Abstract
As the frequency of melanoma increases, current treatment strategies are struggling to significantly impact patient survival. One of the critical issues in designing efficient therapies is understanding the composition of heterogeneous melanoma tumors in order to target cancer stem cells (CSCs) and drug resistant subpopulations. In this review, we summarize recent findings pertinent to the reemergence of the embryonic Nodal signaling pathway in melanoma and its significance as a prognostic biomarker and therapeutic target. In addition, we offer a novel molecular approach to studying the functional relevance of Nodal-expressing subpopulations and their CSC phenotype.
The incidence and mortality rates of melanoma have been increasing over the last few decades with the most recent statistics reporting the highest lifetime risk of developing melanoma as 1 in 50 for Caucasians (American Cancer Society; http://www.cancer.org/cancer/skincancer-melanoma/detailedguide/melanoma-skin-cancer-key-statistics). Especially noteworthy is that metastatic melanoma, a disease of poor prognosis, is highly resistant to conventional chemotherapies, with a poor clinical outcome.1 Despite significant efforts devoted to advancing new therapies to improve patient survival, including the recent development of inhibitors to RAS, RAF and MAP-ERK kinase (MEK), not all melanoma cells respond in a predictable manner -- primarily due to the conundrum of tumor cell heterogeneity.2 Thus, a primary focus of cancer researchers is to better understand cellular heterogeneity within tumors, such as melanoma, with a special emphasis on identifying and ultimately targeting tumor cells exhibiting stem cell properties -- known as the cancer stem cell phenotype, generally associated with drug resistance.3–5
Recognizing that cancer cells can exploit normally dormant embryonic pathways to promote tumorigenicity and metastasis presents an unique therapeutic opportunity.6–7 Studying embryonic signaling pathways in melanoma has led to the discovery that the embryonic morphogen Nodal is reexpressed in the aggressive phenotype.6,8–10 Interestingly, Nodal is a member of the TGF-β superfamily and is a critical factor in normal embryonic development, including maintenance of pluripotency in human embryonic stem cells (hESCs), initiation of mesoderm formation and regulation of left-right asymmetry.11 In humans, Nodal expression is largely restricted to embryonic tissues, including the trophoblast and the developing mammary gland, but is generally lost in most normal adult tissues. Therefore, studies addressing the role of Nodal in cancer progression have focused on the mechanisms underlying its reexpression in tumor cells and the translational relevance of targeting Nodal-positive tumor cells as a novel therapy.12,13
Recent findings have revealed that Nodal is a critical regulator of melanoma growth, plasticity and tumorigenicity, and holds promise as a new biomarker for metastatic potential.9–10,14 Similar observations have been reported in gliomas and carcinomas of the breast, prostate, endometrium, liver and pancreas.15–20 However, with any new discovery, there are associated challenges. In the case of Nodal, research in this field for human cells and tissues has been hampered by inconsistencies and sometimes incorrect information available in public databases, and by lackluster reagents commercially available and the associated disparate results.21 Furthermore, because Nodal is a secreted protein that can influence cellular behavior in an autocrine and a paracrine manner via a diffusion gradient,22 it has been particularly challenging to assess the extent and influence of Nodal-expressing tumor cells vs. tumor cells where Nodal is simply adherent to the cell surface, thus compromising our ability to directly measure the functional relevance of Nodal-expressing subpopulations of melanoma cells. Fortunately for this avenue of discovery, molecular detection probes have been developed recently which allow live cell sorting, imaging and assessment of Nodal (both independently and together with other critical biomarkers) in tumor cells,23,24 thereby advancing our ability to understand the significance of Nodal-expressing subpopulations in heterogeneous melanomas.
