Abstract
We investigated the relationship between prenatal maternal urinary concentrations of phthalate metabolites and neonatal behavior in their 295 children enrolled in a multiethnic birth cohort between 1998 and 2002 at the Mount Sinai School of Medicine in New York City. Trained examiners administered the Brazelton Neonatal Behavioral Assessment Scale (BNBAS) to children within 5 days of delivery. We measured metabolites of 7 phthalate esters in maternal urine that was collected between 25 and 40 weeks’ gestation. All but two phthalate metabolites were over 95% detectable. We summed metabolites on a molar basis into low and high molecular weight phthalates. We hypothesized the existence of sex-specific effects from phthalate exposure a priori given the hormonal activity of these chemicals. Overall we found few associations between individual phthalate metabolites or their molar sums and most of the BNBAS domains. However, we observed significant sex-phthalate metabolite interactions (p < 0.10) for the Orientation and Motor domains and the overall Quality of Alertness score. Among girls, there was a significant linear decline in adjusted mean Orientation score with increasing urinary concentrations of high molecular weight phthalate metabolites (B = -0.37, p = 0.02). Likewise, there was a strong linear decline in their adjusted mean Quality of Alertness score (B = -0.48, p < 0.01). In addition, boys and girls demonstrated opposite patterns of association between low and high molecular weight phthalate metabolite concentrations and Motor performance, with some indication of improved Motor performance with increasing concentration of low molecular weight phthalate metabolites among boys. This is the first study to report an association between prenatal phthalate exposure and neurological effects in humans or animals, and as such requires replication.
Keywords: phthalates, behavior, neonatal, neurodevelopment
Introduction
Concern is mounting that a family of chemicals, the phthalate diesters, may pose human health risks based on numerous animal studies and a limited number of observational studies in humans. Among the reported human associations with phthalate exposure are increased waist circumference and insulin resistance (Stahlhut et al., 2007; Hatch et al., 2008); DNA damage in human sperm (Hauser et al., 2007); semen quality (Hauser et al., 2006; Zhang et al., 2006); and decreased anogenital distance in male infants (Swan et al., 2005). The observed risks are generally presumed to be related to the endocrine altering properties of these chemicals, although the exact nature of the hormonal effects may be in part dependent on exposure dose. One area of concern specifically to neurodevelopment is the observed correlations between phthalates exposure and circulating thyroid hormone. Low serum free thyroxine (T4) was associated with high urinary concentration of monobutyl phthalate, a metabolite of dibutyl phthalate in a cohort of pregnant women during the second trimester (Huang et al., 2007), and with urinary mono(2-ethylhexyl) phthalate (MEHP) in adult males (Meeker et al., 2007).
Human exposure to phthalates occurs through a variety of consumer and personal care products. High molecular weight phthalates make plastics pliable, and therefore are included in a number of commonly encountered products like vinyl flooring, medical devices, wall coverings, and food containers. Low molecular weight phthalates are often found in personal care products that carry a scent, such as cosmetics, lotions and perfumes (Hauser et al., 2006).
The Mount Sinai Children's Environmental Health Center was developed to investigate the role of prenatal toxicant exposure on childhood growth and neurodevelopment. We have previously reported associations between pesticide and PCB exposure on size measures at birth (Berkowitz et al., 2004; Wolff et al., 2007) and abnormal neonatal reflexes (Engel et al., 2007). In addition, we have reported the relationship between prenatal phthalate and phenol exposure and size measures at birth (Wolff et al., 2008). Here we describe the relationship between prenatal phthalate exposures and neonatal behavior.
Materials and Methods
The Mount Sinai Children's Environmental Health Cohort study is a prospective multiethnic cohort that enrolled primiparous women presenting for prenatal care at the Mount Sinai prenatal clinic and two private practices with singleton pregnancies, and who delivered at Mount Sinai Hospital between May 1998 and July 2001 (Berkowitz et al., 2003; Berkowitz et al., 2004). Four hundred and seventy nine mother-infant pairs were successfully recruited during pregnancy. Of these women, seventy five women were excluded for reasons detailed elsewhere (Engel et al., 2007) leaving 404 for whom birth data were available.
We administered a questionnaire to participants during their third trimester of pregnancy to obtain information on sociodemographic characteristics, medical history, and lifestyle factors. A sample of maternal urine was obtained between 25 and 40 weeks gestation. Delivery characteristics and birth outcomes were obtained from a perinatal database within the Mount Sinai Department of Obstetrics, Gynecology and Reproductive Science. The Brazelton Neonatal Behavioral Assessment Scale (BNBAS) was administered before hospital discharge (n = 311) by one of four examiners (Engel et al., 2007). Examiners were either trained and certified by the Brazelton Institute or trained by a certified examiner. Examinations were performed in a quiet, semi-darkened, warm room adjacent to the neonatal nursery, or in the mother's private room if available. The BNBAS was not administered if the infant was admitted to the Neonatal Intensive Care Unit, delivered and discharged over a weekend, the parent refused, the baby was not testable, or study personnel were unavailable (Engel et al., 2007). This study was approved by the Institutional Review Board of Mount Sinai School of Medicine. The involvement of the Centers for Disease Control and Prevention (CDC) laboratory was limited and determined not to constitute engagement in human subjects research.
From the 311 subjects on whom the BNBAS was administered, sufficient maternal urine sample was available on 295 (95%) subjects to obtain phthalate measurements. These maternal urine samples were analyzed by the CDC for 10 phthalate metabolites. Methods and quality control procedures have been previously described (Kato et al., 2005; Silva et al., 2008). To limit the influence of multiple testing on our findings, phthalate metabolites were grouped into categories defined by molecular weight, as they each represent similar molecular structure, biological activity, and sources of exposure (Wolff et al., 2008). These groupings were monoester metabolites of high-(>250 dalton) and low-(<250 dalton) molecular weight biomarkers. We also examined the molar sum of di(2-ethylhexyl) phthalate (DEHP) metabolites, however results were very similar to the total high molecular weight monoester grouping, of which they are the major contributing metabolites. To account for urine dilution, we included logcreatinine in models where metabolites were continuous variables, and to create quartile cut points we used creatinine corrected concentrations (uM/g creatinine). All models were restricted to observations in which urinary creatinine values were greater than 20 mg/dL in order to eliminate measures from extremely dilute urine specimens.
