test, Fisher's exact test and linear regression analysis. Recurrence free survival was obtained using the Kaplan-Meier method and a log-rank test. Multivariate analysis was performed using the Cox-proportional hazards model. p-values of .05 or less were considered to be statistically significant.
RESULTS
Tumor sprouting
The clinicopathologic characteristics of 204 patients are described in Table 1. Among 204 thyroidectomy specimens, lymphovascular invasion was observed in 10 patients (5%) and tumor sprouting was observed in 101 patients (50%). D2-40 immunohistochemical staining failed to reveal lymphatic endothelial cells in and around tumor sproutings. The mean size of tumor sproutings was 0.08 cm (range, 0.01 to 0.3 cm) and the mean number was 3.52 (range, 1 to 20). Distance of tumor sprouting from the main tumor ranged from 0.1 to 1.0 cm (mean, 0.3 cm). Characteristics of tumor sprouting (number, size, and distance) were closely inter-correlated with each other (p<.001). The number of metastatic lymph nodes was also correlated with the number, size, and distance of tumor sprouting (p<.001). In other words, the greater the number, size, and distance of tumor sprouting, the greater the number of metastatic lymph nodes. No linear relationship between the patient's age, tumor size, number of tumors, number of desmoplastic foci, and characteristics of tumor sprouting was observed.
Table 1.
Clinicopathologic characteristics of patients
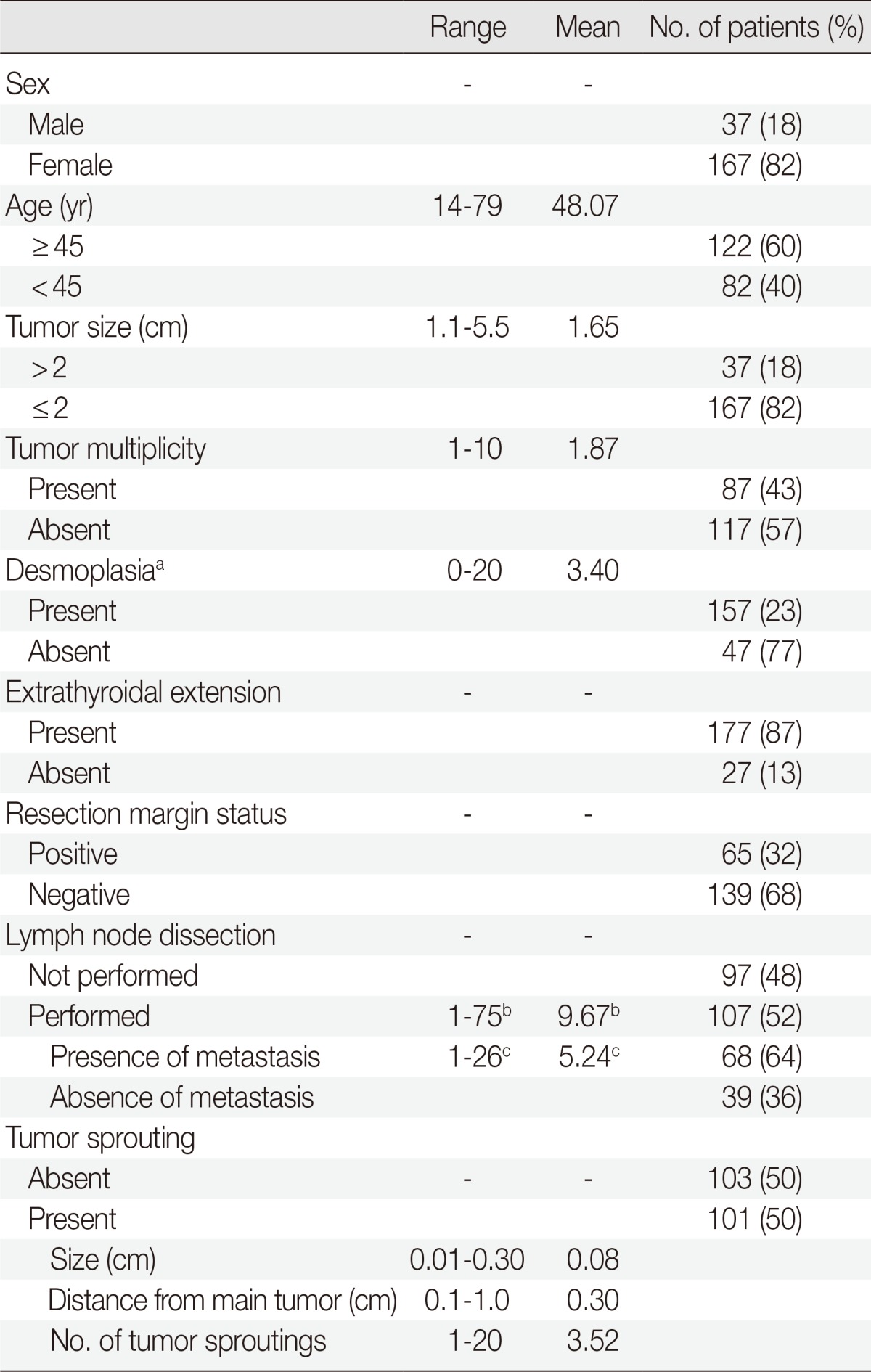
aNumber of desmoplastic areas at ×100 magnification; bRange and mean number of dissected lymph nodes; cRange and mean number of metastatic lymph nodes.