MELANOMA TUMOR CELL PLASTICITY
The aggressive melanoma phenotype has been described as plastic and multipotent, similar in many respects to embryonic stem cells.10 However, dissimilar to normal embryonic progenitors, these tumor cells lack critical regulators, resulting in the aberrant activation of embryonic signaling pathways, which maintains their plastic phenotype and promotes unregulated growth.6,25 Of special note are the phenotypes expressed by aggressive melanoma cells that are associated with hESCs and endothelial cells/progenitors, in which their respective molecular signatures correlate with functional plasticity.26 An example of an endothelial phenotype found in advanced stage melanoma cells is demonstrated by the expression of Vascular Endothelial Cadherin (CD144) and de novo formation of vascular perfusion networks, in vitro and in vivo, revealing a transendothelial functionality resulting in tumor perfusion.27 Interestingly, only subpopulations of melanoma cells express CD144, specifically the tumor cells forming vasculogenic networks.28,29
The definition for cancer stem cells (CSCs) has expanded to include the capacity for differentiation and self-renewal for subpopulations of tumor cells originally described as having stem cell-like properties.30 However, unlike many solid tumors that consist of defined, isolatable subpopulations of CSCs, melanoma cells can generate reversible stem-like cells through “phenotype switching”.5 In this manner, melanoma cells can switch between a migratory, stem-like state that may be resistant to therapy, and a highly proliferative state that leads to tumor growth. Further support for the phenotype switching paradigm includes a recent study showing at least 25% of tumor cells isolated from melanoma patients were able to establish new tumors.31 Moreover, similar to normal developmental events where cell fate determination is profoundly influenced by the microenvironment, phenotypic changes in tumor cells can be similarly influenced. Indeed, an excellent example of this phenomenon has been shown in studies where metastatic melanoma cell subpopulations have been reprogrammed by the embryonic microenvironments of hESCs and chick neural crest -- to assume a more differentiated melanocyte phenotype.32
TARGETING EMBRYONIC SIGNALING IN AGGRESSIVE MELANOMA
The significance of Nodal expression in melanoma, as well as other tumor models, is of special interest to researchers, pathologists and oncologists, based on the emerging body of evidence supporting its potential as a biomarker for tumor progression and a viable target for therapy.9–10,14–20 Immunohistochemical studies have shown that Nodal protein is absent in normal skin, is seen in only a few cells in radial growth phase (RGP) melanomas, and is robustly detected in vertical growth phase (VGP) melanomas and metastases,6,9–10,14 as demonstrated in representative tissues shown in Figure 1. Additional findings have indicated the localization of Nodal in vascular networks in advanced stages of melanoma progression, and have associated the expression with therapeutic resistance.1 Thus, these expression studies suggest that Nodal may be a promising biomarker for not only melanoma progression but also responsiveness to therapy.
Figure 1.
Immunohistochemical analysis of Nodal in human cutaneous melanoma tissue representative of the radial growth phase (RGP; A), vertical growth phase (VGP; B) and a lymph node metastasis (C). Nodal staining is shown is reddish brown (arrows) in A, and the Isotype control is pictured in the inset; (A; bar = 50 μm); Nodal staining is reddish brown in VGP (B; original magnification=63×; and Nodal staining is reddish brown in a lymph node metastasis (C; original magnification=63×).
In vitro studies have provided some clarity to the Nodal signaling cascade employed by aggressive melanoma cells, which differs from that described for hESCs.6,25 Nodal propagates its signal by binding to and activating the heteromeric complex composed of the EGF-like glycoprotein co-receptor Cripto-1 and Types I and II activin-like kinase receptors, leading to activation and nuclear translocation of the Smad2/3/4 complex, where it regulates the expression of downstream genes involved in stem cell maintenance and plasticity, including Nodal.11 Down-regulation of Nodal signaling results in the commensurate diminution of pSmad-2.6 In addition, Nodal signals using a feed forward process, thus underscoring its importance as an autocrine and paracrine regulator of cellular activity.25 Surprisingly, cell surface expression of Nodal's coreceptor, Cripto-1, has been detected in only a small subpopulation of melanoma cells, indicating a primary role for Cripto-1-independent signaling of Nodal in melanoma, in addition to intriguing role(s) for Cripto-1-positive melanoma cells with CSC properties.9 Furthermore, it is important to address the intracellular role for Cripto-1, which is quite robust in melanoma and may suggest that this protein is involved in facilitating this complex signaling cascade in unique ways.
