Abstract
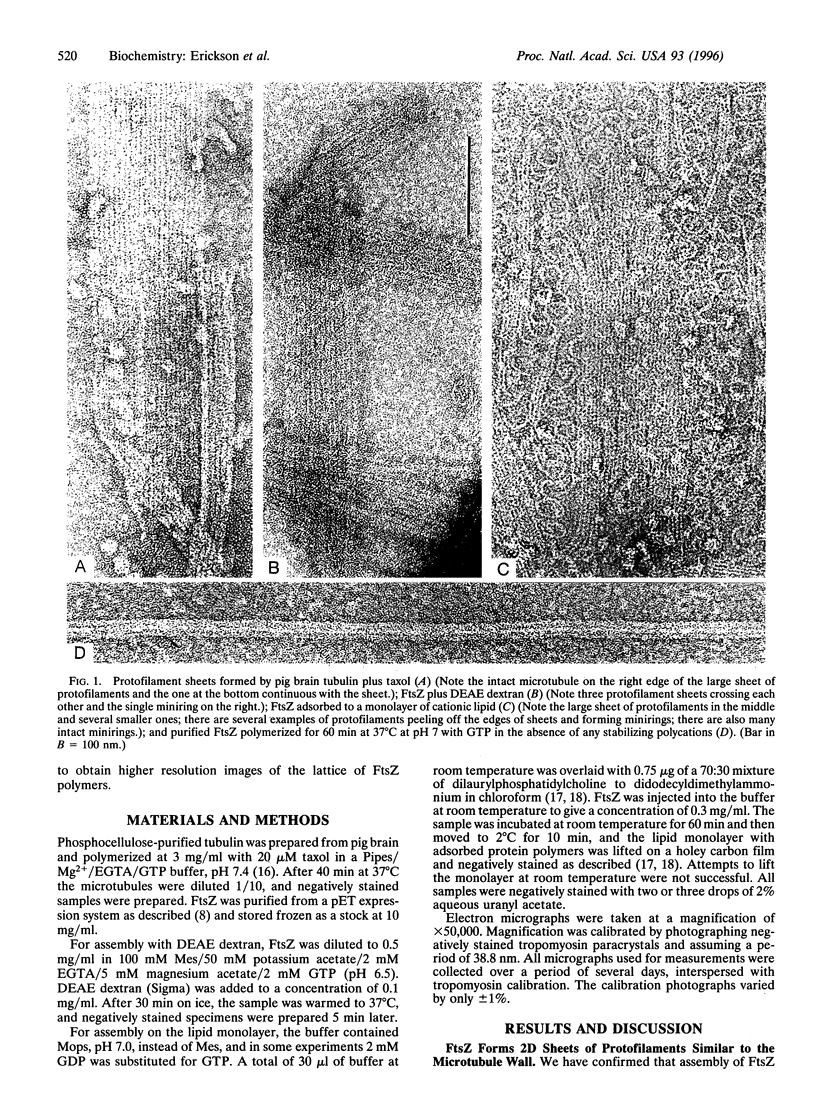
The bacterial cell division protein FtsZ is a homolog of tubulin, but it has not been determined whether FtsZ polymers are structurally related to the microtubule lattice. In the present study, we have obtained high-resolution electron micrographs of two FtsZ polymers that show remarkable similarity to tubulin polymers. The first is a two-dimensional sheet of protofilaments with a lattice very similar to that of the microtubule wall. The second is a miniring, consisting of a single protofilament in a sharply curved, planar conformation. FtsZ minirings are very similar to tubulin rings that are formed upon disassembly of microtubules but are about half the diameter. This suggests that the curved conformation occurs at every FtsZ subunit, but in tubulin rings the conformation occurs at either beta- or alpha-tubulin subunits but not both. We conclude that the functional polymer of FtsZ in bacterial cell division is a long thin sheet of protofilaments. There is sufficient FtsZ in Escherichia coli to form a protofilament that encircles the cell 20 times. The similarity of polymers formed by FtsZ and tubulin implies that the protofilament sheet is an ancient cytoskeletal system, originally functioning in bacterial cell division and later modified to make microtubules.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bramhill D., Thompson C. M. GTP-dependent polymerization of Escherichia coli FtsZ protein to form tubules. Proc Natl Acad Sci U S A. 1994 Jun 21;91(13):5813–5817. doi: 10.1073/pnas.91.13.5813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai K., Lutkenhaus J. The proper ratio of FtsZ to FtsA is required for cell division to occur in Escherichia coli. J Bacteriol. 1992 Oct;174(19):6145–6151. doi: 10.1128/jb.174.19.6145-6151.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson H. P. Assembly of microtubules from preformed, ring-shaped protofilaments and 6-S tubulin. J Supramol Struct. 1974;2(2-4):393–411. doi: 10.1002/jss.400020228. [DOI] [PubMed] [Google Scholar]
- Erickson H. P. FtsZ, a prokaryotic homolog of tubulin? Cell. 1995 Feb 10;80(3):367–370. doi: 10.1016/0092-8674(95)90486-7. [DOI] [PubMed] [Google Scholar]
- Erickson H. P. Microtubule surface lattice and subunit structure and observations on reassembly. J Cell Biol. 1974 Jan;60(1):153–167. doi: 10.1083/jcb.60.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson H. P., Voter W. A. Polycation-induced assembly of purified tubulin. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2813–2817. doi: 10.1073/pnas.73.8.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman A. A., Chrétien D., Arnal I., Wade R. H. Structural changes accompanying GTP hydrolysis in microtubules: information from a slowly hydrolyzable analogue guanylyl-(alpha,beta)-methylene-diphosphonate. J Cell Biol. 1995 Jan;128(1-2):117–125. doi: 10.1083/jcb.128.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschner M. W., Williams R. C., Weingarten M., Gerhart J. C. Microtubules from mammalian brain: some properties of their depolymerization products and a proposed mechanism of assembly and disassembly. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1159–1163. doi: 10.1073/pnas.71.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombillo V. A., Stewart R. J., McIntosh J. R. Minus-end-directed motion of kinesin-coated microspheres driven by microtubule depolymerization. Nature. 1995 Jan 12;373(6510):161–164. doi: 10.1038/373161a0. [DOI] [PubMed] [Google Scholar]
