Abstract
In this study, we investigated the possible antidepressant-like effect of I. paraguariensis in rats. Rats were treated for four weeks with an aqueous extract of I. paraguariensis in drinking water, following the traditional preparation of this beverage. After the period of treatment, behavioral (elevated plus-maze, open field test, and forced swimming test) and biochemical parameters (lipid peroxidation assay, thiol content, vitamin C levels, and monoamine oxidase activity) were evaluated. Animals were also analyzed on forced swimming test after 24 hours of I. paraguariensis intake. An additional group was injected with selegiline 24 hours and 30 minutes before forced swimming test as positive control. HPLC analysis revealed the profile of I. paraguariensis extract. I. paraguariensis reduced the immobility time on forced swimming test without significant changes in locomotor activity in the open field test. Any anxiolytic/anxiogenic effect of I. paraguariensis was observed in rats through the elevated plus-maze test. The antidepressant-like effect of I. paraguariensis was not accompanied by inhibitory effect on monoamine oxidase activity. There were no significant alterations on lipid peroxidation, thiol content, and vitamin C levels among the groups. In conclusion, aqueous extract of I. paraguariensis decreases the time of immobility in rats suggesting an antidepressant-like effect.
1. Introduction
Depression is a psychiatric illness with a high prevalence in humans reaching 21% of the worldwide population [1]. It is well known that pathophysiology of depression involves a dysfunction in monoamine neurotransmitter circuits in the central nervous system [2]. The treatments of depression have monoamines, their receptors and transporters as a target [2]. There are several classes of synthetic drugs available for treating depression in humans: tricyclics, monoamine oxidase inhibitors, selective serotonin reuptake inhibitors, and atypical antidepressants as mirtazapine, for example [3]. However, even the more recent synthetic antidepressants, like atypical ones, have numerous side effects. These undesirable effects compromise the life quality of the patients and, consequently, their clinical use by causing the relapse of the treatment and the recurrence of the symptoms. Furthermore, about 30% of patients do not present remission under therapy with these drugs, which leads to the association of more than one class of antidepressants beyond other classes of drugs, as atypical antipsychotics. All of these pharmacological combinations predispose the patient to severe side effects [4].
Alternative to synthetic antidepressant drugs, the population uses some phytochemical preparations with relative success. Medicinal herbs have been marketed to treat depression [5], such as Hypericum perforatum commonly known as St. John's wort. H. perforatum has been used for treating mild to moderate forms of depression and its antidepressant effect is attributed to inhibition either of monoamine oxidase (MAO) or serotonin reuptake [6, 7]. Therefore, the search for other natural compounds present in everyday life of the population could be interesting as auxiliary in the antidepressant treatment with fewer side effects than conventional therapy.
In this context, Yerba mate (Ilex paraguariensis) is a beverage commonly consumed in South America especially in Argentina, Brazil, Uruguay, and Paraguay. It is a stimulating beverage traditionally consumed as infusion locally known as “chimarrão” or “mate” [8]. Lately, the I. paraguariensis has gained rapid penetration into the worldwide markets, either as tea itself or as an ingredient in the industries of food and dietary supplement [9]. The I. paraguariensis has a range of biological activities which are attributed to its high polyphenol content. In addition to flavonoids as quercetin and rutin and phenolic compounds as chlorogenic and caffeic acids, yerba mate is also rich in caffeine and saponins [10]. The literature data have demonstrated that I. paraguariensis can improve the cognition of rats treated with acute administration of hydroalcoholic extract probably through its antagonist's action on adenosine receptors [11]. Another study showed that an infusion of I. paraguariensis can improve the memory of rats treated with haloperidol and this effect was related to an indirect modulation of oxidative stress [12]. Oxidative damage is implicated in the pathogenesis of various neuropsychiatric disorders including major depression [13–15]. However, besides innumerous studies about pharmacological properties of I. paraguariensis, any study was drawn to investigate if this plant has antidepressant-like effects.
In the present study, we evaluated the possible antidepressant-like effect of I. paraguariensis by using forced swimming test (FST) in rats. As a large quantity of antidepressant drugs act through monoamine oxidase inhibition, we investigated if the I. paraguariensis could modify the activity of this enzyme. Therefore, other behavioral and oxidative stress parameters were also evaluated considering the possible effects of I. paraguariensis.
2. Material and Methods
2.1. Chemical, Apparatus, and General Procedures
All chemical were of analytical grade. Acetonitrile, formic acid, gallic acid, chlorogenic acid, and caffeic acid were purchased from Merck (Darmstadt, Germany). Quercetin, theobromine, caffeine, rutin, catechin, and kaempferol were acquired from Sigma Chemical Co. (St. Louis, MO, USA). High performance liquid chromatography (HPLC-DAD) was performed with a Shimadzu Prominence Autosampler (SIL-20A) HPLC system (Shimadzu, Kyoto, Japan), equipped with Shimadzu LC-20AT reciprocating pumps connected to a DGU 20A5 degasser with a CBM 20A integrator, SPD-M20A diode array detector, and LC solution 1.22 SP1 software.