It is critical to note that while Nodal is reactivated in aggressive melanoma cells, Lefty, the natural inhibitor of Nodal, is methylated resulting in its silencing.25,33 Therefore, Nodal signaling in melanoma, and other cancers, is highly unregulated. Several studies in melanoma have focused on down-regulating Nodal expression using a variety of experimental approaches, both in vitro and in vivo, and then measuring the outcome on tumor cell phenotype, biological function, tumorigenesis and metastatic potential. Collectively, the findings generated indicate that down-regulating Nodal results in: 1) suppression of the plastic phenotype, including down-regulation of CD144, and upregulation of Tyrosinase and Melan-A (melanocyte pathway genes); 2) decreased invasive potential and proliferative capacity in vitro; 3) diminished clonogenic potential in soft agar; and 4) significant reduction in tumorigenicity in vivo, accompanied by apoptosis.6,8,12 Using anti-Nodal antibody treatment in a preclinical mouse model bearing pulmonary melanoma metastases, targeted apoptosis was induced in the lung metastases.9 In an orthotopic model, melanoma palpable tumors were injected intratumorally with Lefty isolated from stem cells, resulting in apoptosis of Nodal-positive tumor cells.9 Collectively, these findings implicate Nodal as a potential new therapeutic target supported by compelling proof-of-principle evidence.
STUDYING THE FUNCTIONAL RELEVANCE OF NODAL SUBPOPULATIONS
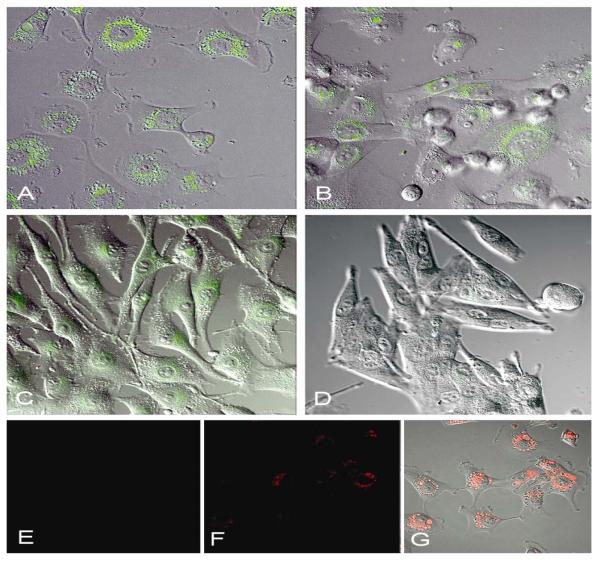
To better understand the significance of Nodal expression in subpopulations of heterogeneous melanoma, and recognizing that Nodal as a secreted protein does not easily allow experimental sorting for subsequent biological assays, we investigated various molecular detection methods that might address our challenge. After discrete testing of viable Nodal sequences that could be effectively conjugated with spherical nucleic acid gold nanoparticles consisting of highly oriented oligonucleotide sequences, many of which are hybridized to a reporter containing a distinct fluorophore label complementary to the corresponding Nodal mRNA target, called SmartFlare RNA detection probes,23,24 we pursued several preliminary experiments examining the specificity and suitability of this approach for our studies. Treatment of three human metastatic melanoma and one non-aggressive melanoma cell line(s) with Cy3-labeled SmartFlares prepared to detect Nodal mRNA showed remarkable specificity of Nodal expression (by confocal microscopy) predominantly in the C8161, MV3 and SK-MEL28 metastatic cells, and not in the UACC1273 non-aggressive melanoma (Figure 2A–D). Equally noteworthy is that the Cy3-labeled scrambled sequence SmartFlare (representing a negative control) shows no fluorescent signal in the tumor cells (Figure 2E), while a positive control demonstrates SmartFlare uptake by tumor cells (Figure 2F).
Figure 2.