- Lutkenhaus J. FtsZ ring in bacterial cytokinesis. Mol Microbiol. 1993 Aug;9(3):403–409. doi: 10.1111/j.1365-2958.1993.tb01701.x. [DOI] [PubMed] [Google Scholar]
- Mandelkow E. M., Mandelkow E., Milligan R. A. Microtubule dynamics and microtubule caps: a time-resolved cryo-electron microscopy study. J Cell Biol. 1991 Sep;114(5):977–991. doi: 10.1083/jcb.114.5.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIlvain J. M., Jr, Burkhardt J. K., Hamm-Alvarez S., Argon Y., Sheetz M. P. Regulation of kinesin activity by phosphorylation of kinesin-associated proteins. J Biol Chem. 1994 Jul 22;269(29):19176–19182. [PubMed] [Google Scholar]
- Melki R., Carlier M. F., Pantaloni D., Timasheff S. N. Cold depolymerization of microtubules to double rings: geometric stabilization of assemblies. Biochemistry. 1989 Nov 14;28(23):9143–9152. doi: 10.1021/bi00449a028. [DOI] [PubMed] [Google Scholar]
- Mukherjee A., Dai K., Lutkenhaus J. Escherichia coli cell division protein FtsZ is a guanine nucleotide binding protein. Proc Natl Acad Sci U S A. 1993 Feb 1;90(3):1053–1057. doi: 10.1073/pnas.90.3.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee A., Lutkenhaus J. Guanine nucleotide-dependent assembly of FtsZ into filaments. J Bacteriol. 1994 May;176(9):2754–2758. doi: 10.1128/jb.176.9.2754-2758.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osteryoung K. W., Vierling E. Conserved cell and organelle division. Nature. 1995 Aug 10;376(6540):473–474. doi: 10.1038/376473b0. [DOI] [PubMed] [Google Scholar]
- RayChaudhuri D., Park J. T. Escherichia coli cell-division gene ftsZ encodes a novel GTP-binding protein. Nature. 1992 Sep 17;359(6392):251–254. doi: 10.1038/359251a0. [DOI] [PubMed] [Google Scholar]
- Taylor K. A., Taylor D. W. Formation of 2-D paracrystals of F-actin on phospholipid layers mixed with quaternary ammonium surfactants. J Struct Biol. 1992 Mar-Apr;108(2):140–147. doi: 10.1016/1047-8477(92)90013-z. [DOI] [PubMed] [Google Scholar]
- Taylor K. A., Taylor D. W. Formation of two-dimensional complexes of F-actin and crosslinking proteins on lipid monolayers: demonstration of unipolar alpha-actinin-F-actin crosslinking. Biophys J. 1994 Nov;67(5):1976–1983. doi: 10.1016/S0006-3495(94)80680-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voter W. A., Erickson H. P. Tubulin rings: curved filaments with limited flexibility and two modes of association. J Supramol Struct. 1979;10(4):419–431. doi: 10.1002/jss.400100405. [DOI] [PubMed] [Google Scholar]
- Wolf S. G., Mosser G., Downing K. H. Tubulin conformation in zinc-induced sheets and macrotubes. J Struct Biol. 1993 Nov-Dec;111(3):190–199. doi: 10.1006/jsbi.1993.1049. [DOI] [PubMed] [Google Scholar]
- de Boer P., Crossley R., Rothfield L. The essential bacterial cell-division protein FtsZ is a GTPase. Nature. 1992 Sep 17;359(6392):254–256. doi: 10.1038/359254a0. [DOI] [PubMed] [Google Scholar]