2.2. Quantification of Phenolics and Flavonoids Compounds by HPLC-DAD
Reverse phase chromatographic analyses were carried out under gradient conditions using C18 column (4.6 mm × 250 mm) packed with 5 μm diameter particles. The mobile phase was water containing 1% formic acid (A) and acetonitrile (B), and the composition gradient was 13% of B until 10 min and changed to obtain 20%, 30%, 50%, 60%, 70%, 20%, and 10% B at 20, 30, 40, 50, 60, 70, and 80 min, respectively [16], with slight modifications. I. paraguariensis infusion was analyzed by dissolving in ethanol at a concentration of 20 mg/mL. The presence of nine antioxidants compounds was investigated, namely, gallic acid, chlorogenic acid, caffeic acid, catechin, quercetin, rutin, kaempferol, caffeine, and theobromine. Identification of these compounds was performed by comparing their retention time and UV absorption spectrum with those of the commercial standards. The flow rate was 0.7 mL/min, injection volume 50 μL and the wavelengths were 254 nm for gallic acid, 270 nm for theobromine, 280 nm for catechin and caffeine, 327 nm for caffeic and chlorogenic acids, and 366 nm for quercetin, rutin, and kaempferol. All the samples and mobile phase were filtered through 0.45 μm membrane filter (Millipore) and then degassed by ultrasonic bath prior to use. Stock solutions of standards references were prepared in the HPLC mobile phase at a concentration range of 0.045–0.300 mg/mL for kaempferol, quercetin, catechin, rutin, caffeine, and theobromine and 0.030–0.250 mg/mL for gallic, caffeic, and chlorogenic acids. The chromatography peaks were confirmed by comparing their retention time with those of reference standards and by DAD spectra (200 to 400 nm). Calibration curve for gallic acid was Y = 12539x + 1305.3 (r = 0.9997); catechin: Y = 12851x + 1289.5 (r = 0.9998); chlorogenic acid: Y = 13079x + 1195.8 (r = 0.9992); caffeic acid: Y = 11978x + 1326.2 (r = 0.9994); caffeine: Y = 13276x + 1293.6 (r = 0.9995); theobromine: Y = 12473x + 1275.8 (r = 0.9996); rutin: Y = 12763 + 1265.7 (r = 0.9999); quercetin: Y = 11780x + 1362.6 (r = 0.9995); and kaempferol: Y = 12583x + 1238.9 (r = 0.9997). All chromatography operations were carried out at ambient temperature and in triplicate. The limit of detection (LOD) and limit of quantification (LOQ) were calculated based on the standard deviation of the responses and the slope using three independent analytical curves. LOD and LOQ were calculated as 3.3 and 10 σ/S, respectively, where σ is the standard deviation of the response and S is the slope of the calibration curve [17].
2.3. Animals
Male Wistar rats (with 2 months of age) weighing 200 to 250 g from our breeding colony were kept in cages with five animals each with continuous access to food and water or infusion of I. paraguariensis. The room housing the cages was temperature-controlled (22 ± 2°C) and on a 12 h light/dark cycle with the lights going on at 7:00 a.m. Animals were maintained and used in accordance to the guidelines of the Brazilian Association for Laboratory Animal Science (Ethics Committee Approval number A011-09).
2.4. Experimental Design
I. paraguariensis was obtained from local supermarkets in a form of herb consumed by population. The extract was prepared as infusion like “chimarrão” or “mate.” Herbal commercial samples (25 g) were weighed and put into 500 mL of hot water (70°C) [12]. The infusion was filtered using filter paper and then cooled to room temperature. The yield of mate infusion was 30.45%. An aliquot of the infusion was subjected to qualitative analyses by HPLC. The extracts of mate were daily prepared and offered to the animals in place of drinking water during four weeks [12] ad libitum. Any difference of liquid ingestion was observed between the groups (data not shown). The dose of dry extract per rat was calculated with the values obtained in gravimetric assay and estimated at 2.31 g/kg/day. After four weeks, behavioral analysis of locomotor activity and anxiety was evaluated in animals receiving water (n = 11) or I. paraguariensis (n = 9). Our intent with elevated plus-maze and open field tests was to avoid any possible false positive result in the FST, since the alterations in locomotion or anxiety could modify the response of the animals in FST. After this evaluation, the group that received water was subdivided into two groups. One group of animals received two administrations of selegiline (10 mg/kg, i.p., dissolved in 0.9% NaCl), 24 hours and 30 minutes, before FST [18]. This group was the positive control to antidepressant activity and MAO inhibition. Thus, the experimental groups were named as: control (n = 6), I. paraguariensis (n = 9), and selegiline (n = 5). After the behavioral analysis, the animals were killed by decapitation and the brains were immediately excised and used in biochemical assays.
2.5. Behavioral Analysis
2.5.1. Elevated Plus-Maze
To evaluate possible alterations in anxiety-like state caused by treatment with I. paraguariensis, animals were exposed to an elevated plus-maze apparatus [19, 20]. The percentage of time spent on open arm and the percentage of the entries into the open arms were calculated as follows: time spent or number of entries into the open arm/total time or total number of the entries into closed and open arms × 100, respectively.
2.5.2. Open Field Test
To analyze possible changes in spontaneous locomotor and exploratory activity caused by treatment with I. paraguariensis, the animals were placed individually in the center of a circular open field arena divided into nine parts [21]. The effect of drugs on behavior was examined after I. paraguariensis treatment (on day 30). The number of rearing and the number of line crossings were measured over 5 min. Sections of open field test were evaluated 1 hour after elevated plus maze test.
2.5.3. Forced Swimming Test
This experiment was performed using the FST according to the method previously published by Porsolt et al. [22, 23]. The effects of I. paraguariensis on FST were investigated after 24 hours and 4 weeks of treatment. Male rats were placed into a cylinder with a diameter of 40 cm containing a column of 17 cm of water at 27°C. The animals were trained 24 hours before the test for 5 min. Twenty-four hours later, the animals were exposed to the same experimental conditions for 5 min. A rat was judged to be immobile whenever it remained floating in the water, in an upright position, making only small movements to keep its head above the water. The immobility time was taken.
2.6. Tissue Preparations
Rats were killed about 1 hour after the last session of behavioral. The brains were immediately excised and put on ice. Then, they were homogenized in 10 volumes (w/v) of 10 mM Tris-HCl, pH 7.4. The homogenates were centrifuged at 4000 ×g for 10 min to yield a low-speed supernatant fraction (S1) that was used for the biochemical assays.
2.7. Biochemical Assays
2.7.1. Lipid Peroxidation Assay
To evaluate the participation of lipid peroxidation in the action of I. paraguariensis or selegiline, thiobarbituric acid reactive species (TBARS) were determined as described by [24]. In brief, samples were incubated at 100°C for 1 hour in a medium containing 8.1% sodium dodecyl sulfate, 1.4 M acetic acid, pH 3.4, and 0.6% thiobarbituric acid. The pink chromogen produced in the reaction was measured spectrophotometrically at 532 nm. Results were expressed as nmol of TBARS/g of tissue.