Aggressive metastatic melanoma cells C8161 (A), MV3 (B), SK-MEL28 (C) and non-aggressive melanoma cells UACC1273 (D) were treated with 100pM Cy3-labeled SmartFlares prepared to detect Nodal mRNA for 16 hours in culture, then the live cells were imaged using a Zeiss LSM 700 confocal microscope equipped with a LSM 700 XL S1 incubation system for live cell imaging. The detection of Nodal mRNA as a Cy3 fluorescent green signal is seen in the aggressive melanoma cells (A, B, C), while little-to-no signal is seen in the non-aggressive UACC1273 cells (D). A Cy3-labeled scrambled sequence SmartFlare representing a negative control shows no green signal in C8161 cells (E) while a positive uptake control demonstrating that SmartFlares are taken up by the C8161 cells (Cy5-labeled, red fluorescent) is shown by the red fluorescent signal in these same cells (F) and with red fluorescence and differential interference contrast (DIC) imaging (G). (All magnifications=40×.)
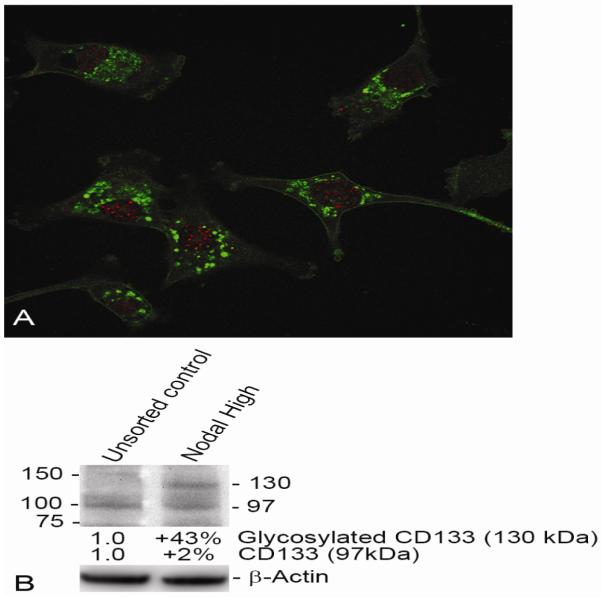
After determining the specificity of Nodal SmartFlare detection in human melanoma cells, a series of sorting experiments was conducted. Following treatment of metastatic melanoma cells with Nodal mRNA SmartFlares, a FACSAria II Cell Sorter was used to separate Nodal-high-expressing tumor cells vs. Nodal-low-expressing tumor cells. These two subpopulations were further analyzed for Nodal expression by real-time RT-PCR and Western blot, which revealed an excellent correlation of Nodal expressed at the gene and protein level(s) between the sorted tumor cell subpopulations (Figure 3A, B). These two subpopulations sorted from three metastatic melanoma cell lines were also seeded into soft agar to assess clonogenic/tumorigenic potential. After three weeks in the soft agar assay, the Nodal-high-expressing subpopulations formed significantly more tumor colonies compared with the Nodal-low-expressing subpopulations indicating the importance of Nodal in the formation of tumors (Figure 4). Lastly, we measured whether CD133, an important biomarker for CSCs and drug resistance,34,35 could be detected in the Nodal-high-expressing melanoma cells. Confocal immunofluorescence microscopy shows CD133 protein localized in many of the Nodal-expressing tumor cells (Figure 5A). Western blot analysis confirms a 43% increase in the glycosylated form of CD133 in Nodal-high-expressing melanoma cells vs. the unsorted heterogeneous control group (Figure 5B).
Figure 3.
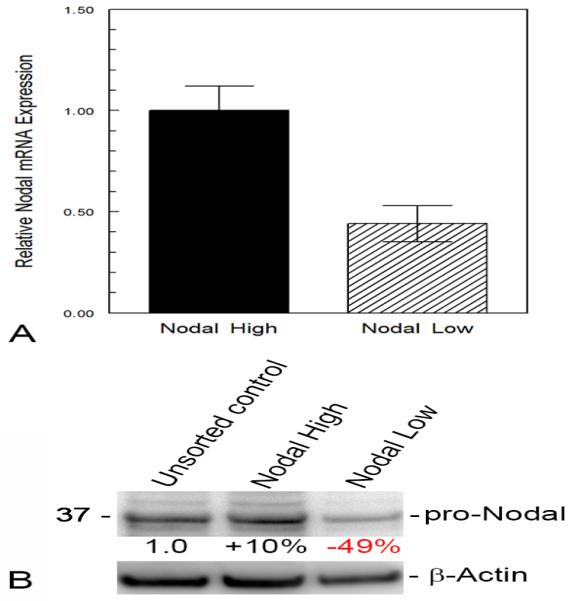
C8161 cells were treated with Nodal mRNA SmartFlares for 16 hours, then sorted into subpopulations based on a high or low fluorescent signal representing Nodal expressing (Nodal High) and Nodal deficient (Nodal Low) subpopulations using a BD Bioscience FACSAria II Cell Sorter. The relative expression of Nodal mRNA in the two subpopulations was determined by real-time polymerase chain reaction (RT-PCR) assay (A) and the relative expression of Nodal protein (detected at 39 kilodaltons as pro-Nodal) determined by Western blot analysis (B). The differences in pro-Nodal Protein in the High and Low Nodal sorted cells was measured densitometrically relative to the unsorted cells after correction for protein loaded in each lane using β-Actin protein as a control.