The BNBAS includes 28 behavioral items and 18 primitive reflexes. It was scored using the seven-cluster scoring method developed by Lester et al. (Lester et al., 1982) which divides infant behavior into seven domains: habituation (number of subjects with complete data for this cluster = 177), ability to respond to and inhibit discrete stimuli while asleep; orientation (n = 269), attention to visual and auditory stimuli and quality of overall alertness; motor (n = 295), motor performance and quality of movement and tone; range of state (n = 294), a measure of infant arousal and state lability; regulation of state (n = 293), ability to regulate state in the face of increasing levels of stimulation; autonomic stability (n = 294), signs of stress related to homeostatic adjustments of the central nervous system; and number and type of abnormal primitive reflexes (n = 292). Details of the Lester scoring method have been previously described (Lester et al., 1982; Brazelton and Nugent, 1995; Young et al., 2005). It should be noted that the infant must be asleep at the start of the exam in order for the habituation domain to be completed. Therefore, as is typical, there are many more infants who are missing information on this domain. There are also seven supplementary items that are designed to describe qualitative aspects of the infant's performance across the entire examination: Cost of Attention, the extent to which the motor and physiological systems are stressed; Quality of Alertness, overall quality of the infant's responsiveness; Examiner Facilitation, amount of help necessary from the examiner to facilitate the infant's optimal performance; General Irritability, infant response to non-aversive stimuli; Robustness and Endurance, ability to maintain energy throughout examination; State Regulation, availability of clear, well-organized states and the quality of fluctuation between them; and Examiner's Emotional Response, the degree to which the examiner finds the examination stressful. Each of the Lester summary scales and all of the supplementary items rate infant behavior on a scale from 1 to 9, with 1 being the worst and 9 being the best, except the number of abnormal reflexes which is a count.
Data were analyzed using SAS version 9.1 (Cary, NC). Generalized linear models were used to analyze relationships between biomarker concentrations and each domain except abnormal reflexes. Poisson regression was used to analyze the relationship between biomarker concentrations and the number of abnormal reflexes. In Poisson models, when necessary, we corrected for overdispersion by introducing a scale parameter estimated by the deviance divided by degrees of freedom, as overdispersion may cause underestimation of the standard errors of regression estimates. Backward elimination was used to arrive at the final adjusted models. Covariates were eliminated if their exclusion caused less than a 10% change in the beta coefficient of the full model. The following were considered as potential confounders or effect modifiers: maternal age (continuous), race (white or non-white), marital status (single, married, living with baby's father), education (< high school, high school, some college, ≥ college degree), cesarean delivery (yes/no), delivery anesthesia (yes/no), infant age at examination (continuous), infant sex (male/female), infant jaundice (yes/no), and smoking (yes/no), alcohol (yes/no), caffeine (yes/no) or illicit drug use (yes/no) during pregnancy, urinary creatinine, and examiner. Because we previously reported an association between prenatal organophosphate pesticide urinary concentrations and neonatal behavior (Engel et al., 2007), we additionally examined confounding by urinary organophosphate metabolite concentration. Gestational age at delivery and birthweight were not evaluated for confounding because they are potentially causal intermediates; however, models restricting the population to gestational ages at or above 37 weeks and birthweight over 2500 grams were examined to be sure that these few observations were not overly influential.
All phthalate biomarkers and creatinine were included as log-linear terms in the initial model selection stage. Subsequently we examined effects by creatinine corrected quartiles of exposure in order to make fewer assumptions about the shape of the relationship. However, in the case of no effect, we report only the log-linear term. Infant sex-phthalate interactions were examined using the log-linear term for phthalate level. We did not examine additional interactions by infant age at exam, as has been previously described (Young et al., 2005; Engel et al., 2007), because of the difficulty in interpreting multiple interactions simultaneously, and because our sample size would be insufficient to support three-way interactions. We focused on interactions at less than the p = 0.2 level.
Results
Maternal and infant characteristics of the population tested have been previously reported (Engel et al., 2007). To summarize, the majority of participants were Black or Latina women aged 25 years or younger who were unmarried at the time of enrollment and had a relatively low educational attainment (Table 1). Severely preterm births (< 32 weeks) were excluded by design; therefore, most delivered babies at term (92.9%) with birthweights over 2500 grams (97.8%). All babies were evaluated by 5 days of age, the majority by day 2 (Table 1). Median phthalate biomarker concentrations in this population were within the range of those reported for the 1999-2000 National Health and Nutrition Examination Survey for US adults aged 20-39 (Silva et al., 2004). Most metabolites were detectable in over 95% of our subjects (Table 2).
Table 1.
Characteristics of the Population in a Multiethnic Pregnancy Cohort (n = 295), Mount Sinai Hospital 1998- 2002
| Characteristic | N | % |
|---|---|---|
| Maternal Age (years) | ||
| < 20 | 102 | 34.6 |
| 20 - 24 | 97 | 32.9 |
| 25 -29 | 36 | 12.2 |
| 30+ | 60 | 20.3 |
| Race | ||
| White | 60 | 20.3 |
| Black | 80 | 27.1 |
| Latina | 152 | 51.5 |
| Other | 3 | 1.0 |
| Education | ||
| < High School | 93 | 31.5 |
| HS graduate | 56 | 19.0 |
| Some college | 76 | 25.8 |
| Bachelors + | 70 | 23.7 |
| Marital Status | ||
| Married | 84 | 28.5 |
| Living w/ baby's father | 73 | 24.8 |
| Single | 138 | 46.8 |
| Infant Age at BNBAS (days) | ||
| 1 | 147 | 50.3 |
| 2 | 101 | 34.6 |
| 3+ | 44 | 15.1 |
| Smoke during pregnancy | 53 | 18.0 |
| Infant gender (Female) | 122 | 44.5 |
| Neonatal jaundice | 29 | 9.8 |
| Creatinine < 20mg/dL | 21 | 7.1 |
| Mean | SD | |
|---|---|---|
| Gestational age at delivery (weeks) | 39.3 | 1.5 |
| Birthweight (grams) | 3288 | 429 |
| Habituation | 7.00 | 1.59 |
| Motor | 5.29 | 0.69 |
| Orientation | 6.60 | 1.57 |
| Range of State | 3.68 | 0.83 |
| Regulation of State | 6.06 | 1.33 |
| Autonomic Stability | 6.24 | 1.23 |
| Number of Abnormal Reflexes | 0.68 | 1.20 |
Table 2.