2.7.2. SH Levels
The total SH (TSH) and non-protein-SH (NPSH) content from samples were determined as described by [25]. For the nonprotein thiol groups (NP) determination, the samples of S1 were precipitated with 200 μL of 10% trichloroacetic acid followed by centrifugation. The colorimetric assay was carried out in phosphate buffer 1 M, pH 7.4. The reaction was measured spectrophotometrically at 412 nm. Results were expressed as μg/g of tissue.
2.7.3. Vitamin C Levels
Cerebral vitamin C (ascorbic acid (AA)) levels were determined as described by Jacques-Silva et al. [26]. Brain homogenates were precipitated with 1 volume of 10% trichloroacetic acid followed by centrifugation. An aliquot of 300 μL of the supernatants was mixed with 2,4-dinitrophenylhydrazine (4.5 mg/mL), CuSO4 (0.075 mg/mL), and trichloroacetic acid 13.3% (final volume 1 mL) and incubated for 3 h at 37°C. Then 1 mL of H2SO4 65% (v/v) was added to the medium. The ascorbic acid levels were measured spectrophotometrically at 520 nm and calculated using a standard curve (1.5–4.5 μM ascorbic acid freshly prepared in sulfuric acid).
2.7.4. MAO Activity Assay
MAO activity was determined by measuring the kynuramine oxidation to 4-hydroxyquinoline [27–29]. The samples were preincubated at 37°C for 10 min with the irreversible and selective inhibitor clorgyline (250 nM) or pargyline (250 nM) to assay MAO-A or MAO-B activity, respectively. After 10 min, kynuramine was added as a nonselective substrate at concentrations equal to the corresponding Km value (45 μM for MAO-A and 30 μM for MAO-B). The reaction was incubated during 30 min at 37°C. After this time, the reaction was stopped with trichloroacetic acid (TCA) 10%. The samples were centrifuged at 5.000 ×g for 5 min. It was added to supernatant 1 M NaOH. The reaction was measured by fluorimetric method, using 315 nm (excitation) and 380 nm (emission). The results are represented as fluorescence intensity/mg protein.
2.7.5. Protein Quantification
The total protein content in homogenates (S1) was determined by the method of Lowry and Rosebrough [30], using bovine serum albumin as standard.
2.8. Statistical Analysis
Data were analyzed by unpaired t-test or one-way ANOVA, followed by Tukey's post hoc test when appropriate. Significance was considered when P < 0.05.
3. Results
3.1. HPLC Analysis
HPLC fingerprinting of Ilex paraguariensis infusion revealed the presence of the gallic acid (t R = 10.17 min; peak 1), catechin (t R = 16.23 min; peak 2), chlorogenic acid (t R = 22.56 min; peak 3), caffeic acid (t R = 24.97 min; peak 4), caffeine (t R = 31.48 min; peak 5), theobromine (t R = 37.12 min; peak 6), rutin (t R = 41.73 min; peak 7), quercetin (t R = 50.14 min; peak 8), and kaempferol (t R = 57.39 min; peak 9) (Figure 1 and Table 1).
Figure 1.
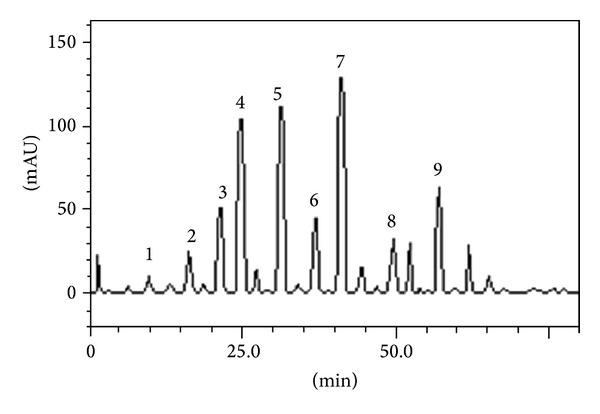
Representative high performance liquid chromatography profile of Ilex paraguariensis infusion,detection of UV was at 327 nm. Gallic acid (peak 1), catechin (peak 2), chlorogenic acid (peak 3), caffeic acid (peak 4), caffeine (peak 5), theobromine (peak 6), rutin (peak 7), quercetin (peak 8), and kaempferol (peak 9).
Table 1.
Composition of Ilex paraguariensis infusion.
| Compounds | Ilex paraguariensis |
LOD μg/mL |
LOQ μg/mL |
|
|---|---|---|---|---|
| mg/g | % | |||
| Gallic acid | 0.65 ± 0.01a | 0.06 | 0.016 | 0.052 |
| Catechin | 1.74 ± 0.03b | 0.17 | 0.029 | 0.095 |
| Chlorogenic acid | 5.31 ± 0.01c | 0.53 | 0.008 | 0.027 |
| Caffeic acid | 12.26 ± 0.03d | 1.22 | 0.035 | 0.115 |
| Caffeine | 13.47 ± 0.01d | 1.34 | 0.015 | 0.049 |
| Theobromine | 5.03 ± 0.01c | 0.50 | 0.007 | 0.023 |
| Rutin | 17.82 ± 0.03e | 1.78 | 0.026 | 0.086 |
| Quercetin | 3.16 ± 0.01b | 0.31 | 0.032 | 0.104 |
| Kaempferol | 7.58 ± 0.02f | 0.75 | 0.019 | 0.063 |
Results are expressed as mean ± standard deviations (SD) of three determinations.
Averages followed by different letters differ by Tukey's test at P < 0.05.
3.2. Effects of Treatment with I. paraguariensis on Elevated Plus-Maze Test in Rats
I. paraguariensis did not cause any significant effect on the number of head dipping, percentage of the time spent, and number of entries on the open arms of elevated plus-maze apparatus (Table 2).
Table 2.
Effects of I. paraguariensis on elevated plus-maze test in rats during 5 minutes.
| Head dipping (5 min) |
Entry into open arms (%) | Time spent into open arms (%) | |
|---|---|---|---|
| Control | 6.07 ± 1.60 | 17.93 ± 4.32 | 9.67 ± 3.40 |
| I. paraguariensis | 6.13 ± 0.96 | 18.10 ± 3.19 | 14.91 ± 5.31 |
Values are represented as means ± SEM (control, n = 11; I. paraguariensis, n = 9).