Figure 4.
The mRNA Nodal SmartFlare sorted and unsorted cells representing the Unsorted, Nodal High and Nodal Low subpopulations of C8161, MV3 and SK-MEL28 cells were also analyzed for clonogenicity (the ability to form colonies from individual cells), using a soft agar assay. Suspended cells were introduced into soft agar where individual cells became set in place in the solidifying agar. After three weeks, colonies grown from individual cells were imaged using dark-field microscopy from Unsorted (A), Nodal High (B) and Nodal Low (C) sorted populations and appear as discrete spots against a uniform background (C8161 cells shown in representative fields). The colonies were counted in each sample and the Relative Colony Formation of Nodal High and Nodal Low sorted subpopulations as a percentage vs. the Unsorted cells determined for C8161 (D), MV3 (E) and SK-MEL28 (F) cells. (n=3; *p<0.05.)
Figure 5.
A Nodal High population of C8161 cells sorted using Nodal mRNA SmartFlares was plated on glass coverslips and fixed and permeabilized with methanol. The cells were then treated with a rabbit primary antibody to Nodal (Santa Cruz; H-110) and a mouse primary antibody to CD133 (Millipore; 17A6.1), followed by an Alexa dye 488-conjugated secondary antibody against the rabbit antibody (green; Nodal) and Alexa dye 594-conjugated secondary antibody against the mouse antibody (red; CD133). CD133 expression appears enriched in the Nodal High sorted subpopulation (A) and Western blot analysis of the Unsorted vs. the Nodal High subpopulations for CD133 protein expression shows an increase in the amount of CD133 glycosylated CD133 (130 kilodalton) protein in the Nodal High selected subpopulation vs. the Unsorted subpopulation (B; corrected for the amount of protein loaded per lane using β-Actin protein as a control, see Figure 3).
These data indicate for the first time, that using the SmartFlare detection technology, it is possible to perform live cell imaging and sorting of melanoma subpopulations producing Nodal and then analyzing their biological capability in the soft agar assay. Most promising is the ability to also examine other biomarkers concurrently, such as CD133. Although CD133 was originally classified as a marker of hematopoietic progenitors and neural stem cells, recent compelling reports have shown CD133 to be a critical marker for CSCs in a number of cancer types, including a prominent role in melanoma associated with drug resistance and vasculogenic networks.34,35 Indeed, some investigators have suggested CD133 as a viable molecular therapeutic target for metastatic melanoma,34 and we now have the tools in hand to rigorously test this hypothesis.
CONCLUSION
We appreciate that the challenges facing the successful treatment of melanoma have given rise to the era of personalized medicine and targeted therapies. For researchers and oncologists, the collective goal is to recognize which tumor cells have the potential to escape immune surveillance, metastasize, and become resistant to chemotherapy. As modest advances have been made in the treatment of melanoma, the most critical questions to address focus on CSCs, tumor initiating cells, phenotype switching, and acquired drug resistance -- all part of the conundrum associated with tumor cell heterogeneity. The real challenge now is determining how to study melanoma heterogeneity. Can every tumor cell propagate a melanoma or is this tumorigenic property exclusive to specific subpopulations? What are the unique biomarkers that will definitively answer this question? Do we have the proper research tools to perform the necessary experiments?