Correlation between tumor sprouting and clinicopathologic characteristics
In the analysis of a total of 204 patients, presence of tumor sprouting was correlated with lymph node metastasis (p<.001), resection margin status (p=.002) and lymphovascular invasion (p=.001). Lymph node metastasis showed an association with male sex (p=.025), tumor size larger than 2 cm (p=.001), positive resection margin (p=.015), and lymphovascular invasion (p<.001). Lymphovascular invasion was more common in the male sex (p=.019). Correlations between tumor recurrence and tumor sprouting (p=.004), lymph node metastasis (p=.001), lymphovascular invasion (p=.040), resection margin status (p=.016), and tumor multiplicity (p=.049) were observed (Table 2).
Table 2.
Tumor sprouting and clinicopathologic characteristics of 204 papillary thyroid carcinoma patients
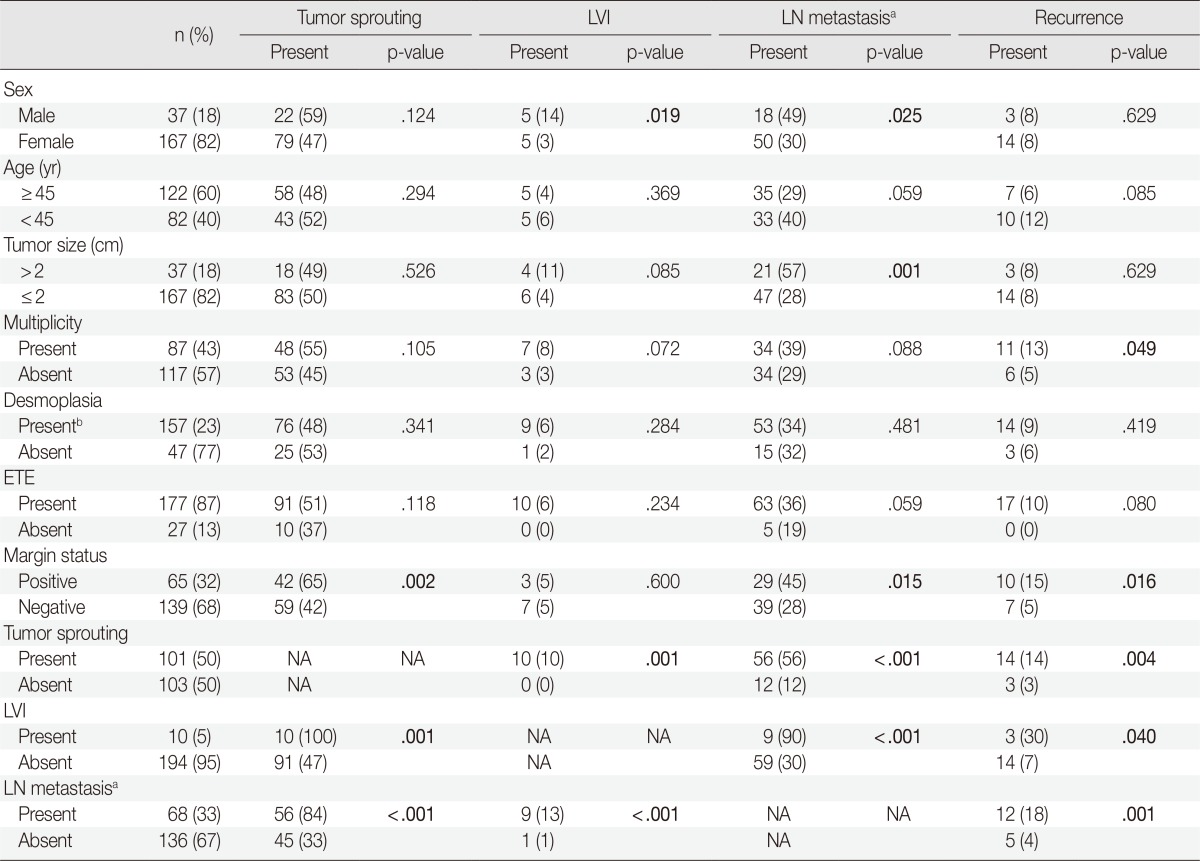
LVI, lymphovascular invasion; LN, lymph node; ETE, extrathyroidal extension; NA, not applicable.