Phthalate Metabolite Biomarker Urinary Concentrations in a Multiethnic Pregnancy Cohort, Mount Sinai Hospital 1998- 2002 (n = 295)
| Biomarker | Limit of Detection (μg/L) | % Detectable | Median (μmoles/L) | Interquartile Range (μmoles/L) | Median (μg/L) | Interquartile Range (μg/L) | ||
|---|---|---|---|---|---|---|---|---|
| Σ Low-molecular weight | 100.00% | 2.23 | 0.95 | 5.65 | ||||
| MMP | 0.15 | 61.69% | 0.01 | 0.00 | 0.02 | 1.7 | 0.7 | 3.8 |
| MEP | 0.40 | 99.66% | 1.99 | 0.78 | 5.28 | 385.8 | 151.5 | 1025.2 |
| MBP | 0.40 | 100.00% | 0.16 | 0.07 | 0.33 | 36.2 | 16.6 | 73 |
| MiBP | 0.26 | 97.63% | 0.03 | 0.01 | 0.05 | 6.2 | 2.6 | 12.2 |
| Σ High-molecular weight | 99.66% | 0.46 | 0.22 | 0.90 | ||||
| MBzP | 0.11 | 99.66% | 0.09 | 0.03 | 0.20 | 23.8 | 8.7 | 50.9 |
| MECPP | 0.25 | 99.66% | 0.12 | 0.05 | 0.23 | 35.8 | 15.9 | 71.8 |
| MEHHP | 0.32 | 99.32% | 0.07 | 0.03 | 0.14 | 19.6 | 9.7 | 41.2 |
| MEOHP | 0.45 | 98.98% | 0.06 | 0.03 | 0.13 | 17.9 | 8.3 | 37.4 |
| MEHP | 0.90 | 91.19% | 0.02 | 0.01 | 0.05 | 6.1 | 2.7 | 14.5 |
| MCPP | 0.16 | 98.31% | 0.01 | 0.01 | 0.03 | 3.4 | 1.9 | 6.4 |
Monomethyl phthalate (MMP), monoethyl phthalate (MEP), monobenzyl phthalate (MBzP), mono-n-butyl phthalate (MnBP), mono-isobutyl phthalate (MiBP), mono(2-ethylhexyl) phthalate (MEHP), mono(2-ethyl-5-oxohexyl) phthalate (MEOHP), mono(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP), mono(2-ethyl-5-carboxypentyl) phthalate (MECPP), and mono(3-carboxypropyl) phthalate (MCPP)
There were few notable associations between low or high molecular weight phthalate molar sums (Table 3). There was a slight positive, though non-significant, association between prenatal low molecular weight phthalate concentrations and improved motor performance overall (B = 0.05, p = 0.06). These models examined overall effects of phthalates. We hypothesized a priori that phthalate effects may be sex specific. Therefore, we examined these models, additionally considering the interaction between phthalate metabolite concentration and sex on each of the BNBAS outcomes. Orientation (sex-log high molecular weight phthalate interaction p = 0.06) and motor (sex - log low molecular weight phthalate interaction p = 0.09) domains both appeared to show sex specific effects (Figures 1 and 2).
Table 3.
Relationship between Third Trimester Urinary Concentrations of Phthalate Metabolite Biomarkers and Neonatal Behavior in a Multiethnic Pregnancy Cohort, Mount Sinai Hospital 1998 – 2002
| BNBAS Domain by Lester Scoring method1 | N | Sum of Low Molecular Weight Phthalates | Sum of High Molecular Weight Phthalates | ||
|---|---|---|---|---|---|
| β | 95% CI | β | 95% CI | ||
| Habituation2 | 162 | 0.05 | −0.13, 0.23 | 0.01 | −0.24, 0.25 |
| Orientation3 | 249 | −0.01 | −0.16, 0.15 | −0.11 | −0.31, 0.09 |
| Range of state | 273 | 0.01 | −0.07, 0.08 | 0.06 | −0.04, 0.16 |
| Motor2 | 274 | 0.05 | −0.01, 0.11 | 0.02 | −0.06, 0.09 |
| Regulation of state2 | 272 | 0.04 | −0.08, 0.17 | −0.09 | −0.24, 0.07 |
| Autonomic stability4 | 273 | 0.08 | −0.03, 0.19 | 0.07 | −0.06, 0.21 |
| Number of Abnormal Reflexes5 | 259 | 0.04 | −0.07 | ||
| Relative Risk | 1.05 | 0.93, 1.17 | 0.93 | 0.82, 1.06 | |
All models were restricted to observations with creatinine values greater than 20 mg/dL and are adjusted for the log urinary creatinine value and the examiner who administered the BNBAS.
Additionally adjusted for drug use during pregnancy.
Additionally adjusted for race.
Additionally adjusted for smoking during pregnancy.
Additionally adjusted for maternal education and prenatal dialkylphosphate pesticide level.
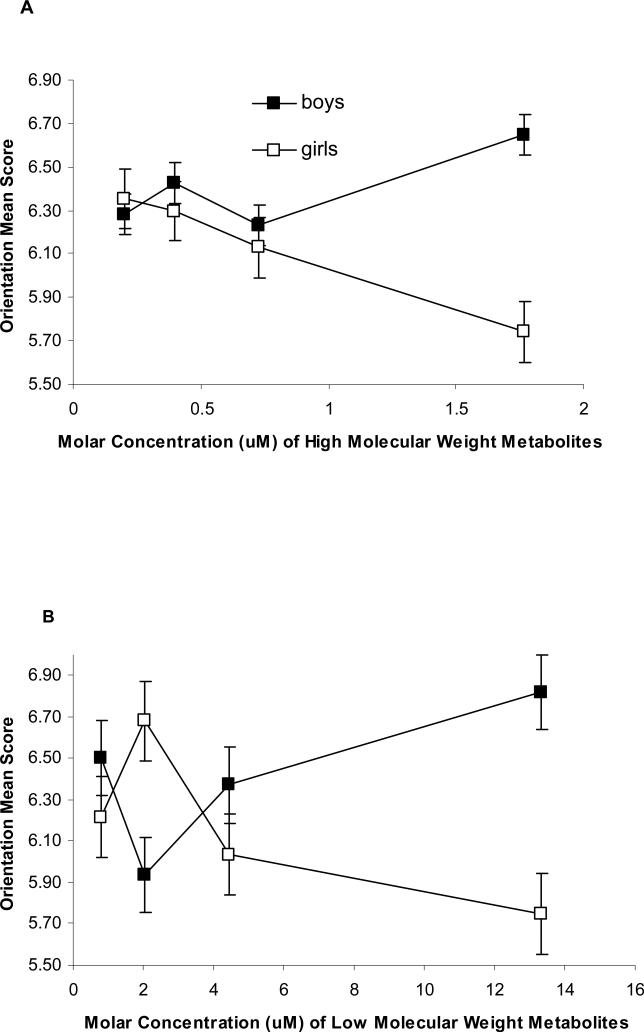
Figure 1.
Prenatal High (A) and Low Molecular Weight (B) Phthalate Metabolite Concentrations (in μM) in maternal urine and Performance on the Orientation Domain of the Brazelton Neonatal Assessment Scale
Legend: Median molar phthalate metabolite concentration within quartiles is plotted against the adjusted mean orientation score for each quartile. Models are adjusted as in Table 3, and include a sex and sex-phthalate interaction term. There was an inverse, linear association between high molecular weight phthalates and mean score on the orientation BNBAS domain among girls. Boys and girls had similar trends for high molecular weight phthalates below 1µM. Although boys and girls showed opposite patterns of effect for low molecular weight phthalates, there were no significant associations, either overall or sex-stratified.