The plastic phenotype of aggressive melanoma has presented a significant challenge in the detection and targeting of tumor cells exhibiting stem cell-like characteristics -- indicative of CSCs. As the molecular signaling pathways underlying tumor cell plasticity become more transparent, our ability to strategically target this elusive phenotype will be enhanced. In this regard, a promising, emerging area of focus is the targeting of embryonic signaling pathways that reemerge in aggressive tumors cells, such as melanoma -- in the absence of regulatory checkpoints.
Worthy of particular attention is the recent discovery of the embryonic morphogen Nodal in melanoma, which has been shown to underlie unregulated cell growth, tumorigenicity, metastasis and the CSC phenotype. Experimental down-regulation of Nodal in melanoma results in the suppression of this phenotype and its associated aggressive behavior. However, it is important to note that the majority of these studies utilized conventional technologies of immunohistochemistry, Western blot analyses, and RT-PCR to measure Nodal expression, but have suffered from the inability to specifically sort Nodal-expressing subpopulations within heterogeneous cell lines for further functional investigation. Now, with the development of novel, molecular SmartFlares specific for Nodal mRNA, melanoma subpopulations are easily sortable and viable for functional analyses -- in real time. This approach allowed the revelation that CD133, an important biomarker for CSCs and drug resistance, is enhanced in Nodal-expressing subpopulations of melanoma, which may shed new light on Nodal's role in melanoma progression and possible options for combinatorial multi-target therapies -- to take advantage of molecularly based approaches to target all aspects of melanoma heterogeneity.
Acknowledgements
Research was supported in part by NIH/NCI CA121205
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial disclosures and conflict of interest: The authors (E Seftor, R Seftor, Kirsammer, Gilgur and Hendrix) have no financial disclosures or conflicts to report related to the technology presented. D Weldon is the R&D Manager at EMD Millipore responsible for the production of SmartFlares. Patents related to therapeutically targeting Nodal in tumor cells have been awarded to E Seftor, R Seftor and Hendrix.
REFERENCES
- 1.Schatton T, Frank MH. Cancer stem cells and human malignant melanoma. Pigment Cell Melanoma Res. 2007;21:39–55. doi: 10.1111/j.1755-148X.2007.00427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thakur MD, Stuart DD. The evolution of melanoma resistance reveals therapeutic opportunities. doi: 10.1158/0008-5472.CAN-13-1633. Epub 2013 October 4, 10.1158/0008-5472.CAN-13-1633. Available from: www.cancerres.aacrjournlals.org. [DOI] [PubMed]
- 3.Williams SA, Anderson WC, Santaguida MT, Dylla SJ. Patient-derived xenografts, the cancer stem cell paradigm, and cancer pathobiology in the 21st century. Lab Invest. 2013;93:970–982. doi: 10.1038/labinvest.2013.92. [DOI] [PubMed] [Google Scholar]
- 4.Malanchi I, Santamarie-Martinez A, Susanto E, Peng H, Lehr H-A, Delaloye J-F, et al. Interactions between cancer stem cells and their niche govern metastatic colonization. Nature. 2011;481(7379):85–99. doi: 10.1038/nature10694. [DOI] [PubMed] [Google Scholar]
- 5.Hoek KS, Goding CR. Cancer stem cells versus phenotype-switching in melanoma. Pigment Cell Res. 2010;23:746–759. doi: 10.1111/j.1755-148X.2010.00757.x. [DOI] [PubMed] [Google Scholar]
- 6.Topczewska JM, Postovit LM, Margaryan NV, Sam A, Hess AR, Wheaton WW, et al. Embryonic and tumorigenic pathways converge via Nodal signaling: Role in melanoma aggressiveness. Nature Medicine. 2006;12:925–932. doi: 10.1038/nm1448. [DOI] [PubMed] [Google Scholar]
- 7.Takebe N, Ivy SP. Controversies in cancer stem cells: Targeting embryonic signaling pathways. Clin Cancer Res. 2010;16(12):3106–3112. doi: 10.1158/1078-0432.CCR-09-2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Postovit LM, Margaryan NV, Seftor E, Hendrix MJC. Role of Nodal signaling and the microenvironment underlying melanoma plasticity. Pigment Cell Melanoma Res. 2008;21:348–357. doi: 10.1111/j.1755-148X.2008.00463.x. [DOI] [PubMed] [Google Scholar]
