Abstract
Four appetitive conditioning experiments studied generalization between compound conditional stimuli (AB) and their elements (e.g., A or B). In Experiments 1 and 2, rats received conditioning with A and AB and then extinction with either A or AB. During subsequent testing, there was more generalization of extinction (nonresponding) from the compound (AB) to the element (A) than from the element (A) to the compound (AB). This asymmetry was consistent with earlier results involving temporal discrimination learning in which short and long temporal intervals played the roles of A and AB. In Experiment 3, rats received conditioning with either A or AB and then testing with A and AB. Consistent with elemental models of conditioning, there was more generalization of conditioned responding from A to AB than from AB to A. Experiment 4 found that these asymmetries in the generalization of extinction (Experiments 1–2) and conditioning (Experiment 3) both contribute to the feature-positive effect. Overall, the parallel between the current findings and previous results with temporal discrimination learning supports an associative analysis of interval timing. Implications for elemental and configural theories of conditioning and generalization are also discussed.
Contemporary conditioning theories make different predictions regarding the generalization between stimulus compounds (e.g., Stimuli A and B presented together as AB) and their elements (e.g., A or B presented alone). For example, Pearce’s “configural” learning model (Pearce, 1987, 1994) assumes that an animal will learn about the entire pattern of stimuli present on any conditioning trial. When element A is paired with an unconditional stimulus (US), the animal will learn an association between Stimulus A and the US. When compound AB is paired with a US, the animal will learn an association between Stimulus AB and the US. The amount of generalization to the other stimulus is assumed to be a function of the similarity between the conditioned and the test stimulus. Since similarity between an element (A) and compound (AB) is the same whether A is tested after conditioning of AB or AB is tested after conditioning of A, any generalization from AB to A or A to AB should be equivalent and symmetrical.
In contrast, “elemental” learning models such as the Rescorla-Wagner model (Rescorla & Wagner, 1972) and Wagner’s subsequent SOP (Sometimes Opponent Process) model (Wagner, 1981, 2003, 2008) predict a fundamental asymmetry in generalization between elements and compounds. When A and B are conditioned together (AB+), they will acquire separate associations with the US that will compete for the total amount of conditioning that is supported by the US. Therefore, when A is removed from AB and tested on its own, it will elicit less responding than the AB compound. In contrast, when A is conditioned alone (A+), adding B to A during a generalization test with AB will have no influence on associative strength, and hence cause no change in responding. Wagner’s most recent formulations (2003, 2008; see also Harris, 2006) predict that some generalization decrement may occur when elements of the added B stimulus replace conditioned elements in A. But the decrement in responding when switching from A+ to AB will generally be less than that when switching from AB+ to A.
Several laboratories have tested these predictions. In animal conditioning (Brandon, Vogel, & Wagner, 2000; Gonzalez, Quinn, & Fanselow, 2003) and in human causal judgment experiments (Glautier, 2004; Wheeler, Amundson, & Miller, 2006), asymmetrical generalization has been observed: Switching from compound to element has yielded a larger loss of responding than switching from element to compound (see also related results by Rescorla, 1999). Although these data are consistent with elemental models, there is contrasting evidence of symmetrical generalization under some conditions in animal conditioning (Young, 1984, reported in Pearce, 1987) and in human associative learning (Thorwart & Lachnit, 2009).
The present experiments provide new tests of generalization between compounds and elements. Their focus was the generalization of extinction. They started as an extension of recent research that studied how animals generalize (and discriminate) between different temporal intervals. Bouton and García-Gutiérrez (2006) and Bouton and Hendrix (2011) asked whether rats can use the interval between successive conditioning trials (the inter-trial interval or ITI) to predict whether or not the next tone conditional stimulus (CS) would be paired with a food pellet US. For example, in several experiments, different groups of rats received a 10-s tone CS separated half the time by long ITIs (e.g., 16 min) and half the time by short ITIs (e.g., 4 min). For Long+/Short− groups, tone presentations that followed the long ITI were reinforced while tones that followed the short ITI were not. Rats learned these discriminations readily; for example, they quickly learned to check the foodcup where the US was delivered in the presence of the tone more when it followed the long interval than when it followed the short interval. However, other rats received Short+/Long− discriminations. For these rats, tones that followed the short interval (e.g., 4 min) were reinforced whereas those that followed the long interval (e.g., 16 min) were not. In contrast to animals given the Long+/Short− discrimination, rats given Short+/Long− often did not learn to respond differentially to the tone. Thus, although the ITI could clearly serve as a discriminative cue, discrimination learning was asymmetrical; the Long+/Short− discrimination was learned more rapidly than Short+/Long−. Other experiments have produced a similar asymmetry when timing has occurred within CSs instead of during ITIs (Kyd, Pearce, Haselgrove, Amin, & Aggleton, 2007; Todd, Winterbauer, & Bouton, 2010).
Bouton and García-Gutiérrez (2006) and Bouton and Hendrix (2011) noted that this asymmetry was not anticipated by most models of interval timing. However, it is consistent with the idea that the passage of time involves exposure to a series of hypothetical “temporal elements” in which element A is followed by B (which is followed by C, etc.). According to this approach, a short interval might be represented by A, and a longer interval might be represented by A and B. In these terms, the Long+/Short− discrimination takes the form of an AB+/A−, or “feature-positive” discrimination, while the Short+/Long discrimination takes the form of A+/AB−, a “feature-negative” discrimination. Long+/Short− discriminations might be easier than the Short+/Long− discriminations because they are a new example of the feature-positive effect: Feature-positive discriminations are known to be easier to learn than feature-negative discriminations (e.g., Jenkins & Sainsbury, 1970; Hearst, 1978, 1984). Bouton and Hendrix (2011) tested and failed to confirm several alternative explanations, and consistent with the temporal elements concept, they found a strong feature-positive effect when a noise and light CSs were substituted for the hypothetical temporal elements and studied in a serial compound conditioning experiment.