aPatients who had not undergone lymph node dissection are regarded as absence of lymph node metastasis; bTumors with one or more than one ×100 microscopic fields showing desmoplasia.
Analysis of correlations between the clinicopathologic factors was done in the lymph node dissection group (n=107). The presence of tumor sprouting showed an association with lymph node metastasis (p<.001) and lymphovascular invasion (p=.009). Lymph node metastasis was correlated with lymphovascular invasion (p=.014) and male sex (p=.037) and recurrence was correlated with lymph node metastasis (p=.017) and tumor multiplicity (p=.039) (Table 3).
Table 3.
Tumor sprouting and clinicopathologic characteristics of 107 papillary thyroid carcinoma patients with lymph node dissection
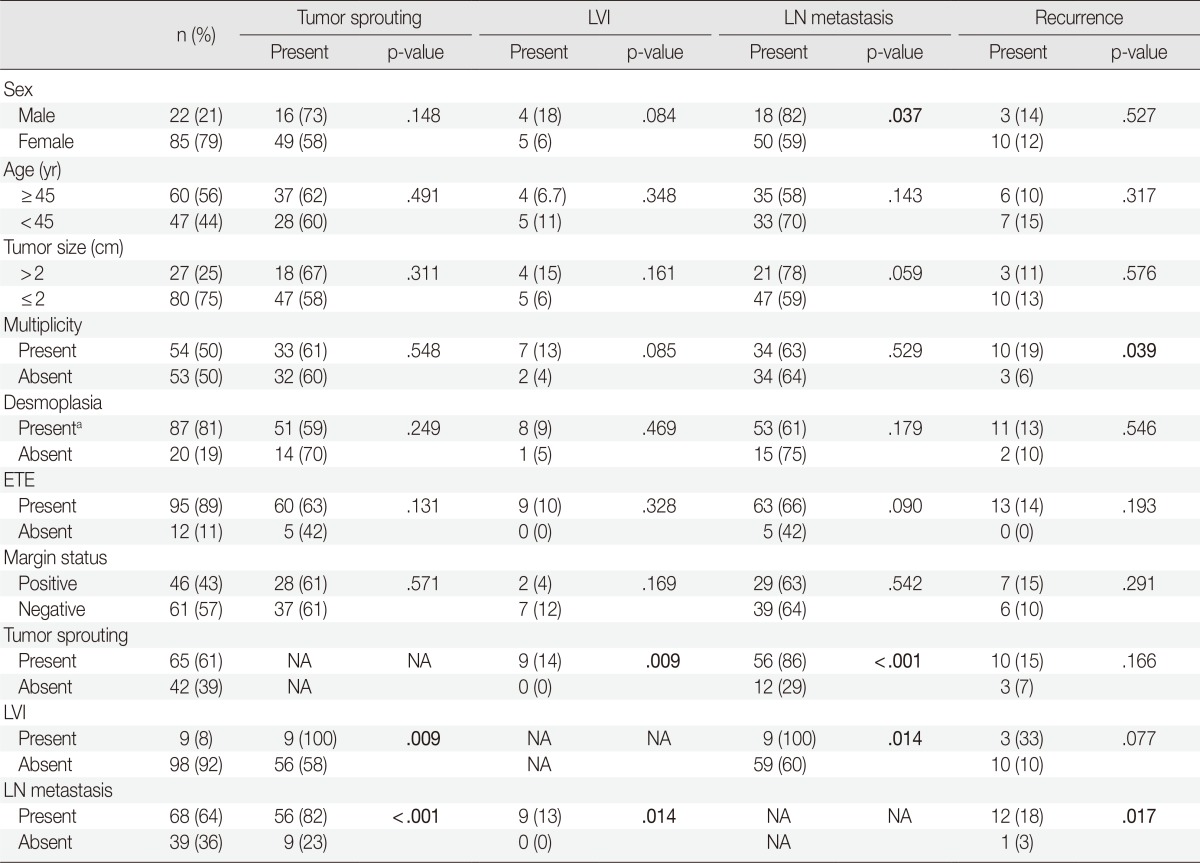
LVI, lymphovascular invasion; LN, lymph node; ETE, extrathyroidal extension; NA, not applicable.