3.3. Effects of Treatment with I. paraguariensis on Locomotor and Exploratory Activity in Rats
I. paraguariensis treatment did not cause any effect on locomotor activity represented by number of crossings (Figure 2(a)). However, the number of rearing on the open field test was observed, I. paraguariensis caused a decrease in the number of rearing (P < 0.05; Figure 2(b)) in the open field test after 4 weeks of treatment.
Figure 2.
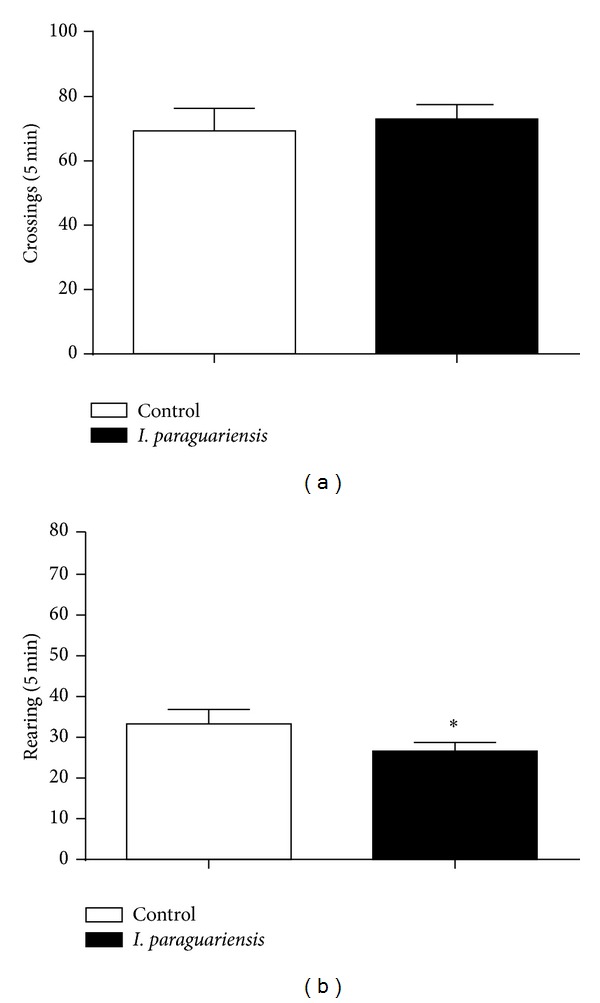
Effects of I. paraguariensis in the open field test in rats after 4 weeks of treatment. (a) Number of crossings and (b) rearing in 5 min. Values of number of crossings and rearing are represented by means ± SEM; control, n = 11; I. paraguariensis, n = 9. *Significant differences from control group.
3.4. Effects of Treatment with I. paraguariensis on Forced Swimming Test in Rats
To verify the possible antidepressant-like effects of I. paraguariensis, the FST was used. Statistical analyses revealed that aqueous extract of I. paraguariensis caused a significant reduction of immobility time in FST in relation to control group either when administered during 24 hours (F(2,11) = 18.73, P < 0.05; Figure 3(a)) or 4 weeks (F(2,19) = 25.45, P < 0.05; Figure 3(b)). Furthermore, selegiline, a MAO inhibitor, caused a significant reduction in immobility time in relation to control and I. paraguariensis group in this test (P < 0.05; Figure 3).
Figure 3.
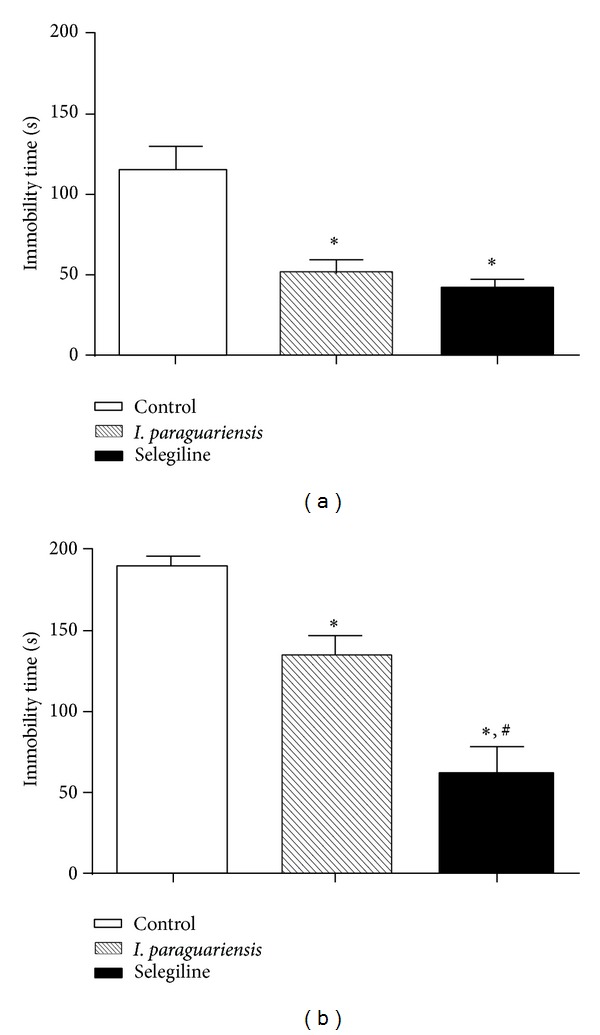
Effects of I. paraguariensis and selegiline on forced swimming test after (a) 24 hours and (b) 4 weeks of treatment with I. paraguariensis or two administrations (24 hours and 30 min before the test) of selegiline (10 mg/kg; i.p.). Values of immobility time are represented by means ± SEM (a); control, n = 4; I. paraguariensis, n = 5; and selegiline, n = 5; (b) control, n = 6; I. paraguariensis, n = 9; and selegiline, n = 5 (one-way ANOVA followed by Tukey's multiple comparison test). * represents significant differences from control group and # represents significant differences from I. paraguariensis group.