- 9.Strizzi L, Postovit LM, Margaryan NV, Lipavsky A, Gadiot J, Blank C, et al. Nodal as a biomarker for melanoma progression and a new therapeutic target for clinical intervention. Expert Rev Dermatol. 2009;4:67–78. doi: 10.1586/17469872.4.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Strizzi L, Hardy KM, Kirsammer GT, Gerami P, Hendrix MJC. Embryonic signaling in melanoma: potential for diagnosis and therapy. Lab Invest. 2011;91:819–824. doi: 10.1038/labinvest.2011.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schier AF, Shen MM. Nodal signalling in vertebrate development. Nature. 2000;403:385–389. doi: 10.1038/35000126. [DOI] [PubMed] [Google Scholar]
- 12.Hardy KM, Kirschmann DA, Seftor EA, Margaryan NV, Postovit LM, Strizzi L, et al. Regulation of the embryonic morphogen Nodal by Notch4 facilitates manifestation of the aggressive melanoma phenotype. Cancer Res. 2010;70:10340–10350. doi: 10.1158/0008-5472.CAN-10-0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Quail DF, Taylor MJ, Walsh LA, Dieters-Castator D, Das P, Jewer M, et al. Low oxygen levels induce the expression of the embryonic morphogen Nodal. Mol Biol Cell. 2011;22(24):4809–4821. doi: 10.1091/mbc.E11-03-0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu L, Harms PW, Pouryazdanparast P, Kim DS, Ma L, Fullen DR. Expression of the embryonic morphogen Nodal in cutaneous melanocytic lesions. Mod Pathol. 2010;23:1209–1214. doi: 10.1038/modpathol.2010.101. [DOI] [PubMed] [Google Scholar]
- 15.Lee CC, Jan HJ, Lai JH, Ma HI, Hueng Dy, Lee YC, et al. Nodal promotes growth and invasion inhuman gliomas. Oncogene. 2010;29:3110–3123. doi: 10.1038/onc.2010.55. [DOI] [PubMed] [Google Scholar]
- 16.Strizzi L, Hardy KM, Margaryan NV, Hillman DW, Seftor EA, Chen B, et al. Potential for the embryonic morphogen Nodal as a prognostic and predictive biomarker in breast cancer. Breast Cancer Res. 2012;14(R75):1–12. doi: 10.1186/bcr3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lawrence MG, Margaryan NV, Loessner D, Collins A, Kerr KM, Turner M, et al. Reactivation of embryonic Nodal signaling is associated with tumor progression and promotes the growth of prostate cancer cells. Prostate. 2011 doi: 10.1002/pros.21335. 71-1198-1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Papageorgiou I, Nicholis PK, Wang F, Lackmann M, Makanji Y, Salamonsen LA, et al. Expression of Nodal signaling components in cycling human endometrium and in endometrial cancer. Reprod Biol Endocrinol. 2009;7:122–133. doi: 10.1186/1477-7827-7-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun C, Sun L, Jiang K, Gao D-M, Kang X-N, Wang C, et al. NANOG promotes liver cancer cell invasion by inducing epithelial-mesenchymal transition through NODAL/SMAD3 signaling pathway. Int J Biochem Cell Biol. 2013;45:1099–1108. doi: 10.1016/j.biocel.2013.02.017. [DOI] [PubMed] [Google Scholar]
- 20.Lonardo E, Hermann PC, Mueller MT, Huber S, Balic A, Miranda-Lorenzo I, et al. Nodal/Activin signaling drives self-renewal and tumorigenicity of pancreatic cancer stem cells and provides a target for combined drug therapy. Cell Stem Cell. 2011;9(5):433–446. doi: 10.1016/j.stem.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 21.Strizzi L, Hardy KM, Kirschmann DA, Ahrlund-Richter L, Hendrix MJC. Nodal expression and detection in cancer: Experience and challenges. Cancer Res. 2012;72(8):1915–1920. doi: 10.1158/0008-5472.CAN-11-3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muller P, Rogers KW, Jordan BM, Lee JS, Robson D, Ramanathan S, et al. Differential diffusivity of Nodal and Lefty underlies a reaction-diffusion patterning system. Science. 2012;336(6082):721–724. doi: 10.1126/science.1221920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prigodich AE, Randeria PS, Briley WE, Kim NJ, Daniel WL, Golhohann DA, et al. Multiplexed Nanoflares: mRNA detection in live cells. Analytical Chem. 2012;84:2062–2066. doi: 10.1021/ac202648w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simultaneous detection of a surface protein and its mRNA in living cells by conventional and imaging flow cytometry. EMD Millipore Application Note. 2013 Available from: www.emdmillipore.com/SmartFlare.