It should be noted that the asymmetry inherent in the feature-positive effect is more consistent with elemental models (e.g., Rescorla & Wagner, 1972) than with configural models (Pearce, 1987, 1994). This is at least partly because, as noted above, elemental models predict less generalization of excitation from AB+ to A trials in the feature-positive discrimination than from A+ to AB trials in the feature-negative discrimination. Such a pattern would allow the feature-positive discrimination to develop more rapidly. The Pearce model once again predicts symmetrical generalization, and without modification does not predict the feature-positive effect (see Lotz, Uengoer, Koenig, Pearce, & Lachnit, 2012, for a recent discussion).
Bouton and García-Gutiérrez (2006) also reported a second asymmetry in generalization between temporal intervals that can be considered independently of the one just described. They ran another experiment in which rats initially received trials in which a 10-s tone CS was reinforced after both long (16-min) and short (4-min) ITIs. In a subsequent extinction phase, different groups received nonreinforced trials with the tone separated by either a 4-min or 16-min ITI. When the rats were then tested 16 min later, rats that had received extinction with the 4-min ITI showed responding to the tone (a form of spontaneous recovery), but rats extinguished with the 16-min ITI did not. Since extinction after 16-min ITIs allows spontaneous recovery after much longer intervals (e.g., 72 hrs; Moody, Sunsay, & Bouton, 2006), the results were consistent with the idea that the ITI was encoded as part of the extinction context, and that extinction with the 4-min ITI did not generalize to the 16-min ITI. However, Bouton and García-Gutiérrez also reported that in two unpublished experiments, testing with a 4-min retention interval did not yield a recovery of responding when extinction had been conducted with a 16-min ITI. Thus, a similar mismatch between the extinction ITI and the test interval was not sufficient to yield recovered responding. In generalization terms, extinction with a long ITI generalized to a short ITI, whereas extinction with a short ITI did not generalize to a long ITI.
The upper part of Table 1 illustrates this result in schematic form. During conditioning, reinforced trials occurred after both 4-min and 16-min intervals (4+ and 16+); during extinction nonreinforced trials then occurred with either 4-min or 16-min intervals (4− or 16−); and during the final test phase test trials occurred after either 4-min or 16-min intervals. The bolded number in the test column (16) indicates the one condition in which test responding (and thus generalization decrement) was observed. The lower part of Table 1 then translates the ITIs into their hypothetical temporal elements. As before, the short interval is A, and the longer interval is AB. As the table indicates, if there is a parallel between temporal intervals and explicit compounds and elements, then in a compound conditioning experiment run along these lines, responding to AB should still be strong after extinction of A, but responding to A should be weak after extinction with AB. Put another way, there should be more generalization of extinction from AB to A than from A to AB.
Table 1.
Generalization tests of extinction over long and short temporal intervals (ITIs) and explicit compound and elemental CSs
| Conditioning | Extinction | Test |
|---|---|---|
| Temporal Intervals | ||
| 4+, 16+ | 4− | 4, 16 |
| 4+, 16+ | 16− | 4, 16 |
| Explicit CSs | ||
| A+, AB+ | A− | A, AB |
| A+, AB+ | AB− | A, AB |
Note: 4 and 16 denote ITIs; A and B denote explicit conditional stimuli; + = reinforced
trials; − = nonreinforced trials; bold font denotes predicted generalization decrement.
To our knowledge, this prediction is new and has never been tested. It is interesting from the parallel between temporal intervals and CS compounds and elements suggested by the temporal elements hypothesis. But it is also significant because the models of generalization just considered appear to predict different outcomes. Here again, the Pearce model predicts that generalization of extinction will be symmetrical; there should be an equal increase in responding to AB after A’s extinction and to A after AB’s extinction. In contrast, elemental models would again predict asymmetrical generalization, such that there should be more generalization of extinction learning from A to AB than from AB to A. For example, in SOP theory (Wagner, 1981, 2003, 2008), extinction with the AB compound would cause A and B to share the available extinction (inhibitory) learning. But notice that this predicted asymmetry is the opposite of what the temporal elements parallel predicts. Thus, almost any outcome of an experiment like the one illustrated in Table 1 would be informative, and we therefore set out to run it in our laboratory. We used our appetitive conditioning procedure with noise and light CSs in the role of elements A and B (see also Bouton & Hendrix, 2011).
Experiment 1
The design of Experiment 1 is illustrated in the lower half of Table 1. Rats first received appetitive conditioning with 10-s noise and light CSs (counterbalanced) in a phase that intermixed A+ and AB+ trials. The compound trials were simultaneous, such that the onsets and offsets of A and B occurred at the same time. At the end of conditioning, different groups received extinction trials with either A alone (A−) or the AB compound (AB−) before being tested with both A and AB. If there is a parallel between time and explicit CSs, as suggested by the temporal elements hypothesis, the results of Bouton and García-Gutiérrez (2006) would predict responding to AB after A-− training but little responding to A after AB-−. As noted above, such an outcome would seem inconsistent with current approaches to compound-element generalization (e.g., Pearce, 1987, 1994; Harris, 2006; Rescorla & Wagner, 1972; Wagner, 1981, 2003, 2008).
In an unpublished experiment, we confirmed the asymmetry predicted by the temporal elements view in a serial procedure in which A preceded and then overlapped with B (A was a 20-s stimulus whereas B was a 10-s stimulus that, when compounded with A, came on during its latter half). Such a procedure modeled the way that temporal elements might unfold in time (e.g., Desmond & Moore, 1988; see Bouton & Hendrix, 2011, for discussion). There was indeed more generalization of extinction from AB to A than from A to AB. However, that result could have occurred because extinction with the serial AB compound involved exposure to 10 s of A alone as well as 10 s of AB on each trial; the animals were thus arguably extinguished with both A and AB. It would be more surprising to observe the same result with a standard procedure in which A and B were presented simultaneously. In the present experiment, we therefore used the same design with a simultaneous procedure in which A and AB were both 10 s and the onset and offset of B coincided perfectly with that of A during AB.