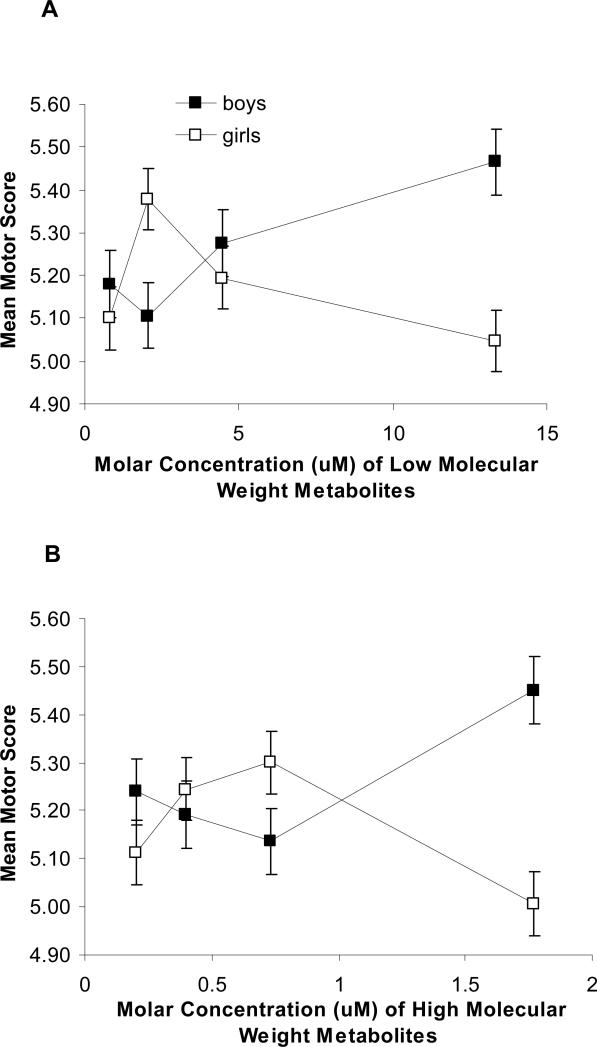
Figure 2.
Relationship between Prenatal Low (A) and High (B) Molecular Weight Phthalate Metabolite Concentrations in Maternal Urine and Performance on the Motor Domain of the Brazelton Neonatal Assessment Scale
Figure 2 Legend: Median molar phthalate metabolite concentration within quartiles is plotted against the adjusted mean motor score for each quartile. Models are adjusted as in Table 3, and include a sex and sex-phthalate interaction term. Among boys, there appeared to be a slight positive association between increasing LMWP phthalate concentrations and improved motor performance (Figure 2A) (B = 0.09, p = 0.01) although it was non-linear at the second quartile. Overall, neither boys nor girls demonstrated monotonic relationships.
There was an inverse, linear association between log high molecular weight phthalates (Figure 1A, interaction p = 0.06) and mean score on the Orientation BNBAS domain. Among girls, there was an adjusted mean 0.37 point decline (p = 0.02) in orientation score for each log micromolar increase in high molecular weight phthalate metabolite concentration in maternal prenatal urine. Although the decline in mean orientation score among girls and boys for log high molecular weight phthalates were very similar for metabolite concentrations less than 1μM, substantially elevated mean orientation scores in the highest quartile of exposure among boys prevented an unstratified log-linear term for phthalates from accurately characterizing the overall effect. Although boys and girls showed opposite patterns of effect for log low molecular weight phthalates (Figure 1C), there were no significant associations, either overall or sex-stratified (sex - log low molecular weight phthalate interaction p = 0.10).
We observed an interaction among sex, low molecular weight phthalates and motor performance (interaction p = 0.09). Among boys, there appeared to be a positive association between increasing low molecular weight metabolite concentrations and improved motor performance (Figure 2) (B = 0.09, p = 0.01) although it was non-linear at the second quartile. Among girls there were no significant associations between low or high molecular weight metabolite concentrations and motor performance. Overall, neither boys nor girls demonstrated monotonically increasing or decreasing relationships between high or low molecular weight metabolite concentrations and motor performance (Figure 2), but the patterns of association were intriguing in that they were mirror images.
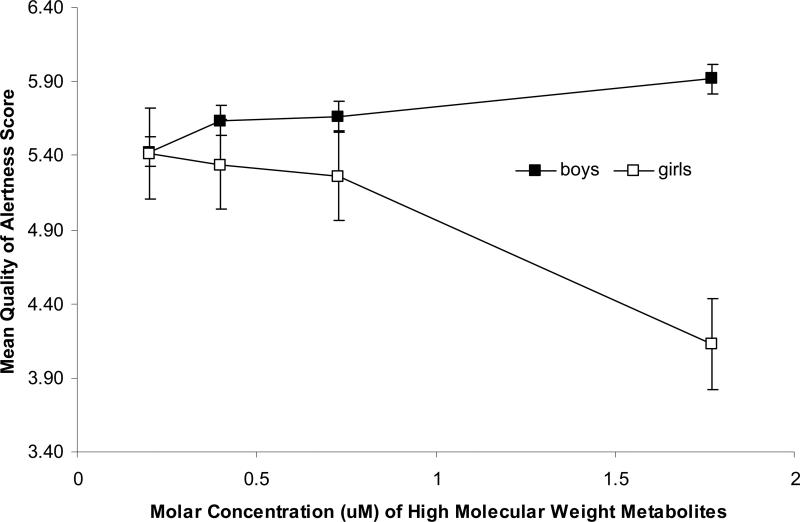
The orientation domain measures the infant's ability to attend to visual and auditory stimuli and alertness. Therefore, to better understand the association, we further examined the Cost of Attention and Quality of Alertness supplementary items. There was no association between any of the phthalate metabolite molar sums and Cost of Attention overall, and no interactions with infant sex. For Quality of Alertness, there was no association between low molecular weight phthalates overall, and no interaction between low molecular weight phthalates and infant sex. However, there were strong main effects, and strong sex interactions between high molecular weight phthalates and Quality of Alertness (p = 0.03 for high molecular weight phthalates). The relationship between high molecular weight phthalates and Quality of Alertness appeared to be limited to girls. Among girls, there was a 0.48 point decline (95% CI -0.83, -0.12) per log unit increase in the sum of high molecular weight phthalates (Figure 3). Very similar results were found for DEHP metabolites. There were no substantial differences to the results when race was instead included as a three-level class variable (Caucasian, African American and Hispanic).
Figure 3.