- 25.Postovit L-M, Margaryan NV, Seftor EA, Kirschmann DA, Lipavsky A, Wheaton WW, et al. Human embryonic stem cell microenvironment suppresses the tumorigenic phenotype of aggressive cancer cells. Proc Natl Acad Sci, USA. 2008;105(11):4329–4334. doi: 10.1073/pnas.0800467105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hendrix MJC, Seftor EA, Hess AR, Seftor REB. Vasculogenic mimicry and tumour-cell plasticity: Lessons from melanoma. Nature Rev Cancer. 2003;3:411–421. doi: 10.1038/nrc1092. [DOI] [PubMed] [Google Scholar]
- 27.Hendrix MJC, Seftor REB, Seftor EA, Gruman LM, Lee LML, Nickoloff BJ, et al. Transendothelial function of human metastatic melanoma cells: Role of the microenvironment in cell-fate determination. Cancer Res. 2002;62:665–668. [PubMed] [Google Scholar]
- 28.Hendrix MJC, Seftor EA, Meltzer PS, Gardner LMG, Hess AR, Kirschmann DA, et al. Expression and functional significance of VE-cadherin in aggressive human melanoma cells: Role in vasculogenic mimicry. Proc Natl Acad Sci, USA. 2001;98(14):8018–8023. doi: 10.1073/pnas.131209798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Demou ZN, Hendrix MJC. Microgenomics profile the endogenous angiogenic phenotype in subpopulations of aggressive melanoma. J Cell Biochem. 2008;105:562–573. doi: 10.1002/jcb.21855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rasheed ZA, Kowalski J, Smith BD, Matsui W. Concise review: Emerging concepts in clinical targeting of cancer stem cells. Stem Cells. 2011;29(6):883–887. doi: 10.1002/stem.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quintana E, Shackleton M, Foster HR, Fullen DR, Sabel MS, Johnson TM, et al. Phenotypic heterogeneity among tumorigenic melanoma cells from patients that is reversible and not hierarchically organized. Cancer Cell. 2010;18:8510–8523. doi: 10.1016/j.ccr.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hendrix MJC, Seftor EA, Seftor REB, Kasemeier-Kulesa J, Kulesa PM, Postovit L-M. Reprogramming metastatic tumour cells with embryonic microenvironments. Nature Rev. Cancer. 2007;7:246–255. doi: 10.1038/nrc2108. [DOI] [PubMed] [Google Scholar]
- 33.Costa FF, Seftor EA, Bischof JM, Kirschmann DA, Strizzi L, Arndt K, et al. Epigenetically reprogramming metastatic tumor cells with an embryonic microenvironment. Epigenomics. 2009;1(2):387–398. doi: 10.2217/epi.09.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rappa G, Fodstad O, Lorico A. The stem cell-associated antigen CD133 (Prominin-1) is a molecular therapeutic target for metastatic melanoma. Stem Cells. 2008;26:3008–3017. doi: 10.1634/stemcells.2008-0601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lai C-Y, Schwartz BE, Shu M-Y. CD133+ melanoma subpopulations contribute to perivascular niche morphogenesis and tumorigenicity through vasculogenic mimicry. Cancer Res. 2012;72(19):5111–5118. doi: 10.1158/0008-5472.CAN-12-0624. [DOI] [PMC free article] [PubMed] [Google Scholar]