Method
Subjects
The subjects were 32 naive, female Wistar rats purchased from the Charles River Laboratories (St. Constance, Que.). They were between 75 and 90 days old at the start of the experiment and were individually housed in suspended wire mesh cages in a room on a 12:12 light:dark cycle. The rats were food-deprived to 80% of their initial body weights throughout the experiment. The experiment was run in two replications of 16 rats that each involved 8 rats per group.
Apparatus
The apparatus consisted of two sets of four conditioning chambers housed in separate rooms of the laboratory. Each chamber was housed in its own sound attenuation chamber. All boxes measured 30.5 cm × 24.1 × 23.5 cm (l × w × h). The side walls and ceiling were made of clear acrylic plastic, while the front and rear walls were made of brushed aluminum. The floor was made of stainless steel grids (0.48 in diameter). A recessed 5.08 cm × 5.08 cm food cup was centered in the front wall approximately 2.5 above the level of the floor. Infrared photocells positioned in the cup just behind the plane of the wall monitored entries into the food cup. A 28-V panel light (2.5 cm in diameter) was attached to the wall 10.8 cm above the floor and 6.4 cm to the left of the food cup. The two sets of four boxes had unique features that allow them to be used as different contexts (although they were not used in that capacity here). In one set of boxes, one side wall had black diagonal stripes, 3.81 cm wide and 3.81 cm apart. The ceiling had similarly spaced stripes oriented in the same direction. The grids of the floor were mounted on the same plane and were spaced 1.6 cm apart (center-to-center). The other set of boxes had no distinctive visual cues, and the grids of the floor were staggered such that odd- and even-numbered grids were mounted in two separate planes, one 0.5 cm above the other.
The chambers were illuminated by two 7.5-W incandescent bulbs mounted to the ceiling of the sound attenuation chamber, approximately 34.9 cm from the grid floor. There were two CSs, each of which came on for 10 s whenever it was presented. One CS was an intermittent white noise (pulsed 4 times/s) presented through the speaker mounted to the ceiling of the sound attenuation chamber. The noise was 70 dB(A) above a 65–66 dB(A) background. The other CS was a flashing (0.4-s on alternated with 0.1-s off) of the 28-V panel light that was mounted on the wall above and to the left of the food cup. The US was provided by two 45 mg food pellets (Traditional formula, Research Diets, New Brunswick, NJ) delivered 0.2 s apart. The apparatus was controlled by computer equipment located in an adjacent room.
Procedure
Magazine Training
All rats were first assigned to a box and trained to retrieve food pellets from the food cup. Each rat received approximately 30 food pellets evenly distributed during a 20-min session.
Conditioning
On each of the next 6 days, the rats received a 42-min conditioning session. In each session, a 10-s CS was paired with the US on 16 trials with a variable 2-min intertrial interval (ITI) defined as the period between the offset of a CS and the beginning of the next pre-CS period (± 25%). There were eight trials each of A and AB; A and B were counterbalanced over noise and light, so that half the rats received noise as A and light as B and half received the reverse. For all rats, A and AB trials were double alternated (so that trial type did not predict the next type of trial), but always started with the compound (AB).
Extinction and generalization test
On the following day, all rats received a single extinction and test session. The session began with four conditioning trials in the usual order (AB+, AB+, A+, and A+), followed by eight extinction trials, and then four test trials (two each of A and AB). The ITI was again a variable 2-min. Sixteen rats (8 in each replication) received extinction with A (counterbalanced over noise and light) and 16 rats (8 in each replication) received extinction with the AB compound. Testing then began without interruption (i.e., after another 2-min ITI). There were four nonreinforced test trials presented in the order of A, AB, AB, A for half the rats from each group and AB, A, A, and AB for the other half. This sequence was orthogonal to stimulus assignment (noise or light as CS A).
Data analyses
The computer counted magazine entries during each 10-s CS and during the 10-s period that preceded the CS (the “pre-CS period”). The primary measure of conditioned responding was elevation scores of the form e=c – p, where c represents the number of responses recorded during the CS and p the number of responses in the corresponding pre-CS period. Elevation scores have been used extensively in this preparation (e.g., Bouton & Sunsay, 2003; Brooks & Bouton, 1993) because they separate CS responding from baseline responding. Elevation scores and pre-CS responses were analyzed with parallel analyses of variance (ANOVAs), using a rejection criterion of p < .05. We routinely included CS Counterbalancing (whether light and noise or noise and light served as A and B) as a factor because the noise often elicited more responding than the light, and it was important to assess whether key results depended on counterbalancing assignment. Planned comparisons examined whether there was a difference in responding to A and AB (i.e., generalization decrement) after each extinction treatment.
Results
Acquisition
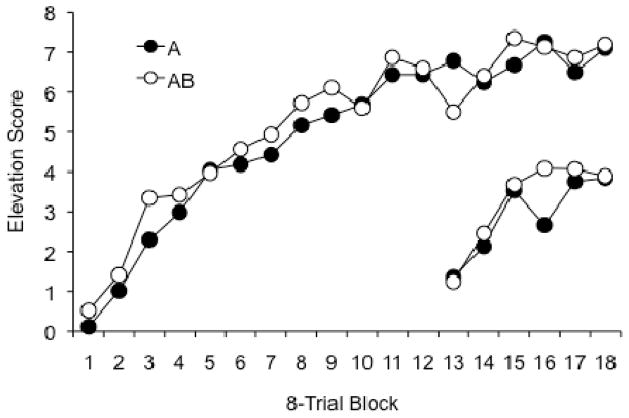
The results of the acquisition phase are shown in Figure 1. The data were consistent over replications (statistical analyses indicated no interactions with the replication factor), so the figure and the statistical tests reported here combined the data over replications. The rats learned to respond in the presence of both A and AB. A Stimulus (A or AB) x CS Counterbalancing (whether light and noise or noise and light as A and B) x Session ANOVA revealed significant effects of Session, F (5, 150) = 30.94, MSE = 3.39, and of Stimulus, F (1,30) = 11.41, MSE = 1.30. None of the other factors or interactions were significant. The results confirm that responding developed steadily during training, and that there was more responding to the AB compound than to A alone.