3.5. Effects of I. paraguariensis on MAO-A and MAO-B Activity
Since we detected a reduction in immobility in forced swimming test in rats treated with I. paraguariensis, we verified if this effect was caused by a possible inhibitory effect of I. paraguariensis on MAO-A or MAO-B activity (Figure 4). Selegiline administration was a positive control and caused a significant reduction in MAO-B activity (F(2,11) = 78.80, P < 0.05; Figure 4(c)). However, the activities of either MAO-A or MAO-B were not modified after treatment with I. paraguariensis (Figure 4).
Figure 4.
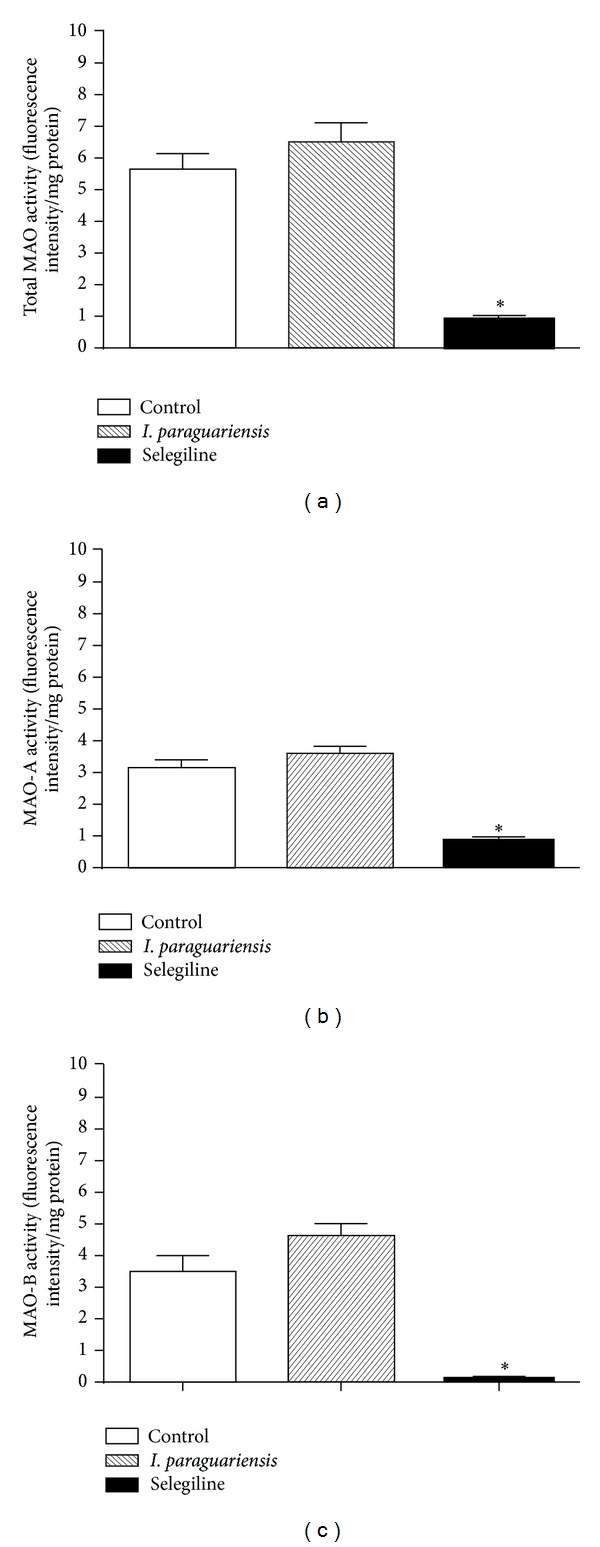
Effects of I. paraguariensis or selegiline on monoamine oxidase activity after 4 weeks of treatment with I. paraguariensis or two administrations (24 hours and 30 min before the test) of selegiline (10 mg/kg; i.p.). Values represent means ± SEM; control, n = 6; I. paraguariensis, n = 9; and selegiline, n = 5. * represents significant differences from control group and I. paraguariensis group.
3.6. Effects of I. paraguariensis on Oxidative Stress Parameters
As I. paraguariensis and selegiline are known by their antioxidant activity, we carried out assays for lipid peroxidation, levels of nonprotein SH, and ascorbic acid to verify possible changes in oxidative stress parameters that could modulate the MAO activity or could be a signal of protection/toxicity of both tested drugs. However, there was no significant difference among the groups in TBARS, nonprotein SH, and ascorbic acid levels in rats under treatment with I. paraguariensis (Table 3).
Table 3.
Effects of I. paraguariensis or selegiline treatments on oxidative stress parameters.
| TBARS (nmol of MDA/g tissue) |
Nonprotein—SH (μmol/g tissue) |
Vitamin C (μg of ascorbic acid/g tissue) |
|
|---|---|---|---|
| Control | 148.30 ± 26.78 | 6.33 ± 1.22 | 608.70 ± 73.30 |
| I. paraguariensis | 157.60 ± 14.83 | 5.99 ± 0.61 | 643.10 ± 27.09 |
| Selegiline | 210.20 ± 44.01 | 3.69 ± 0.23 | 739.20 ± 47.80 |
Mean ± SEM; with control, n = 6; I. paraguariensis, n = 9; selegiline, n = 5.
4. Discussion
The present study aimed to evaluate the possible antidepressant-like effect of I. paraguariensis in rats. We demonstrated that the treatment with I. paraguariensis decreased the immobility time on FST at 24 hours and this effect was the same after four weeks; a behavioral parameter was used to investigate the antidepressant potential of the drugs. Furthermore, the reduction in time of immobility by I. paraguariensis on FST was not associated with effects neither on locomotor nor on anxiolytic/anxiogenic activity. The antidepressant-like effect of I. paraguariensis was not associated with inhibitory effects on monoamine oxidase activity.