Prenatal High Molecular Weight Phthalates Metabolite Concentrations in Maternal Urine and Examiner Assessment of the Quality of Alertness Supplementary Item on the Brazelton Neonatal Behavioral Assessment Scale
Figure 3 Legend: Median molar phthalate metabolite concentration within quartiles is plotted against the adjusted mean orientation score for each quartile. Models are adjusted for examiner and maternal race. The relationship between high molecular weight phthalate metabolite concentrations and Quality of Alertness appeared to be limited to girls. Among girls, there was an adjusted mean 0.48 point decline (95% CI -0.83, -0.12) per log unit increase in the sum of high molecular weight metabolites.
Discussion
We report associations between maternal urinary phthalate metabolite concentrations in the third trimester and neonatal behavior measured within five days of birth using the BNBAS. Specifically, there were strong, inverse associations between increasing concentrations of high molecular weight phthalates metabolite concentrations and orientation among girls. Similarly, there was an inverse association between high molecular weight metabolite concentrations and Quality of Alertness among girls. Among boys, there appeared to be a slight positive association between increasing low molecular weight metabolite concentrations and motor performance, although it was non-linear. According to our expectation, we did observe significant sex-phthalate interactions for BNBAS scales, which is consistent with the hypothesis that phthalates are hormonally active and may exert sex-specific effects (Wolff et al., 2008). These associations are intriguing in that they constitute the first reported association between phthalates and neurodevelopment. As such, replication is essential. Moreover, studies investigating the role of phthalates in neurodevelopment should be conducted in animals under more controlled conditions than those that can be achieved in observational studies.
The limitations of this study should be considered. First, the clinical or preclinical utility of a single assessment of infant behavior shortly after delivery is unclear. The BNBAS, though frequently used in studies examining prenatal drug (Richardson et al., 1996; Datta-Bhutada et al., 1998; Myers et al., 2003) and toxicant exposure (Jacobson et al., 1984; Rogan et al., 1986; Lonky et al., 1996; Stewart et al., 2000; Young et al., 2005; Engel et al., 2007; Fenster et al., 2007; Sagiv et al., 2008), was primarily designed as a screening tool to examine behavioral organization and detect gross neurological abnormalities at birth. We hypothesized that phthalate effects may be sex-specific based on the literature, although without regard to specific BNBAS domains, which represent general CNS organization. Previous studies of endocrine disruptors and BNBAS performance have noted effects in multiple domains, including Habituation (Lonky et al., 1996; Stewart et al., 2000), Motor Performance (Rogan et al., 1986), Range of State (Jacobson et al., 1984; Engel et al., 2007), Autonomic Stability (Jacobson et al., 1984; Lonky et al., 1996; Stewart et al., 2000), Alertness and Quality of Alertness (Sagiv et al., 2008), and abnormal primitive reflexes (Jacobson et al., 1984; Rogan et al., 1986; Lonky et al., 1996); therefore, there was plausibility for a range of findings. The long term consequences of abnormal neonatal behavior is understudied. However, there is evidence linking neonatal behavior to infant temperament (Tirosh et al., 1992), childhood behavioral problems (Ohgi et al., 2003; Canals et al., 2006) and developmental disabilities (Ohgi et al., 2003) . Therefore, the long-term implications of our findings are unclear.
In addition, phthalate metabolites have short half-lives and our exposure measurements rely on a single urine specimen taken during the 3rd trimester. However, if sources and patterns of exposure (for example residential pesticide use or exposure from a food source) are unchanged, we can assume that a single measurement reflects a typical measurement at any time during pregnancy. This is supported by several studies of urinary phthalate levels over days to months (Hauser et al., 2004; Teitelbaum et al., 2008). Even so, there is probable misclassification of both exposure and outcome in this study, although they are likely to be independent of one another.
Given the dearth of mechanistic studies relating phthalates to neural development, the mechanisms underlying the associations we report have not been established. Although there is little information about bioavailability to the fetus, the literature indicates that fetal exposure occurs and that phthalates with four-to-six carbon ester moieties exhibit antiandrogenicity (Committee on the Health Risks of Phthalates, 2008). Consequently, most of the research on phthalate esters focuses on their association with aberrant male reproductive development (Swan et al., 2005). Phthalate monoesters have been shown to affect fetal Leydig cell function, resulting in impaired androgen activity during a critical period of fetal sex differentiation (Swan et al., 2005). Androgens play a role in brain development in both sexes (Colciago et al., 2006). Treatment of rat dams with DEHP during gestation and lactation at doses that overlap with estimated exposure in the general human population induced changes in brain aromatase activity (encoded by CYP19) in male and female offspring. Aromatase catalyzes the conversion of testosterone to estradiol, which is critical for brain sexual differentiation (Andrade et al., 2006).
Additionally, the role of phthalates is testosterone biosynthesis may be mediated, at least in part, through peroxisome proliferator-activated receptors (PPARs), which in turn control fatty acid, synthesis critical for brain development. PPARs are members of the nuclear receptor superfamily and consist of three isoforms, namely PPARα, PPARß, and PPARγ. In response to ligand activation, PPARs heterodimerize with retinoid-X-receptor-α, interact with co-activators and peroxisome proliferator-response elements found in the promoter region of target genes, and modulate expression of target genes (Shearer and Hoekstra, 2003). PPARs activated by phthalates may also repress gene expression in a DNA-binding-independent manner through the recruitment of corepressors or by interfering with other DNA-associated transcription factors (Corton and Lapinskas, 2005). Phthalate monoesters are also thought to induce rodent hepatocarcinogenesis by activating PPARα (Peters et al., 1997; Hays et al., 2005), though this may not be directly relevant to human carcinogenesis. Finally, PPARs have been found in the developing neural tube (Braissant and Wahli, 1998). They are involved in adipogenesis and lipid homeostasis (Peters et al., 2000; Latini et al., 2008) and may affect neurodevelopment by altering lipid metabolism in the fetal brain (Xu et al., 2007).
Several studies have reported possible antagonistic effects of phthalates on the thyroid gland in vivo and thyroid tissue in vitro (Hinton et al., 1986; Price et al., 1988; Poon et al., 1997; Sugiyama et al., 2005; Pereira et al., 2007). Low serum free T4 was associated with high urinary concentration of monobutyl phthalate, a metabolite of dibutyl phthalate in a cohort of pregnant women during the second trimester (Huang et al., 2007), and with urinary MEHP in adult males (Meeker et al., 2007). In addition, an investigation of the effects of six phthalates on the transcriptional activity of a sodium/iodide symporter (NIS) reported that dibutyl phthalate appeared to down-regulate the human NIS promoter. This evidence suggests that some phthalates may modulate transcriptional activity resulting in decreased levels of T4 (Breous et al., 2005).