Figure 1.
Mean responding to the element (A) and the compound (AB) during the acquisition phase of Experiment 1.
Extinction and testing
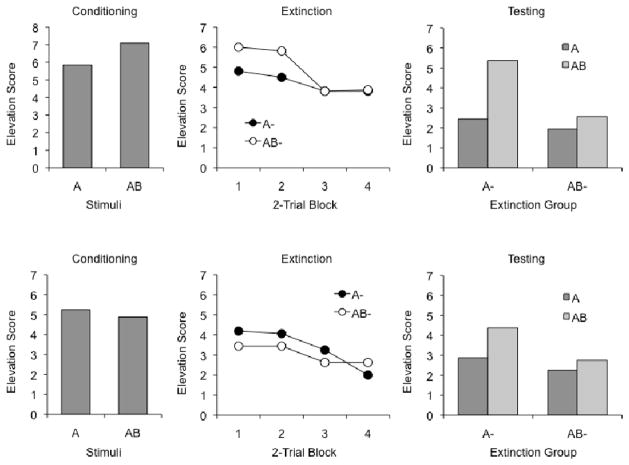
The results of the test session are shown in Figure 2. The left panel summarizes responding to A and AB at the start of the session; there was no difference in responding to the compound and element on these trials. A Stimulus (A or AB) x CS Counterbalancing (light and noise or noise and light as A and B) ANOVA indicated no main effects or interactions, ps ≥ .35. The middle panel of Figure 2 shows responding during the four 2-trial blocks of extinction. Here again, there was no evidence of different levels of responding to A and AB. A Group (A− or AB−) x CS Counterbalancing x Trial-block ANOVA uncovered an effect of trial block, F (3,84) = 5.04, MSE = 4.63, but no other main effects or interactions, ps ≥ .07. The Group effect did not approach significance, F (1, 28) < 1.
Figure 2.
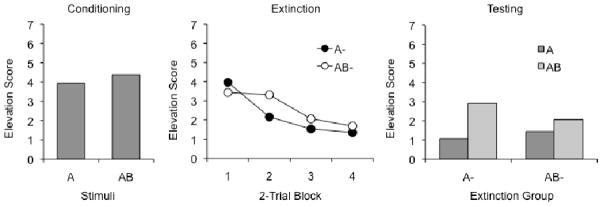
Mean responding during testing in Experiment 1. Left: Responding during the final reinforced trials with CSs AB and A. Center: Responding during the extinction trials. Right: Responding during the final generalization tests.
The crucial test trials with A and AB after extinction are shown in the panel at right. The results were consistent with the temporal elements view: Extinction with AB left little responding to either A or AB, whereas extinction with A resulted in little responding to A, but significantly more to AB. This conclusion was supported by statistical analysis. A Group x Test Stimulus x CS Counterbalancing ANOVA indicated no difference between Groups, F (1, 28) < 1, although there was a reliable overall main effect of Test Stimulus, F (1, 28) = 9.82, MSE = 69.48, indicating more responding to AB than to A. No other main effects or interactions approached significance, Fs ≤ 1.01. The Group x Test Stimulus interaction was not significant, F (1, 28) = 2.39, MSE = 69.48, p = .13 (but see Experiment 2). However, planned Test Stimulus x CS counterbalancing ANOVAs on the data of each group revealed that, for the group extinguished with A alone, there was significantly more responding to AB than to A, F (1, 14) = 23.82, MSE = 15.98. In contrast, for the group extinguished with AB, there was no difference between responding in AB and A, F (1, 14) < 1. No other effects or interactions approached significance in either group, all Fs < 1.
Pre-CS responding
Parallel ANOVAs were run on pre-CS responding in each of the phases. None of the main effects or interactions approached significance, ps ≥ .10, except for the main effect of extinction trial block in the analysis of the extinction data, F (3,90) = 4.43, MSE = 2.35, where responding decreased over trials, from a mean of 1.9 on the first block to 0.5 on last block. Pre-CS responding averaged 1.7 over all the trial blocks of acquisition, 1.1 during extinction, and 0.8 during the final tests of A and AB.
Discussion
The results of Experiment 1 paralleled the results that Bouton and García-Gutiérrez (2006) reported with temporal intervals (ITIs). As in that case, when rats were given initial conditioning with A+ and AB+, extinction of AB generalized more to A than extinction of A generalized to AB. As discussed in the introduction, that result is consistent with the temporal elements analysis of the passage of time, which holds that explicit CSs in the roles of the hypothetical temporal elements should produce the same effects. However, it does not appear to be consistent with either the Pearce model (Pearce, 1987, 1994), which predicts equivalent generalization (or generalization decrement) from A to AB and AB to A, or with elemental models (e.g., Harris, 2006; Rescorla & Wagner, 1972; Wagner, 1981, 2003, 2008), which predict asymmetrical generalization in the form of A to AB being stronger than AB to A.
Experiment 2
A closer analysis of the Rescorla-Wagner model suggests another way to interpret the results of Experiment 1. Ideally, because the intermixed A+ and AB+ trials in Phase 1 constitutes a concurrent blocking design (e.g., Wagner & Saavedra, 1969, reported in Rescorla & Wagner, 1972), the associative strength of A should approach the maximal value supported by the US, while that of B should approach zero. When AB is then extinguished, the associative strengths of each element should decline from their respective starting points until their summed associative strengths approaches zero, the asymptote supported by trials with no US. Since B starts at essentially zero, it will acquire a negative value (inhibition), and ultimately “protect” CS A from full associative loss—A and B will stop losing associative strength when their absolute values become equal (e.g., Rescorla, 2003). Of course, this is the prediction that was not confirmed in Experiment 1. B did not protect A from associative loss; on the contrary, extinction of A in compound with B led to very little responding to A when it was tested alone.