The Literature showed that I. paraguariensis has stimulant effects on central nervous system. Thus, the purpose of this study was to evaluate the antidepressant-like effect of I. paraguariensis. Despite previous some studies show the antidepressant-like activity of flavonoids [31, 32] which are present in the extract of I. paraguariensis, any study has evaluated the possible antidepressant-like activity of it. The present study showed that I. paraguariensis significantly reduced the immobility time on FST either after 24 hours or four weeks of treatment. HPLC analysis revealed the presence of caffeine in our extract. The percentage of caffeine was a little lesser than other ones [33, 34]. These differences can be attributed to the method of extraction and the different trademarks used in these studies. Previous studies demonstrated that caffeine exerts stimulant effects on motor activity of mice and rats [35]. Thus, in order to exclude a false positive, we use the open field test to verify possible alterations in locomotor activity in rats caused by I. paraguariensis. The treatment did not cause any effect on locomotor activity represented by the number of crossings, but when the number of rearing on the open field test was observed, Ilex paraguariensis caused a decrease in this number. These data can suggest that other compounds present in extract are able to decrease the number of rearing since caffeine has no effect on the central control of vertical activity [36]. In the same way, we evaluated if the presence of stimulants compounds like caffeine and theobromine in the extract of I. paraguariensis could cause anxiety. Furthermore, some studies have demonstrated that antidepressant agents can also possess anxiolytic properties in different anxiety animal models [36–38]. Thus, we investigated if I. paraguariensis treatment exerts anxiety or anxiolytic activity by using the plus-maze test. However, any significant effect was observed on elevated plus-maze test.
Monoamine oxidase is an enzyme responsible for degradation of monoamines, such as serotonin, dopamine, and norepinephrine. Moreover, the abnormal activity of the enzyme has been implicated in pathophysiology of depression [39–41]. The inhibition of MAO activity promotes an increase in monoamines and is one important target for the treatment of depression. Monoamine oxidase inhibitors have been used for decades in the treatment of depression and their antidepressant properties result from selective MAO-A inhibition in the central nervous system, which could lead to increased brain levels of 5-HT, NE, and DA [42, 43]. Recently, selegiline, an MAO-B inhibitor, has been used with success in the treatment of patients that are refractory to other antidepressant in a pharmaceutical transdermal preparation to avoid food interactions [44, 45]. We also investigated the involvement of the MAO in the possible antidepressant-like effect of I. paraguariensis. In the present study, MAO activity was not modified after treatment with I. paraguariensis. Thus, the antidepressant-like effect of Ilex paraguariensis represented by decrease in immobility time on FST seems not to be associated with MAO inhibition. Natural products have demonstrated numerous benefits in several animal models suggesting that antioxidant compound could exert protective effects [46–50]. However, there are studies showing that depending on the dose of the antioxidant compounds used, they could be prooxidants, causing toxicity and modulation in MAO activity by redox alteration [51, 52]. Selegiline was used as a positive control in our study because it possesses both MAO inhibitory activity and antioxidant properties [53]. We did not observe significant difference in oxidative stress parameters in this model. However, as we used a total brain homogenate in our study, some effects could better appear if they were analyzed in brain specific region.
5. Conclusion
In conclusion, the present study showed that Ilex paraguariensis presents an important effect on reducing immobility time on forced swimming test which could suggest an antidepressant-like effect of this extract. This effect is not associated with MAO inhibition or alterations in the parameters of oxidative stress. Furthermore, the extract did not show anxiolytic or anxiogenic activity. However, additional studies are needed to better understand the action mechanism of I. paraguariensis.
Acknowledgments
The financial support by PRONEM no. 11/2029-1, CNPq (473365/2009-0), and FINEP Research Grant from “Rede Instituto Brasileiro de Neurociência (IBN-Net)” (01.06.0842-00) are gratefully acknowledged. Alcindo Busanello and Luis Ricardo Peroza are recipients of CAPES-DS fellowship. Caroline Queiroz Leal received a fellowship from CNPq/PIBIC.
Conflict of Interests
The authors declare that they have no conflict of interests.
References
- 1.Schechter LE, Ring RH, Beyer CE, et al. Innovative approaches for the development of antidepressant drugs: current and future strategies. The Journal of American Society for Experimental Neuro Therapeutics. 2005;2(4):590–611. doi: 10.1602/neurorx.2.4.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nemeroff CB, Owens MJ. Treatment of mood disorders. Nature Neuroscience. 2002;5:1068–1070. doi: 10.1038/nn943. [DOI] [PubMed] [Google Scholar]
- 3.Belmaker RH, Agam G. Major depressive disorder. The New England Journal of Medicine. 2008;358(1):55–68. doi: 10.1056/NEJMra073096. [DOI] [PubMed] [Google Scholar]
- 4.Millan MJ. Multi-target strategies for the improved treatment of depressive states: conceptual foundations and neuronal substrates, drug discovery and therapeutic application. Pharmacology and Therapeutics. 2006;110(2):135–370. doi: 10.1016/j.pharmthera.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 5.Chambers CD, Hernandez-Diaz S, Van Marter LJ, et al. Selective serotonin-reuptake inhibitors and risk of persistent pulmonary hypertension of the newborn. The New England Journal of Medicine. 2006;354(6):579–587. doi: 10.1056/NEJMoa052744. [DOI] [PubMed] [Google Scholar]