aTumors with one or more than one ×100 microscopic fields showing desmoplasia.
Recurrence-free survival
The mean recurrence time of 17 recurred patients was 14 months and recurrence time ranged from 3 to 36 months. The primary recurrence sites were regional cervical lymph nodes in 13 patients, the thyroid bed in two patients, and both cervical lymph nodes and the thyroid bed in two patients. One patient developed distant metastases to the lung 27 months after the thyroid bed recurrence. Tumor sprouting in thyroidectomy specimens was observed in 14 (82%) out of 17 recurred patients. Twelve (71%) recurred patients had lymph node metastases at the time of the thyroidectomy.
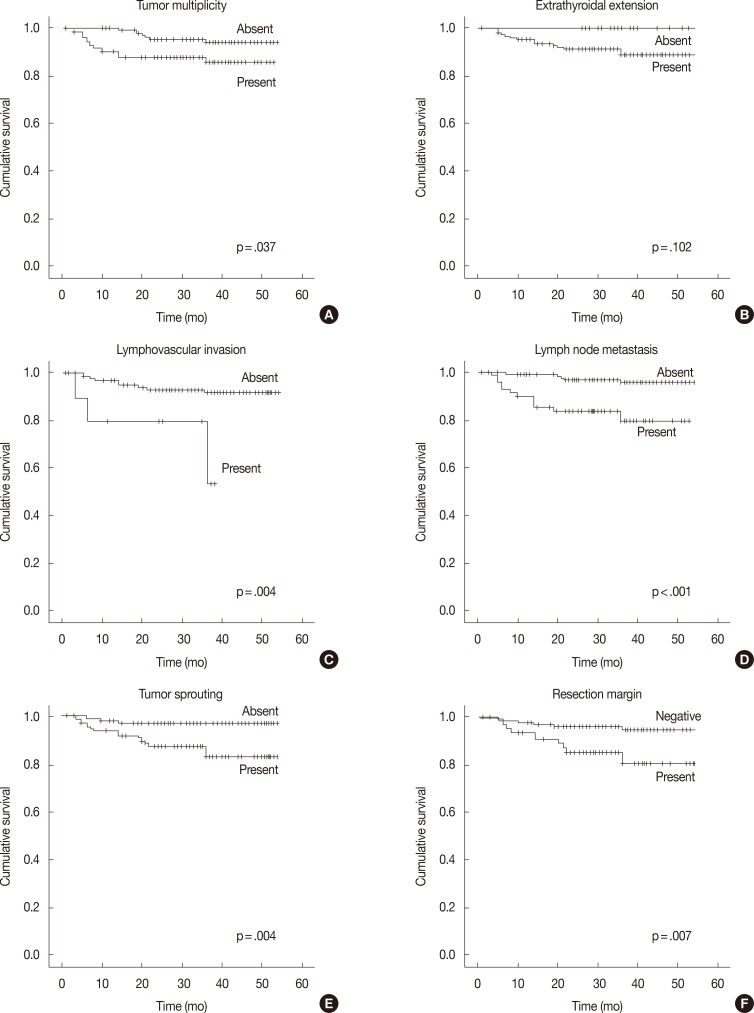
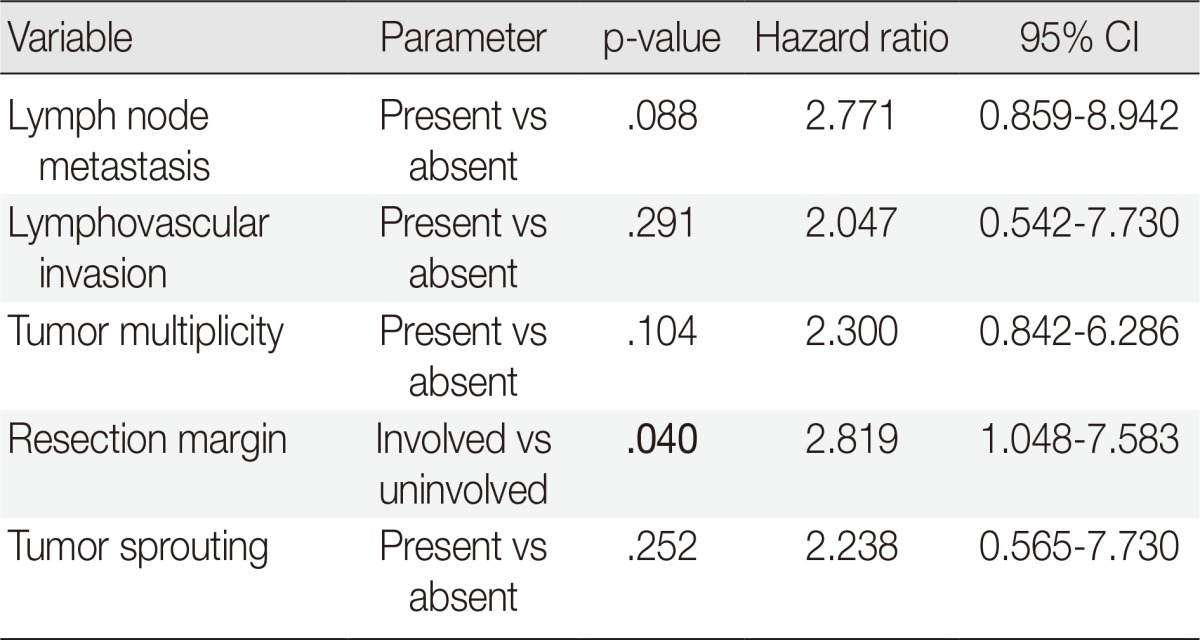
In the univariate survival analysis using a log-rank test, lymph node metastasis (p<.001), tumor sprouting (p=.004), lymphovascular invasion (p=.004), resection margin (p=.009), and tumor multiplicity (p=.037) showed significant associations with recurrence (Fig. 3). Multivariate analysis showed that resection margin (p=.040) is the only independent poor prognostic factor for recurrence (Table 4).
Fig. 3.
Recurrence-free survival analysis of papillary thyroid carcinoma patients. Kaplan-Meier survival plots with tumor multiplicity (A), extrathyroidal extension (B), lymphovascular invasion (C), lymph node metastasis (D), tumor sprouting (E), and resection margin (F) data.
Table 4.
Multivariate analysis results of recurrence-free survival
CI, confidence interval.
DISCUSSION