It is worth noting, however, that when A+ and AB+ trials are intermixed from the beginning of training, early trials with AB+ will give B some associative strength which will then theoretically decline as A acquires enough strength to generate complete blocking of B. Thus, B’s final point of zero associative strength might take extended, and possibly extensive, training. If Phase 1 of Experiment 1 had ended before B reached zero, adding B to A in the compound extinction condition (AB−) could create a larger predictive error than extinction with A alone and therefore yield greater associative loss to A (e.g., Rescorla, 2000). Conceivably, incomplete blocking with B would thus allow the Rescorla-Wagner model to predict the Experiment 1 results, in which extinction with AB caused little responding to A. It would also allow extinction with A to leave high responding to AB, because B would have some associative strength, if incompletely blocked, that would be unaffected by extinction of A.
Experiment 2 was therefore designed to replicate Experiment 1 and further test for the effect of more extensive Phase 1 conditioning. It specifically compared the effects of extinction to A or AB after each had received 48 conditioning trials, as in Experiment 1, or 144 conditioning trials, three times Experiment 1’s amount. As noted above, elemental models, including the Rescorla-Wagner model, would predict greater generalization of extinction from A to AB than AB to A after a conditioning procedure that yielded so little conditioning of B.
Method
Subjects and Apparatus
The subjects were 32 naive female Wistar rats of the same age and from the same supplier as those in the previous experiments. The apparatus, CSs, US, and maintenance conditions were also the same.
Procedure
Magazine Training
Each rat was assigned to a box and trained to retrieve food pellets from the food cup on the day before its conditioning treatment began. There were approximately 30 food pellets distributed evenly throughout a 25-min session.
Conditioning
On Day 1, the 16 rats in the Extended training condition received magazine training (as above). On Day 2, they received the first of nine 76-min daily conditioning sessions. In each of these sessions, there were 16 reinforced presentations of CS A (noise or light, counterbalanced) and 16 reinforced presentations of the simultaneous AB compound. The ITI was variable with a mean of 2 min. As in Experiment 1, the A and AB trials were double alternated; in this case odd-numbered sessions started with AB trials and even-numbered sessions started with A. The 16 rats in the Non-Extended condition received equivalent handling each day until Day 7, when magazine training occurred; they then received conditioning sessions identical to the other subjects on Days 8, 9, and 10. The staggered start allowed all animals to undergo testing on the same day (Day 11) after receiving either 48 (Non-Extended condition) or 144 (Extended condition) A+ and AB+ trials.
Extinction and generalization test
On Day 11, all rats received a single 42 min test session like the one used in Experiment 1. The session began with four conditioning trials (AB+, AB+, A+, then A+) followed by eight extinction trials with either A− or AB−, each for half the animals (n = 8) in the Extended and Non-Extended groups. As usual, the identities of A and B were counterbalanced. The session then concluded with two nonreinforced tests each of A and AB (in a counterbalanced ABBA or BAAB sequence). The trials were separated by a variable 2-min ITI.
Results
Acquisition
The results of the acquisition phase (Figure 3) were similar to those of Experiment 1. There was evidence that the AB compound initially acquired more responding than Stimulus A alone. This was confirmed in both groups by separate Stimulus x CS Counterbalancing x 8-Trial Block ANOVAs. For the Extended-trained group, there were significant effects of Block, F (17, 238) = 18.67, MSE = 7.18, and Stimulus, F (1, 14) = 5.72, MSE = 1.74; AB evoked more responding than A. No other effect or interaction was significant, ps ≥ .12. For the Non-Extended group, there were likewise effects of both Block, F (5, 70) = 8.68, MSE = 4.03, and Stimulus, F (1, 14) = 7.86, MSE = 0.77. In this group, the effects of CS Counterbalancing, F (1, 14) = 7.99, MSE = 22.21, and the Stimulus x Counterbalancing interaction, F (1, 14) = 25.63, MSE = 0.77, were also significant. The latter effects were consistent with the fact that the noise controlled more responding than the light. No other effects approached significance, ps ≥ .12.
Figure 3.
Mean responding to the element (A) and the compound (AB) during the acquisition phase of Experiment 2. The Extended group received conditioning over all 8-trial blocks, whereas the Non-Extended group received conditioning only during blocks 13–18.
Extinction and testing
The results of the test session are shown in Figure 4. The results with the Extended training are at the top, while the results with the Non-Extended procedure are at the bottom. As in Experiment 1, there was a similar level of responding to A and AB when they were tested at the start of the test session (left panels). A Stimulus x Amount of Conditioning (Extended vs. Non-Extended) x CS Counterbalancing ANOVA revealed no effects or interactions, ps ≥ .28; the effect of Amount of Conditioning, F (1, 28) = 3.10, MSE = 18.74, was not significant, p = .09. Also as in Experiment 1, there was no difference in responding to the A and AB compounds during extinction (middle panels): A Stimulus x Amount of Conditioning x CS Counterbalancing x Block ANOVA revealed a main effect of Block, F (3, 72) = 2.97, MSE = 6.31, Amount of Conditioning, F (1, 24) = 4.46, MSE = 13.10, and Counterbalancing, F (1, 24) = 5.67, MSE = 13.10. Once again, there was more responding to the noise than light, and the groups that received Extended training responded more overall than the Non-Extended rats. There were no other effects or interactions, ps ≥ .11.
Figure 4.
Mean responding in the Extended-trained group (top panels) and the Non-Extended-trained group (bottom panels) during testing in Experiment 2. Left panels: Responding during the final reinforced trials with CSs AB and A. Center panels: Responding during the extinction trials. Right panels: Responding during the final generalization tests.