- 6.Istikoglou CL, Mavreas V, Geroulanos G. History and therapeutic properties of Hypericum Perforatum from antiquity until today. Psychiatrike. 2010;21(4):332–338. [PubMed] [Google Scholar]
- 7.Butterweck V. MEchanism of action of St John's wort in depression: what is known? CNS Drugs. 2003;17(8):539–562. doi: 10.2165/00023210-200317080-00001. [DOI] [PubMed] [Google Scholar]
- 8.Mazzafera P. Mate drinking: caffeine and phenolic acid intake. Food Chemistry. 1997;60(1):67–71. [Google Scholar]
- 9.Heck CI, De Mejia EG. Yerba mate tea (Ilex paraguariensis): a comprehensive review on chemistry, health implications, and technological considerations. Journal of Food Science. 2007;72(9):R138–R151. doi: 10.1111/j.1750-3841.2007.00535.x. [DOI] [PubMed] [Google Scholar]
- 10.Bastos DHM, De Oliveira DM, Matsumoto RLT, Carvalho PO, Ribeiro ML. Yerba mate: pharmacological properties, research and biotechnology. Medicinal and Aromatic Plant Science and Biotechnology. 2007;1(1):37–46. [Google Scholar]
- 11.Prediger RDS, Fernandes MS, Rial D, et al. Effects of acute administration of the hydroalcoholic extract of mate tea leaves (Ilex paraguariensis) in animal models of learning and memory. Journal of Ethnopharmacology. 2008;120(3):465–473. doi: 10.1016/j.jep.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 12.Colpo G, Trevisol F, Teixeira AM, et al. Ilex paraguariensis has antioxidant potential and attenuates haloperidol-induced orofacial dyskinesia and memory dysfunction in rats. Neurotoxicity Research. 2007;12(3):171–180. doi: 10.1007/BF03033914. [DOI] [PubMed] [Google Scholar]
- 13.Bilici M, Efe H, Köroğlu MA, Uydu HA, Bekaroğlu M, Değer O. Antioxidative enzyme activities and lipid peroxidation in major depression: alterations by antidepressant treatments. Journal of Affective Disorders. 2001;64(1):43–51. doi: 10.1016/s0165-0327(00)00199-3. [DOI] [PubMed] [Google Scholar]
- 14.Khanzode SD, Dakhale GN, Khanzode SS, Saoji A, Palasodkar R. Oxidative damage and major depression: the potential antioxidant action of selective serotonin-re-uptake inhibitors. Redox Report. 2003;8(6):365–370. doi: 10.1179/135100003225003393. [DOI] [PubMed] [Google Scholar]
- 15.Reis EM, Röpke J, Busanello A, et al. Effect of Hypericum perforatum on different models of movement disorders in rats. Behavioural Pharmacology. 2013;24:623–627. doi: 10.1097/FBP.0b013e3283656d68. [DOI] [PubMed] [Google Scholar]
- 16.Kamdem JP, Olalekana EO, Hassan W, et al. Trichilia catigua (Catuaba) bark extract exerts neuroprotection against oxidative stress induced by different neurotoxic agents in rat hippocampal slices. Industrial Crops and Products. 2013;50:625–632. [Google Scholar]
- 17.Boligon AA, Kubiça TF, Mario DN, et al. Antimicrobial and antiviral activity-guided fractionation from Scutia buxifolia Reissek extracts. Acta Physiologiae Plantarum. 2013;35(7):2229–2239. [Google Scholar]
- 18.Kitamura Y, Kitagawa K, Kimoto S, et al. Selegilin exerts antidepressant-like effects during the forced swim test in adrenocorticotropic hormone-treated rats. Journal of Pharmacological Sciences. 2008;106(4):639–644. doi: 10.1254/jphs.fp0072150. [DOI] [PubMed] [Google Scholar]
- 19.Chopin P, Stenger A, Couzinier JP, Briley M. Indirect dopaminergic effects of tofisopam, a 2,3-benzodiazepine, and their inhibition by lithium. Journal of Pharmacy and Pharmacology. 1985;37(12):917–919. doi: 10.1111/j.2042-7158.1985.tb05003.x. [DOI] [PubMed] [Google Scholar]
- 20.Pellow S, Chopin P, File SE, Briley M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. Journal of Neuroscience Methods. 1985;14(3):149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- 21.Broadhurst PL. The place of animal psychology in the development of psychosomatic research. Fortschritte der Psychosomatischen Medizin. 1960;1:63–69. doi: 10.1159/000386484. [DOI] [PubMed] [Google Scholar]
- 22.Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266(5604):730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- 23.Porsolt RD, Anton G, Blavet N, Jalfre M. Behavioural despair in rats: a new model sensitive to antidepressant treatments. European Journal of Pharmacology. 1978;47(4):379–391. doi: 10.1016/0014-2999(78)90118-8. [DOI] [PubMed] [Google Scholar]
- 24.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Analytical Biochemistry. 1979;95(2):351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 25.Ellman GL. Tissue sulfhydryl groups. Archives of Biochemistry and Biophysics. 1959;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 26.Jacques-Silva MC, Nogueira CW, Broch LC, Flores EM, Rocha JB. Diphenyl diselenide and ascorbic acid changes deposition of selenium and ascorbic acid in liver and brain of mice. Pharmacology and Toxicology. 2001;88(3):119–125. doi: 10.1034/j.1600-0773.2001.d01-92.x. [DOI] [PubMed] [Google Scholar]
- 27.Soto-Otero R, Méndez-Álvarez E, Hermida-Ameijeiras Á, Sánchez-Sellero I, Cruz-Landeira A, Lamas ML-R. Inhibition of brain monoamine oxidase activity by the generation of hydroxyl radicals potential implications in relation to oxidative stress. Life Sciences. 2001;69(8):879–889. doi: 10.1016/s0024-3205(01)01178-x. [DOI] [PubMed] [Google Scholar]
- 28.Sant' Anna GDS, Machado P, Sauzem PD, et al. Ultrasound promoted synthesis of 2-imidazolines in water: a greener approach toward monoamine oxidase inhibitors. Bioorganic and Medicinal Chemistry Letters. 2009;19(2):546–549. doi: 10.1016/j.bmcl.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 29.Villarinho JG, Fachinetto R, Pinheiro F, et al. Antidepressant-like effect of the novel MAO inhibitor 2-(3, 4-dimethoxy-phenyl)-4, 5-dihydro-1H-imidazole (2-DMPI) in mice. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2012;39(1):31–39. doi: 10.1016/j.pnpbp.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 30.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. The Journal of Biological Chemistry. 1951;193(1):265–275. [PubMed] [Google Scholar]