Brain development begins early in the first trimester of pregnancy, at a time when maternal thyroid and associated environmental insults are likely to exert the most damage. Fetal thyroid becomes active only late in gestation. During the first half of pregnancy, the mother is the only source of thyroid hormone available to the fetus, though by approximately 16-20 weeks gestation the fetal gland starts contributing to its own needs (Morreale de Escobar et al., 2004). The consequences of severe hypothyroxinemia during pregnancy are well described. Severe maternal iodine deficiency is associated with neurological cretinism (deafness, mental retardation, cerebral palsy); decreased IQ, and motor deficits in the baby (Morreale de Escobar, 2001; Ohara et al., 2004). However, even mild subclinical forms of maternal hypothyroidism during early gestation may adversely affect fetal neurodevelopment (Haddow et al., 1999; Pop et al., 1999).
Given the ubiquity of phthalates in the environment, the public health impact of even small negative effects of phthalates on neurodevelopment could be significant. Therefore, additional research is urgently needed to replicate these findings, and extend them to measures of childhood behavioral and cognitive development.
Acknowledgements
This research was supported by NIEHS/EPA Children's Center grants ES09584 and R827039, The New York Community Trust, and ATSDR/CDC/ATPM. Dr. Miodovnik was supported by NICHD 5T32HD049311. The authors would like to thank Ella Samandar, James Preau and John A. Reidy (CDC, Atlanta, GA) for technical assistance in measuring the concentrations of phthalate metabolites, and Martha Lievano and Stefanie Meisel for recruiting and following the birth cohort.
Abbreviations
- 1. BNBAS
Brazelton Neonatal Behavioral Assessment Scale
- 2. 95%CI
95% Confidence Interval
- 3. B
Beta
- 4. DNA
Deoxyribonucleic acid
- 5. MEHP
mono(2-ethylhexyl) phthalate
- 6. T4
thyroxine
- 7. PCB
Polychlorinated Biphenyls
- 8. CDC
Centers for Disease Control and Prevention
- 9. DEHP
di(2-ethylhexyl) phthalate
- 10. PPARs
peroxisome proliferator-activated receptors
- 11. NIS
sodium/iodide symporter
- 12. MMP
Monomethyl phthalate
- 13. MEP
monoethyl phthalate
- 14. MBzP
monobenzyl phthalate
- 15. MNBP
mono-n-butyl phthalate
- 16. MiBP
mono-isobutyl phthalate
- 17. MEOHP
mono(2-ethyl-5-oxohexyl) phthalate
- 18. MEHHP
mono(2-ethyl-5-hydroxyhexyl) phthalate
- 19. MECPP
mono(2-ethyl-5-carboxypentyl) phthalate
- 20. MCPP
mono(3-carboxypropyl) phthalate
Footnotes
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the CDC.
References
- Andrade AJ, Grande SW, Talsness CE, Grote K, Chahoud I. A dose-response study following in utero and lactational exposure to di-(2-ethylhexyl)-phthalate (DEHP): non-monotonic dose-response and low dose effects on rat brain aromatase activity. Toxicology. 2006;227(3):185–92. doi: 10.1016/j.tox.2006.07.022. [DOI] [PubMed] [Google Scholar]
- Berkowitz GS, Obel J, Deych E, Lapinski R, Godbold J, Liu Z, Landrigan PJ, Wolff MS. Exposure to indoor pesticides during pregnancy in a multiethnic, urban cohort. Environ Health Perspect. 2003;111(1):79–84. doi: 10.1289/ehp.5619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz GS, Wetmur JG, Birman-Deych E, Obel J, Lapinski RH, Godbold JH, Holzman IR, Wolff MS. In utero pesticide exposure, maternal paraoxonase activity, and head circumference. Environ Health Perspect. 2004;112(3):388–91. doi: 10.1289/ehp.6414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braissant O, Wahli W. Differential expression of peroxisome proliferator-activated receptor-alpha, -beta, and -gamma during rat embryonic development. Endocrinology. 1998;139(6):2748–54. doi: 10.1210/endo.139.6.6049. [DOI] [PubMed] [Google Scholar]
- Brazelton TB, Nugent JK. Neonatal Behavioral Assessment Scale. Mac Keith Press; London: 1995. [Google Scholar]
- Breous E, Wenzel A, Loos U. The promoter of the human sodium/iodide symporter responds to certain phthalate plasticisers. Mol Cell Endocrinol. 2005;244(1-2):75–8. doi: 10.1016/j.mce.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Canals J, Esparo G, Fernandez-Ballart JD. Neonatal behaviour characteristics and psychological problems at 6 years. Acta Paediatr. 2006;95(11):1412–7. doi: 10.1080/08035250600760790. [DOI] [PubMed] [Google Scholar]
- Colciago A, Negri-Cesi P, Pravettoni A, Mornati O, Casati L, Celotti F. Prenatal Aroclor 1254 exposure and brain sexual differentiation: effect on the expression of testosterone metabolizing enzymes and androgen receptors in the hypothalamus of male and female rats. Reprod Toxicol. 2006;22(4):738–45. doi: 10.1016/j.reprotox.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Committee on the Health Risks of Phthalates, N. R. C. Phthalates and Cumulative Risk Assessment The Task Ahead. National Academies Press; Washington, DC: 2008. [PubMed] [Google Scholar]
- Corton JC, Lapinskas PJ. Peroxisome proliferator-activated receptors: mediators of phthalate ester-induced effects in the male reproductive tract? Toxicol Sci. 2005;83(1):4–17. doi: 10.1093/toxsci/kfi011. [DOI] [PubMed] [Google Scholar]
- Datta-Bhutada S, Johnson HL, Rosen TS. Intrauterine cocaine and crack exposure: neonatal outcome. J Perinatol. 1998;18(3):183–8. [PubMed] [Google Scholar]
- Engel SM, Berkowitz GS, Barr DB, Teitelbaum SL, Siskind J, Meisel SJ, Wetmur JG, Wolff MS. Prenatal organophosphate metabolite and organochlorine levels and performance on the Brazelton Neonatal Behavioral Assessment Scale in a multiethnic pregnancy cohort. Am J Epidemiol. 2007;165(12):1397–404. doi: 10.1093/aje/kwm029. [DOI] [PubMed] [Google Scholar]
- Fenster L, Eskenazi B, Anderson M, Bradman A, Hubbard A, Barr DB. In utero exposure to DDT and performance on the Brazelton neonatal behavioral assessment scale. Neurotoxicology. 2007;28(3):471–7. doi: 10.1016/j.neuro.2006.12.009. [DOI] [PubMed] [Google Scholar]