The main route of tumor spreading in PTC is lymphatic vessels and lymphatic invasion of PTC is associated with lymph node metastasis.18,19,20,21,22 Although lymphatic invasion is an important prognostic factor of recurrence and a predictive factor of lymph node metastasis, the definition of lymphatic invasion is somewhat obscure. Some authors define lymphatic invasion as the presence of an endothelialized tumor cluster, while others define it as an endothelialized tumor cluster accompanied by thrombus formation, or as tumor emboli or cancer cells in lymphatic vessels.24,25 The wide range of prevalence of lymphatic invasion in PTC patients may be due to the unclear definition of lymphatic invasion.20,21,22 In pathology literature, the definition of lymphatic invasion is not uniformly accepted and mainly focuses on lymphovascular invasion, which includes vascular invasion of follicular carcinomas.24,25 The absence of a uniformly accepted definition of lymphovascular invasion in PTC explains the wide range (5% to 81%) of lymphovascular invasion prevalence in previous studies.20,22,26,27,28,29 Because lymphatic invasions of PTC may precede lymph node metastasis and recurrence, establishment of a uniform concept of lymphatic invasion is important. In general, the concept is defined as the presence of an endothelialized tumor cluster or the presence of tumor emboli or cancer cells in lymphatic vessels.24,25 Using these strict criteria, we could find only 10 cases with lymphatic invasion in a total of 204 PTC cases, in which 68 pathologically-confirmed lymph node metastasis cases were present.