The final tests of A and AB after extinction are shown in the right-hand panels of the figure. As the figure suggests, the results replicated those of Experiment 1 regardless of the amount of Phase 1 conditioning: There was less generalization of extinction from A to AB than from AB to A. A Test Stimulus (A or AB) x Amount of Conditioning (Extended vs. NonExtended) x Extinction Group (A− or AB−) x CS Counterbalancing ANOVA revealed no main effects or interactions involving the amount of conditioning or counterbalancing factors, ps ≥ .52. There were significant effects of Test Stimulus, F (1, 24) = 4.76, MSE = 6.51, and Extinction Group, F (1, 24) = 6.78, MSE = 4.56. The Test Stimulus x Extinction Group interaction was not reliable, F (1, 24) = 1.69, MSE = 6.51. However, planned comparisons again indicated an effect of Test Stimulus for the groups extinguished with A−, F (1, 15) = 4.51, MSE = 8.73, whereas there was no such effect for the groups extinguished with AB−, F (1, 15) = 1.13, MSE = 2.23. The results thus replicate the pattern in Experiment 1, and suggest that it does not depend on whether the animals had received 48 or 144 conditioning trials with A and AB.
To further evaluate the statistical dependence of the difference in responding to A and AB on the two extinction treatments, we combined the data of all the A− and AB− groups in Experiments 1 and 2 in a single Group x Test Stimulus x CS Counterbalancing ANOVA. Here, the Group x Test Stimulus interaction was reliable, F (1, 60) = 4.20, MSE = 3.93. There were no interactions with the Counterbalancing factor, Fs < 1, although the Counterbalancing main effect was reliable, F (1, 60) = 4.19, MSE = 5.84, indicating more responding overall to the noise than the light. The Group x Test Stimulus interaction, along with the results of the planned comparisons between responding to A and AB in each of the groups in both experiments, confirms that there is indeed a difference in the extent of generalization of extinction from A to AB and from AB to A.
Pre-CS responding
ANOVAs that paralleled the ones on the elevation scores were again conducted on the corresponding pre-CS data. These analyses revealed no main effects or interactions, all ps ≥ .12, except for the following. During acquisition, both the Extended and Non-Extended groups had Stimulus x Block interactions, Fs ≥ 3.12, MSEs ≤ 0.79; there was no consistent pattern in either group. And in extinction, there was an overall effect of Block, F (3, 72) = 11.80, MSE = 3.30, in which the scores decreased over trials. None of these patterns provides an alternative explanation of the CS elevation scores. Pre-CS responding averaged 2.2 during acquisition, 1.7 during extinction, and 2.5 during the final tests of A and AB.
Discussion
The results of this experiment replicated those of Experiment 1, and showed that tripling the number of acquisition trials had no discernible effect on the final outcome. After either 48 or 144 trials with A and AB, extinction of A had less impact on responding to AB than AB had on responding to A. The fact that the amount of training had so little effect on the results of generalization testing suggests that the present generalization results have generality. The fact that Experiment 1’s asymmetrical generalization occurred after such extended training may also be more difficult to reconcile with elemental models.
The results are again consistent with previous results with temporal intervals (Bouton & García-Gutiérrez, 2006), and continue to challenge both configural and elemental models of conditioning.
Experiment 3
The results with generalization of extinction in Experiments 1 and 2 suggest that we should also consider the generalization of conditioned excitation. As noted in the introduction, several experiments have compared the generalization of conditioning from compound to element and from element to compound. Conditioning experiments have suggested better generalization from element to compound in eyeblink conditioning in rabbits (Brandon et al., 2000) and conditioned freezing in rats (Gonzalez et al., 2003). In contrast, Young (1984, described in Pearce, 1987) found a more symmetrical generalization decrement in a conditioned suppression experiment. Given the pattern of generalization in the present Experiments 1 and 2 (greater generalization of extinction from compound to element), it was important to examine the question of generalization of excitation in the appetitive conditioning method used in those experiments.
Experiment 3 involved two groups. After first presenting A and AB without consequence in order to habituate unconditional orienting reactions to them, one group received conditioning with element A and the other received conditioning with the compound AB. After conditioning was complete, we tested responding to A and AB in both groups in a counterbalanced order. For the reasons given at the beginning of this article, configural models predict symmetrical generalization decrement when switching from compound to element and element to compound, whereas elemental models predict stronger generalization decrement when switching from compound to element. Given the results of Experiments 1 and 2, yet another result seemed possible: There might be more generalization from compound to the element than the element to the compound, which is the pattern we observed with extinction.
Method
Subjects and Apparatus
The subjects were 32 female Wistar rats purchased from the same supplier and were the same age as previous subjects. They had previously been used in a conditioned suppression experiment in a different apparatus. In that experiment, they had been food deprived to 80% ad lib weight, trained to lever press for food pellets, and then given Pavlovian fear conditioning with two CSs (a 3000 Hz tone and termination of the houselights) and shock. None of these stimuli were used in the current experiment. The apparatus, CSs, US, and maintenance procedure were the same as those in Experiments 1 and 2.
Procedure
On the first day, all rats were first assigned a box and given magazine training in a 25-min session following the procedure used in the previous experiments. On the following day, they received a 42-min session in which A and AB were each presented without the US eight times. These exposures were designed to habituate unconditional orienting behaviors. The trials were separated by a 2-min variable ITI. The roles of noise and light were counterbalanced. A and AB trials were double alternated starting with the compound (AB).
On each of the four days that followed, the rats received 76-min conditioning sessions, each containing 32 reinforced trials. Half of the animals (n = 16) received A+ trials and the other half received AB+. As usual, the role of noise and light was counterbalanced. On the final day, all rats received a single session that began with four conditioning trials consistent with the previous experience (i.e., A+ or AB+) followed by four nonreinforced tests each with A and AB. The order of presentation of these stimuli was counterbalanced (A, AB, AB, A, A, AB, AB, A or AB, A, A, AB, AB, A, A, AB) over groups. The ITI was variable with a mean of 2 min.