- 31.Butterweck V, Jürgenliemk G, Nahrstedt A, Winterhoff H. Flavonoids from Hypericum perforatum show antidepressant activity in the forced swimming test. Planta Medica. 2000;66(1):3–6. doi: 10.1055/s-2000-11119. [DOI] [PubMed] [Google Scholar]
- 32.Nöldner M, Schötz K. Rutin is essential for the antidepressant activity of Hypericum perforatum extracts in the forced swimming test. Planta Medica. 2002;68(7):577–580. doi: 10.1055/s-2002-32908. [DOI] [PubMed] [Google Scholar]
- 33.Filip R, Sebastian T, Ferraro G, Anesini C. Effect of Ilex extracts and isolated compounds on peroxidase secretion of rat submandibulary glands. Food and Chemical Toxicology. 2007;45(4):649–655. doi: 10.1016/j.fct.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 34.Meinhart AD, Bizzotto CS, Ballus CA, et al. Methylxanthines and phenolics content extracted during the consumption of mate (IIex paraguariensis St. HiI) beverages. Journal of Agricultural and Food Chemistry. 2010;58(4):2188–2193. doi: 10.1021/jf903781w. [DOI] [PubMed] [Google Scholar]
- 35.Nehlig A, Daval J-L, Debry G. Caffeine and the central nervous system: mechanisms of action, biochemical, metabolic and psychostimulant effects. Brain Research Reviews. 1992;17(2):139–169. doi: 10.1016/0165-0173(92)90012-b. [DOI] [PubMed] [Google Scholar]
- 36.Hughes RN, Greig AM. Effects of caffeine, methamphetamine and methylphenidate on reactions to novelty and activity in rats. Neuropharmacology. 1976;15(11):673–676. doi: 10.1016/0028-3908(76)90035-6. [DOI] [PubMed] [Google Scholar]
- 37.De Angelis L. Experimental anxiety and antidepressant drugs: the effects of moclobemide, a selective reversible MAO-A inhibitor, fluoxetine and imipramine in mice. Naunyn-Schmiedeberg's Archives of Pharmacology. 1996;354(3):379–383. doi: 10.1007/BF00171072. [DOI] [PubMed] [Google Scholar]
- 38.Eroğlu L, Güven Ö. The effects of moclobemide on the yohimbine-induced anxiogenic action in the elevated plus-maze. Pharmacological Research. 1998;37(2):137–143. doi: 10.1006/phrs.1997.0275. [DOI] [PubMed] [Google Scholar]
- 39.Lanni C, Govoni S, Lucchelli A, Boselli C. Depression and antidepressants: molecular and cellular aspects. Cellular and Molecular Life Sciences. 2009;66(18):2985–3008. doi: 10.1007/s00018-009-0055-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Manji HK, Quiroz JA, Sporn J, et al. Enhancing neuronal plasticity and cellular resilience to develop novel, improved therapeutics for difficult-to-treat depression. Biological Psychiatry. 2003;53(8):707–742. doi: 10.1016/s0006-3223(03)00117-3. [DOI] [PubMed] [Google Scholar]
- 41.Millan MJ. The role of monoamines in the actions of established and “novel” antidepressant agents: a critical review. European Journal of Pharmacology. 2004;500(1–3):371–384. doi: 10.1016/j.ejphar.2004.07.038. [DOI] [PubMed] [Google Scholar]
- 42.López-Muñoz F, Álamo C, Juckel G, Assion H-J. Half a century of antidepressant drugs—on the clinical introduction of monoamine oxidase inhibitors, tricyclics, and tetracyclics—part I: monoamine oxidase inhibitors. Journal of Clinical Psychopharmacology. 2007;27(6):555–559. doi: 10.1097/jcp.0b013e3181bb617. [DOI] [PubMed] [Google Scholar]
- 43.Robinson DS. Monoamine oxidase inhibitors: a new generation. Psychopharmacology Bulletin. 2002;36(3):124–138. [PubMed] [Google Scholar]
- 44.Pae CU, Bodkin JA, Portland KB, Thase ME, Patkar AA. Safety of selegiline transdermal system in clinical practice: analysis of adverse events from postmarketing exposures. Journal of Clinical Psychopharmacology. 2012;73(5):661–668. doi: 10.4088/JCP.12m07648. [DOI] [PubMed] [Google Scholar]
- 45.Thase ME. The role of monoamine oxidase inhibitors in depression treatment guidelines. Journal of Clinical Psychiatry. 2012;73(1):1–10. doi: 10.4088/JCP.11096su1c.02. [DOI] [PubMed] [Google Scholar]
- 46.Busanello A, Barbosa NBV, Peroza LR, et al. Resveratrol protects against a model of vacuous chewing movements induced by reserpine in mice. Behavioural Pharmacology. 2011;22(1):71–75. doi: 10.1097/FBP.0b013e328341e9b4. [DOI] [PubMed] [Google Scholar]
- 47.Pereira RP, Fachinetto R, De Souza Prestes A, et al. Valeriana officinalis ameliorates vacuous chewing movements induced by reserpine in rats. Journal of Neural Transmission. 2011;118(11):1547–1557. doi: 10.1007/s00702-011-0640-7. [DOI] [PubMed] [Google Scholar]
- 48.Busanello A, Peroza LR, Wagner C, et al. Resveratrol reduces vacuous chewing movements induced by acute treatment with fluphenazine. Pharmacology Biochemistry and Behavior. 2012;101(2):307–310. doi: 10.1016/j.pbb.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 49.Peroza A Busanello LR, Leal CQ, Röpke J, et al. Bauhinia forficata prevents vacuous chewing movements induced by haloperidol in rats and has antioxidant potential in vitro. Neurochemical Research. 2013;38(4):789–796. doi: 10.1007/s11064-013-0981-8. [DOI] [PubMed] [Google Scholar]
- 50.Schaffer LF, Peroza LR, Boligon AA, et al. Harpagophytum procumbens prevents oxidative stress and loss of cell viability in vitro. Neurochemical Research. 2013;38(11):2256–2267. doi: 10.1007/s11064-013-1133-x. [DOI] [PubMed] [Google Scholar]
- 51.Galati G, Chan T, Wu B, O'Brien PJ. Glutathione-dependent generation of reactive oxygen species by the peroxidase-catalyzed redox cycling of flavonoids. Chemical Research in Toxicology. 1999;12(6):521–525. doi: 10.1021/tx980271b. [DOI] [PubMed] [Google Scholar]
- 52.Choi EJ, Chee K-M, Lee BH. Anti- and prooxidant effects of chronic quercetin administration in rats. European Journal of Pharmacology. 2003;482(1–3):281–285. doi: 10.1016/j.ejphar.2003.09.067. [DOI] [PubMed] [Google Scholar]
- 53.Sachdev P, Saharov T, Cathcart S. The preventative role of antioxidants (selegiline and vitamin E) in a rat model of tardive dyskinesia. Biological Psychiatry. 1999;46(12):1672–1681. doi: 10.1016/s0006-3223(99)00091-8. [DOI] [PubMed] [Google Scholar]