During microscopic examination of thyroid resection specimens of PTC patients, we can sometimes see small tumor cluster(s) scattered around the main PTC tumor mass. These tumor clusters lack endothelialization, are not surrounded by lymphatic spaces, and do not exhibit desmoplastic reactions. Although these tumor cell clusters resemble lymphatic tumor emboli, in most cases, ancillary studies such as immunohistochemical staining of CD31, CD34, and D2-40 could not reveal lymphatic endothelial cells. Therefore, we named these tumor clusters 'tumor sprouting' and hypothesized that tumor sprouting would be either a kind of tentacular tumor expansion or lymphatic emboli that could not be easily confirmed by histopathologic examination, and the presence of tumor sprouting and characteristics of tumor sproutings (number, size, and distance from the main mass) would show a correlation with lymph node metastasis.
In this study, we found significant associations between lymph node metastasis, lymphovascular invasion, and tumor sprouting. A linear correlation between the characteristics of tumor sprouting (number, size, and distance from main mass) and the number of metastatic lymph nodes were also observed. In the univariate analysis of recurrence free survival, presence of tumor sprouting was a poor prognostic factor along with lymph node metastasis and lymphovascular invasion. These findings suggest that tumor sprouting can serve as another poor histopathologic prognostic marker that could predict tumor recurrence or lymph node metastasis.
We included only papillary macrocarcinomas with a tumor size that exceeded 1 cm. Because the objective of this study was to examine the correlation between tumor sprouting and lymph node metastasis, papillary microcarcinomas were not included. Lymph node metastasis and tumor sprouting is less frequent in papillary microcarcinomas, although a larger scale patient cohort is essential to verify this correlation. We used recurrence-free survival to evaluate the prognosis of the patients because there was no mortality and only one distant metastasis in our cohort during the follow-up period. A further study with more patients and a long-term follow-up could reveal the effect of tumor sprouting in papillary microcarcinoma and on overall survival.
One representative cross sectional slide was used for the evaluation of tumor sprouting in this study. Inclusion of all slide sections could result in a biased estimation of tumor sprouting number and distance due to the difference in the examined field area as well as differences in tumor size and angle. Tumor clusters too close to the main mass or desmoplastic area were not counted as tumor sprouting because of the difficulty to distinguish these separate tumor clusters from a continuous bulging of the main tumor. To differentially define tumor sprouting from tumor multiplicity, desmoplastic reaction around the tumor cluster and tumor cluster size were adopted as criteria. A desmoplastic reaction around the tumor cluster indicates the extravasation of tumor clusters from lymphatic vessel, so tumor clusters with desmoplastic reactions were counted as multiple tumors regardless of cluster size. Larger tumor clusters often showed desmoplastic reaction in serial sections, where the arbitrarily chosen 0.3 cm was also used as criteria.
Ancillary studies including immunohistochemical staining of cytokeratin 19 (CK19) and HMW-CK were helpful in finding tumor sprouting in the histopathologic slides. All tumor sproutings showed typical nuclear morphology of PTC, but the small size and absence of a peritumoral reaction of tumor sprouting made it difficult to find them on H&E slides. Although CK19 and HMW-CK staining is helpful in finding tumor sproutings, these stains were often positive in benign follicular cells with lymphocytic or Hashimoto's thyroiditis. Confirmation of tumor cell nuclear morphology with H&E stained slides is necessary for the identification of tumor sprouting.
Other clinicopathologic factors mentioned in the literature including tumor size, tumor multiplicity, desmoplasia, extrathyroidal extension, resection margin, lymphovascular invasion, and lymph node metastasis were examined in this study. In the univariate analysis of recurrence free survival, tumor multiplicity, positive resection margin, presence of tumor sprouting, lymphovascular invasion, and lymph node metastasis showed poor prognosis. However, positive resection margin was the only independent poor prognostic factor in the multivariate analysis. Due to a relatively short follow-up period and no mortality during observation, overall survival could not be evaluated. Further studies with a long-term follow-up period may reveal a prognostic impact of tumor sprouting in PTC patients.