- Haddow JE, Palomaki GE, Allan WC, Williams JR, Knight GJ, Gagnon J, O'Heir CE, Mitchell ML, Hermos RJ, Waisbren SE, Faix JD, Klein RZ. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N Engl J Med. 1999;341(8):549–55. doi: 10.1056/NEJM199908193410801. [DOI] [PubMed] [Google Scholar]
- Hatch EE, Nelson JW, Qureshi MM, Weinberg J, Moore LL, Singer M, Webster TF. Association of urinary phthalate metabolite concentrations with body mass index and waist circumference: a cross-sectional study of NHANES data, 1999-2002. Environ Health. 2008;7:27. doi: 10.1186/1476-069X-7-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser R, Meeker JD, Duty S, Silva MJ, Calafat AM. Altered semen quality in relation to urinary concentrations of phthalate monoester and oxidative metabolites. Epidemiology. 2006;17(6):682–91. doi: 10.1097/01.ede.0000235996.89953.d7. [DOI] [PubMed] [Google Scholar]
- Hauser R, Meeker JD, Park S, Silva MJ, Calafat AM. Temporal variability of urinary phthalate metabolite levels in men of reproductive age. Environ Health Perspect. 2004;112(17):1734–40. doi: 10.1289/ehp.7212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser R, Meeker JD, Singh NP, Silva MJ, Ryan L, Duty S, Calafat AM. DNA damage in human sperm is related to urinary levels of phthalate monoester and oxidative metabolites. Hum Reprod. 2007;22(3):688–95. doi: 10.1093/humrep/del428. [DOI] [PubMed] [Google Scholar]
- Hays T, Rusyn I, Burns AM, Kennett MJ, Ward JM, Gonzalez FJ, Peters JM. Role of peroxisome proliferator-activated receptor-alpha (PPARalpha) in bezafibrate-induced hepatocarcinogenesis and cholestasis. Carcinogenesis. 2005;26(1):219–27. doi: 10.1093/carcin/bgh285. [DOI] [PubMed] [Google Scholar]
- Hinton RH, Mitchell FE, Mann A, Chescoe D, Price SC, Nunn A, Grasso P, Bridges JW. Effects of phthalic acid esters on the liver and thyroid. Environ Health Perspect. 1986;70:195–210. doi: 10.1289/ehp.8670195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang PC, Kuo PL, Guo YL, Liao PC, Lee CC. Associations between urinary phthalate monoesters and thyroid hormones in pregnant women. Hum Reprod. 2007;22(10):2715–22. doi: 10.1093/humrep/dem205. [DOI] [PubMed] [Google Scholar]
- Jacobson JL, Jacobson SW, Fein GG, Schwartz PM, Dowler JK. Prenatal Exposure to an Environmental Toxin: A Test of the Multiple Effects Model. Developmental Psychology. 1984;20(4):523–532. [Google Scholar]
- Kato K, Silva MJ, Needham LL, Calafat AM. Determination of 16 phthalate metabolites in urine using automated sample preparation and on-line preconcentration/high-performance liquid chromatography/tandem mass spectrometry. Anal Chem. 2005;77(9):2985–91. doi: 10.1021/ac0481248. [DOI] [PubMed] [Google Scholar]
- Latini G, Scoditti E, Verrotti A, De Felice C, Massaro M. Peroxisome proliferator-activated receptors as mediators of phthalate-induced effects in the male and female reproductive tract: epidemiological and experimental evidence. PPAR Res. 2008;2008:359267. doi: 10.1155/2008/359267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester BM, Als H, Brazelton TB. Regional obstetric anesthesia and newborn behavior: a reanalysis toward synergistic effects. Child Dev. 1982;53(3):687–92. [PubMed] [Google Scholar]
- Lonky E, Reihman J, Darvill T, Mather J, Daly H. Neonatal Behavioral Assessment Scale Performance in Humans Influenced by Maternal Consumption of Environmentally Contaminated Lake Ontario Fish. J. Great Lakes Res. 1996;22(2):198–212. [Google Scholar]
- Meeker JD, Calafat AM, Hauser R. Di(2-ethylhexyl) phthalate metabolites may alter thyroid hormone levels in men. Environ Health Perspect. 2007;115(7):1029–34. doi: 10.1289/ehp.9852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morreale de Escobar G. The role of thyroid hormone in fetal neurodevelopment. J Pediatr Endocrinol Metab 14 Suppl. 2001;6:1453–62. [PubMed] [Google Scholar]
- Morreale de Escobar G, Obregon MJ, Escobar del Rey F. Role of thyroid hormone during early brain development. Eur J Endocrinol 151 Suppl. 2004;3:U25–37. doi: 10.1530/eje.0.151u025. [DOI] [PubMed] [Google Scholar]
- Myers BJ, Dawson KS, Britt GC, Lodder DE, Meloy LD, Saunders MK, Meadows SL, Elswick RK. Prenatal cocaine exposure and infant performance on the Brazelton Neonatal Behavioral Assessment Scale. Subst Use Misuse. 2003;38(14):2065–96. doi: 10.1081/ja-120025126. [DOI] [PubMed] [Google Scholar]
- Ohara N, Tsujino T, Maruo T. The role of thyroid hormone in trophoblast function, early pregnancy maintenance, and fetal neurodevelopment. J Obstet Gynaecol Can. 2004;26(11):982–90. doi: 10.1016/s1701-2163(16)30420-0. [DOI] [PubMed] [Google Scholar]
- Ohgi S, Arisawa K, Takahashi T, Kusumoto T, Goto Y, Akiyama T, Saito H. Neonatal behavioral assessment scale as a predictor of later developmental disabilities of low birth-weight and/or premature infants. Brain Dev. 2003;25(5):313–21. doi: 10.1016/s0387-7604(02)00233-4. [DOI] [PubMed] [Google Scholar]
- Ohgi S, Takahashi T, Nugent JK, Arisawa K, Akiyama T. Neonatal behavioral characteristics and later behavioral problems. Clin Pediatr (Phila) 2003;42(8):679–86. doi: 10.1177/000992280304200803. [DOI] [PubMed] [Google Scholar]
- Pereira C, Mapuskar K, Vaman Rao C. A two-generation chronic mixture toxicity study of Clophen A60 and diethyl phthalate on histology of adrenal cortex and thyroid of rats. Acta Histochem. 2007;109(1):29–36. doi: 10.1016/j.acthis.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Peters JM, Cattley RC, Gonzalez FJ. Role of PPAR alpha in the mechanism of action of the nongenotoxic carcinogen and peroxisome proliferator Wy-14,643. Carcinogenesis. 1997;18(11):2029–33. doi: 10.1093/carcin/18.11.2029. [DOI] [PubMed] [Google Scholar]