Results
Acquisition
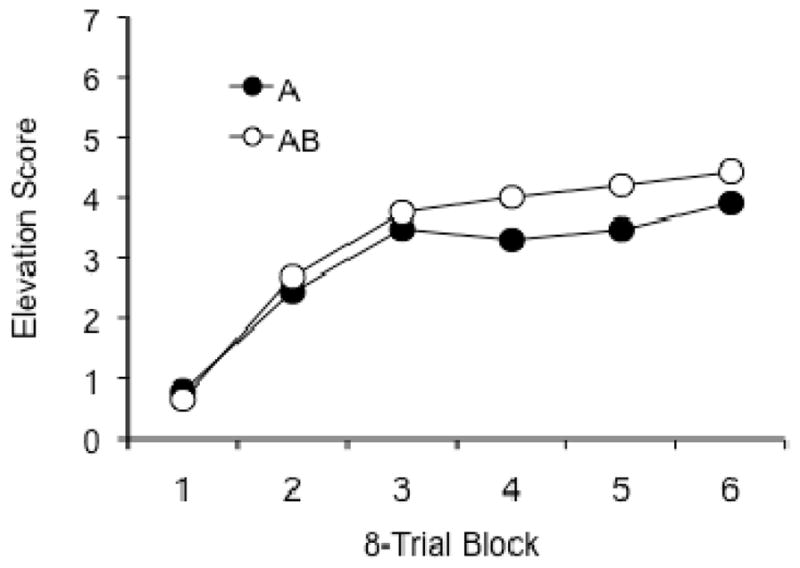
During conditioning (Figure 5), there was a clear increase in responding to both A and AB, but no evidence that they differed in the rates at which they acquired conditioning. A Group x CS Counterbalancing x Trial Block ANOVA revealed significant effects of Trial Block, F (7, 196) = 17.48, MSE = 1.49, and CS Counterbalancing, F (1, 28) = 7.77, MSE = 7.85. The latter effect is consistent with the fact that the noise tended to elicit more foodcup entries than the light. No other main effects or interactions approached statistical reliability, ps ≥ .22.
Figure 5.
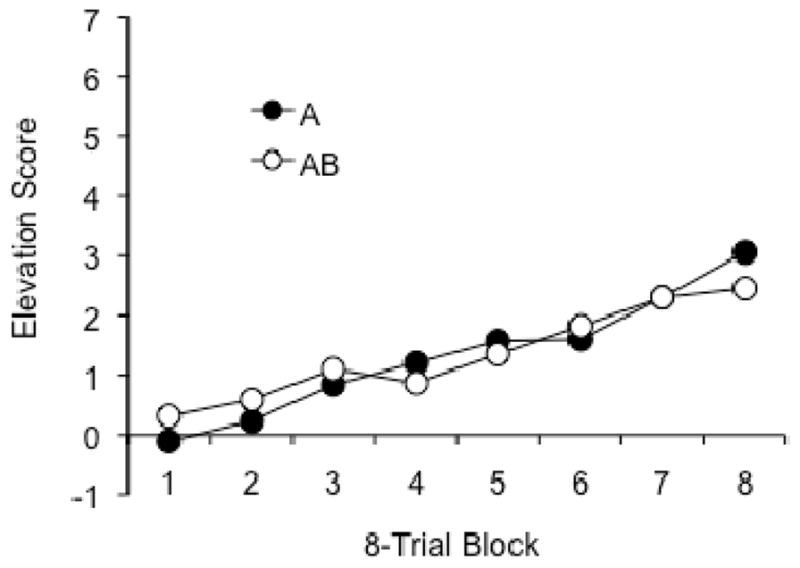
Mean responding in the element-trained (A) and compound-trained (AB) groups during the acquisition phase of Experiment 3.
Generalization test
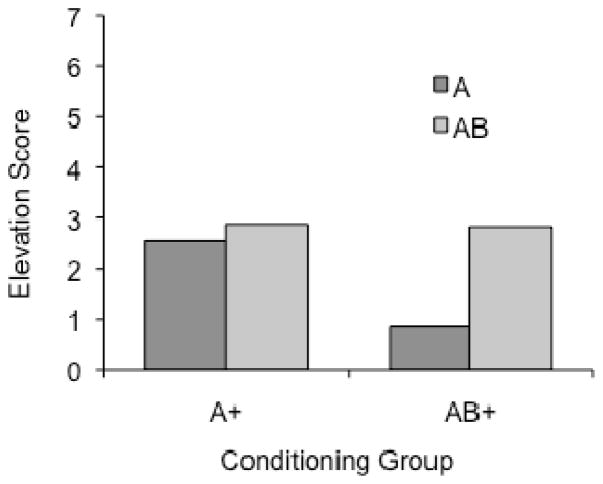
The mean elevation scores during the four tests of A and AB are presented in Figure 6. The results suggested more generalization of conditioning from A to AB than from AB to A. A Test Stimulus x Group x CS Counterbalancing x Test Order ANOVA indicated significant effects of Test Stimulus, F (1, 24) = 8.41, MSE = 5.02, and a Test Stimulus x Group interaction, F (1, 24) = 4.29, MSE = 5.02. The Group effect was not significant, F (1, 24) = 1.11. Test Stimulus x CS Counterbalancing x ANOVAs were also conducted on the results for each group. In the AB+ group, there was an effect of Test Stimulus, F (1, 14) = 6.93, MSE = 4.48, which did not interact with the Counterbalancing factor, F (1, 14) = 4.36, MSE = 4.48, p = .06. In the A+ group, there was no effect of either Test Stimulus, the Counterbalancing factor, or their interaction, Fs (1, 14) ≤ 1.23. Overall, the results indicate a significant generalization decrement when rats trained with AB were tested with A, but not when rats trained with A were tested with AB.
Figure 6.