In summary, presence of tumor sprouting is significantly correlated with lymph node metastasis and recurrence. Evaluation of tumor sprouting in PTC patients could be helpful in predicting tumor recurrence or lymph node metastasis.
Acknowledgments
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2012R1A1A1008668).
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973-2002. JAMA. 2006;295:2164–2167. doi: 10.1001/jama.295.18.2164. [DOI] [PubMed] [Google Scholar]
- 2.Hundahl SA, Cady B, Cunningham MP, et al. U.S. and German Thyroid Cancer Study Group; An American College of Surgeons Commission on Cancer Patient Care Evaluation study. Initial results from a prospective cohort study of 5583 cases of thyroid carcinoma treated in the United States during 1996. Cancer. 2000;89:202–217. doi: 10.1002/1097-0142(20000701)89:1<202::aid-cncr27>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 3.Samaan NA, Schultz PN, Hickey RC, et al. The results of various modalities of treatment of well differentiated thyroid carcinomas: a retrospective review of 1599 patients. J Clin Endocrinol Metab. 1992;75:714–720. doi: 10.1210/jcem.75.3.1517360. [DOI] [PubMed] [Google Scholar]
- 4.Sherman SI, Angelos P, Ball DW, et al. Thyroid carcinoma. J Natl Compr Canc Netw. 2005;3:404–457. doi: 10.6004/jnccn.2005.0021. [DOI] [PubMed] [Google Scholar]
- 5.Hughes DT, Haymart MR, Miller BS, Gauger PG, Doherty GM. The most commonly occurring papillary thyroid cancer in the United States is now a microcarcinoma in a patient older than 45 years. Thyroid. 2011;21:231–236. doi: 10.1089/thy.2010.0137. [DOI] [PubMed] [Google Scholar]
- 6.Kim KM, Park JB, Bae KS, Kang SJ. Analysis of prognostic factors in patients with multiple recurrences of papillary thyroid carcinoma. Surg Oncol. 2012;21:185–190. doi: 10.1016/j.suronc.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 7.Shah JP, Loree TR, Dharker D, Strong EW, Begg C, Vlamis V. Prognostic factors in differentiated carcinoma of the thyroid gland. Am J Surg. 1992;164:658–661. doi: 10.1016/s0002-9610(05)80729-9. [DOI] [PubMed] [Google Scholar]
- 8.Shaha AR. Implications of prognostic factors and risk groups in the management of differentiated thyroid cancer. Laryngoscope. 2004;114:393–402. doi: 10.1097/00005537-200403000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Cushing SL, Palme CE, Audet N, Eski S, Walfish PG, Freeman JL. Prognostic factors in well-differentiated thyroid carcinoma. Laryngoscope. 2004;114:2110–2115. doi: 10.1097/01.mlg.0000149442.22393.e2. [DOI] [PubMed] [Google Scholar]
- 10.Hay ID, Bergstralh EJ, Goellner JR, Ebersold JR, Grant CS. Predicting outcome in papillary thyroid carcinoma: development of a reliable prognostic scoring system in a cohort of 1779 patients surgically treated at one institution during 1940 through 1989. Surgery. 1993;114:1050–1057. [PubMed] [Google Scholar]
- 11.DeGroot LJ, Kaplan EL, McCormick M, Straus FH. Natural history, treatment, and course of papillary thyroid carcinoma. J Clin Endocrinol Metab. 1990;71:414–424. doi: 10.1210/jcem-71-2-414. [DOI] [PubMed] [Google Scholar]
- 12.Shaha AR, Shah JP, Loree TR. Risk group stratification and prognostic factors in papillary carcinoma of thyroid. Ann Surg Oncol. 1996;3:534–538. doi: 10.1007/BF02306085. [DOI] [PubMed] [Google Scholar]
- 13.Buffet C, Golmard JL, Hoang C, et al. Scoring system for predicting recurrences in patients with papillary thyroid microcarcinoma. Eur J Endocrinol. 2012;167:267–275. doi: 10.1530/EJE-12-0105. [DOI] [PubMed] [Google Scholar]