- Peters JM, Lee SS, Li W, Ward JM, Gavrilova O, Everett C, Reitman ML, Hudson LD, Gonzalez FJ. Growth, adipose, brain, and skin alterations resulting from targeted disruption of the mouse peroxisome proliferator-activated receptor beta(delta). Mol Cell Biol. 2000;20(14):5119–28. doi: 10.1128/mcb.20.14.5119-5128.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon R, Lecavalier P, Mueller R, Valli VE, Procter BG, Chu I. Subchronic oral toxicity of di-n-octyl phthalate and di(2-Ethylhexyl) phthalate in the rat. Food Chem Toxicol. 1997;35(2):225–39. doi: 10.1016/s0278-6915(96)00064-6. [DOI] [PubMed] [Google Scholar]
- Pop VJ, Kuijpens JL, van Baar AL, Verkerk G, van Son MM, de Vijlder JJ, Vulsma T, Wiersinga WM, Drexhage HA, Vader HL. Low maternal free thyroxine concentrations during early pregnancy are associated with impaired psychomotor development in infancy. Clin Endocrinol (Oxf) 1999;50(2):149–55. doi: 10.1046/j.1365-2265.1999.00639.x. [DOI] [PubMed] [Google Scholar]
- Price SC, Chescoe D, Grasso P, Wright M, Hinton RH. Alterations in the thyroids of rats treated for long periods with di-(2-ethylhexyl) phthalate or with hypolipidaemic agents. Toxicol Lett. 1988;40(1):37–46. doi: 10.1016/0378-4274(88)90181-6. [DOI] [PubMed] [Google Scholar]
- Richardson GA, Hamel SC, Goldschmidt L, Day NL. The effects of prenatal cocaine use on neonatal neurobehavioral status. Neurotoxicol Teratol. 1996;18(5):519–28. doi: 10.1016/0892-0362(96)00062-1. [DOI] [PubMed] [Google Scholar]
- Rogan WJ, Gladen BC, McKinney JD, Carreras N, Hardy P, Thullen J, Tinglestad J, Tully M. Neonatal effects of transplacental exposure to PCBs and DDE. J Pediatr. 1986;109(2):335–41. doi: 10.1016/s0022-3476(86)80397-3. [DOI] [PubMed] [Google Scholar]
- Sagiv SK, Nugent JK, Brazelton TB, Choi AL, Tolbert PE, Altshul LM, Korrick SA. Prenatal organochlorine exposure and measures of behavior in infancy using the Neonatal Behavioral Assessment Scale (NBAS). Environ Health Perspect. 2008;116(5):666–73. doi: 10.1289/ehp.10553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearer BG, Hoekstra WJ. Recent advances in peroxisome proliferator-activated receptor science. Curr Med Chem. 2003;10(4):267–80. doi: 10.2174/0929867033368295. [DOI] [PubMed] [Google Scholar]
- Silva MJ, Barr DB, Reidy JA, Malek NA, Hodge CC, Caudill SP, Brock JW, Needham LL, Calafat AM. Urinary levels of seven phthalate metabolites in the U.S. population from the National Health and Nutrition Examination Survey (NHANES) 1999-2000. Environ Health Perspect. 2004;112(3):331–8. doi: 10.1289/ehp.6723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva MJ, Preau JL, Jr., Needham LL, Calafat AM. Cross validation and ruggedness testing of analytical methods used for the quantification of urinary phthalate metabolites. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;873(2):180–6. doi: 10.1016/j.jchromb.2008.08.017. [DOI] [PubMed] [Google Scholar]
- Stahlhut RW, van Wijngaarden E, Dye TD, Cook S, Swan SH. Concentrations of urinary phthalate metabolites are associated with increased waist circumference and insulin resistance in adult U.S. males. Environ Health Perspect. 2007;115(6):876–82. doi: 10.1289/ehp.9882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart P, Reihman J, Lonky E, Darvill T, Pagano J. Prenatal PCB exposure and neonatal behavioral assessment scale (NBAS) performance. Neurotoxicol Teratol. 2000;22(1):21–9. doi: 10.1016/s0892-0362(99)00056-2. [DOI] [PubMed] [Google Scholar]
- Sugiyama S, Shimada N, Miyoshi H, Yamauchi K. Detection of thyroid system-disrupting chemicals using in vitro and in vivo screening assays in Xenopus laevis. Toxicol Sci. 2005;88(2):367–74. doi: 10.1093/toxsci/kfi330. [DOI] [PubMed] [Google Scholar]
- Swan SH, Main KM, Liu F, Stewart SL, Kruse RL, Calafat AM, Mao CS, Redmon JB, Ternand CL, Sullivan S, Teague JL. Decrease in anogenital distance among male infants with prenatal phthalate exposure. Environ Health Perspect. 2005;113(8):1056–61. doi: 10.1289/ehp.8100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teitelbaum SL, Britton JA, Calafat AM, Ye X, Silva MJ, Reidy JA, Galvez MP, Brenner BL, Wolff MS. Temporal variability in urinary concentrations of phthalate metabolites, phytoestrogens and phenols among minority children in the United States. Environ Res. 2008;106(2):257–69. doi: 10.1016/j.envres.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Tirosh E, Harel J, Abadi J, Berger A, Cohen A. Relationship between neonatal behavior and subsequent temperament. Acta Paediatr. 1992;81(10):829–31. doi: 10.1111/j.1651-2227.1992.tb12112.x. [DOI] [PubMed] [Google Scholar]
- Wolff MS, Engel S, Berkowitz G, Teitelbaum S, Siskind J, Barr DB, Wetmur J. Prenatal pesticide and PCB exposures and birth outcomes. Pediatr Res. 2007;61(2):243–50. doi: 10.1203/pdr.0b013e31802d77f0. [DOI] [PubMed] [Google Scholar]
- Wolff MS, Engel SM, Berkowitz GS, Ye X, Silva MJ, Zhu C, Wetmur J, Calafat AM. Prenatal phenol and phthalate exposures and birth outcomes. Environ Health Perspect. 2008;116(8):1092–7. doi: 10.1289/ehp.11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Agrawal S, Cook TJ, Knipp GT. Di-(2-ethylhexyl)-phthalate affects lipid profiling in fetal rat brain upon maternal exposure. Arch Toxicol. 2007;81(1):57–62. doi: 10.1007/s00204-006-0143-8. [DOI] [PubMed] [Google Scholar]
- Young JG, Eskenazi B, Gladstone EA, Bradman A, Pedersen L, Johnson C, Barr DB, Furlong CE, Holland NT. Association between in utero organophosphate pesticide exposure and abnormal reflexes in neonates. Neurotoxicology. 2005;26(2):199–209. doi: 10.1016/j.neuro.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Zhang YH, Zheng LX, Chen BH. Phthalate exposure and human semen quality in Shanghai: a cross-sectional study. Biomed Environ Sci. 2006;19(3):205–9. [PubMed] [Google Scholar]