- 14.Jeon HM, Lim BJ, Chang HS, Hong S. The definition of minimal extrathyroid extension in thyroid pathology by analyzing sizable intra- and extrathyroid blood vessels. Korean J Pathol. 2012;46:548–553. doi: 10.4132/KoreanJPathol.2012.46.6.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Compton CC, Fielding LP, Burgart LJ, et al. Prognostic factors in colorectal cancer. College of American Pathologists Consensus Statement 1999. Arch Pathol Lab Med. 2000;124:979–994. doi: 10.5858/2000-124-0979-PFICC. [DOI] [PubMed] [Google Scholar]
- 16.Sakuragi N, Takeda N, Hareyama H, et al. A multivariate analysis of blood vessel and lymph vessel invasion as predictors of ovarian and lymph node metastases in patients with cervical carcinoma. Cancer. 2000;88:2578–2583. [PubMed] [Google Scholar]
- 17.Kim S, Park HK, Jung HY, et al. ERG immunohistochemistry as an endothelial marker for assessing lymphovascular invasion. Korean J Pathol. 2013;47:355–364. doi: 10.4132/KoreanJPathol.2013.47.4.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schlumberger MJ. Papillary and follicular thyroid carcinoma. N Engl J Med. 1998;338:297–306. doi: 10.1056/NEJM199801293380506. [DOI] [PubMed] [Google Scholar]
- 19.Lin X, Zhu B, Liu Y, Silverman JF. Follicular thyroid carcinoma invades venous rather than lymphatic vessels. Diagn Pathol. 2010;5:8. doi: 10.1186/1746-1596-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koo BS, Choi EC, Yoon YH, Kim DH, Kim EH, Lim YC. Predictive factors for ipsilateral or contralateral central lymph node metastasis in unilateral papillary thyroid carcinoma. Ann Surg. 2009;249:840–844. doi: 10.1097/SLA.0b013e3181a40919. [DOI] [PubMed] [Google Scholar]
- 21.Chung YS, Kim JY, Bae JS, et al. Lateral lymph node metastasis in papillary thyroid carcinoma: results of therapeutic lymph node dissection. Thyroid. 2009;19:241–246. doi: 10.1089/thy.2008.0244. [DOI] [PubMed] [Google Scholar]
- 22.Kim JM, Kim TY, Kim WB, et al. Lymphovascular invasion is associated with lateral cervical lymph node metastasis in papillary thyroid carcinoma. Laryngoscope. 2006;116:2081–2085. doi: 10.1097/01.mlg.0000242118.79647.a9. [DOI] [PubMed] [Google Scholar]
- 23.Koperek O, Asari R, Niederle B, Kaserer K. Desmoplastic stromal reaction in papillary thyroid microcarcinoma. Histopathology. 2011;58:919–924. doi: 10.1111/j.1365-2559.2011.03791.x. [DOI] [PubMed] [Google Scholar]
- 24.Fletcher CD. Diagnostic histopathology of tumors. 3rd ed. Edinburgh: Churchill Livingstone; 2007. [Google Scholar]
- 25.Lim DJ, Baek KH, Lee YS, et al. Clinical, histopathological, and molecular characteristics of papillary thyroid microcarcinoma. Thyroid. 2007;17:883–888. doi: 10.1089/thy.2007.0001. [DOI] [PubMed] [Google Scholar]
- 26.Forest VI, Clark JR, Ebrahimi A, et al. Central compartment dissection in thyroid papillary carcinoma. Ann Surg. 2011;253:123–130. doi: 10.1097/SLA.0b013e3181fc9644. [DOI] [PubMed] [Google Scholar]
- 27.Kahn C, Simonella L, Sywak M, Boyages S, Ung O, O'Connell D. Postsurgical pathology reporting of thyroid cancer in New South Wales, Australia. Thyroid. 2012;22:604–610. doi: 10.1089/thy.2011.0501. [DOI] [PubMed] [Google Scholar]
- 28.Lim YC, Koo BS. Predictive factors of skip metastases to lateral neck compartment leaping central neck compartment in papillary thyroid carcinoma. Oral Oncol. 2012;48:262–265. doi: 10.1016/j.oraloncology.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 29.Jung YY, Lee CH, Park SY, et al. Characteristic tumor growth patterns as novel histomorphologic predictors for lymph node metastasis in papillary thyroid carcinoma. Hum Pathol. 2013;44:2620–2627. doi: 10.1016/j.humpath.2013.07.025. [DOI] [PubMed] [Google Scholar]