Mean responding during the generalization tests in Experiment 3.
Pre-CS responding
Parallel ANOVAs were once again conducted on the pre-CS data. There were no main effects or interactions evident during testing, ps ≥ .08. The ANOVA on the conditioning phase revealed a main effect of Block, F (7, 196) = 2.80, MSE = 0.67, (pre-CS responding increased over training), as well as a Group x Block, MSE = 0.67, F (7, 196) = 3.05, and Group x Block x Counterbalancing, F (7, 196) = 2.48, MSE = 0.67, interactions. The latter interactions took the form of pre-CS responding in AB+ being lower at the start of training than at the end. In A+, there was more pre-CS responding when A was the noise. Mean pre-CS scores were 2.2 in acquisition and 2.0 during testing.
Discussion
The results suggested generalization decrement when rats conditioned with the compound (AB) were tested with the element (A), but not when rats conditioned with the element (A) were tested with the compound (AB). As rehearsed above, that is the result anticipated by elemental models, but not configural models. The results are compatible with similar tests in the rabbit eyeblink preparation (Brandon et al., 2000) and rat defensive freezing preparation (Gonzalez et al., 2003). Unlike Brandon et al., (2000), but like Gonzalez et al. (2001), we found less evidence that adding stimuli to a trained element caused a generalization decrement. It is notable that the present design included preeexposures to A and AB that were designed so that the rats were not tested with a novel stimulus. Brandon et al. (2000) did not report using stimulus preexposures.
The results of Experiment 3 concerning the generalization of excitation contrast with the generalization of extinction observed in Experiments 1 and 2. Overall, the results suggest that although conditioning generalizes better from A to AB than from AB to A (Experiment 3), the extinction generalizes better from AB to A than from A to AB (Experiments 1 and 2). Although the results of Experiment 3 are consistent with elemental models, (e.g., Harris, 2006; Rescorla & Wagner, 1972; Wagner, 1981, 2003, 2008), the complete pattern of results does not appear to be.
Experiment 4
The patterns of generalization shown in Experiments 1 – 3 might help explain the feature-positive effect (e.g., Hearst, 1978, 1984), a well-known, but incompletely understood, phenomenon in the conditioning literature. To reiterate, previous research suggests that feature-positive discrimination learning (AB+/A−) occurs more rapidly than feature-negative learning (A+/AB−). The asymmetries documented in Experiments 1 – 3 provide two complementary effects that might contribute to this phenomenon. First, the results of Experiment 3 suggest that there will be more generalization of excitation from A+ to AB− in the feature-negative procedure than generalization from AB+ to A in the feature-positive procedure. That asymmetry would make the discrimination between A and AB more difficult in the feature-negative arrangement, and is one of the reasons why elemental models (e.g., Rescorla & Wagner, 1972) account for the feature-positive effect (Bouton & Hendrix, 2011). However, the asymmetry uncovered in Experiments 1 and 2 might also be important. The greater tendency to generalize extinction (nonresponding) from AB− to A in the feature-negative procedure than A− to AB in the feature-positive procedure might also make feature-negative learning more difficult. Although that result is not currently captured by compound conditioning models, it might easily contribute to the feature-positive effect.
Experiment 4 therefore examined the feature-positive effect in the present conditioning preparation. Groups received either AB+/A− or A+/AB−-training; the feature-positive effect would take the form of more rapid discrimination learning in the former group. However, if the effect is driven by weaker generalization of excitation from AB+ to A and stronger generalization of AB− to A, then it should take a specific form: Responding in the AB+ trial type should be higher than in any other type of trial. Because many reports in the feature-positive effect literature report discrimination ratios that combine responding on positive and negative trials in a single number (e.g., Hearst, 1987), it is not always possible to evaluate this possibility. However, Bouton and Hendrix (2011) reported exactly that pattern in a serial compound arrangement in which the onset of B followed the onset of A (which was arranged in order to model the change of hypothetical temporal elements over time). The present experiment investigated AB+/A− and A+/AB− training in both the serial method used by Bouton and Hendrix and with the simultaneous compound conditioning methods used in Experiments 1 – 3.
Method
Subjects
The subjects were 32 naive female Wistar rats of the same age and from the same supplier as those in the previous experiments. The apparatus, CSs, US, and maintenance conditions were also the same.
Procedure
The first session was a magazine training session in which the rats were placed in a chamber and given 30 pellets distributed over 20 min. The experiment proper was then conducted over the next four days. In each daily session, there were eight presentations of A (noise or light, counterbalanced) and eight presentations of AB. Two groups (n = 8) received a Simultaneous compound arrangement in which the onset and offset of A and B were simultaneous and all stimulus presentations were 10 s in duration. (This is the method used in Experiments 1 – 3.) For Group Sim FP (simultaneous feature-positive), the AB trials ended in the US and the A trials did not; for Group Sim FN (simultaneous feature-negative), the A trials ended in the US and AB did not. Two other groups (n = 8) received a Serial compound arrangement in which A was always 20 s in duration; on AB trials, the 10-s B was presented during the last 10 s of the 20-s A. (This is the method used by Bouton and Hendrix, 2011). For Group Ser FP (serial feature-positive), the AB trials ended in the US and the A trials did not. For Group Ser FN (serial feature-negative), the A trials ended in the US and the AB trials did not. For all groups, the interval between trial offsets was variable with a mean of 2 min. Trial types were double-alternated, and each session started with a reinforced trial.
Elevation scores were determined the usual way for the Sim groups. For the Ser groups, data analysis focused on the second 10 s of each 20-s trial, i.e., the segment when CS B was specifically present or absent. Elevation scores were calculated by subtracting responses made during the 10-s period before onset of A from the responses in this 10-s CS period.
Results
The results are shown in Figure 7. There was a strong feature-positive effect with either conditioning procedure. While the FP groups learned the discriminations rapidly, there was little responding in the FN groups with the number of conditioning trials used here. In fact, neither FN group demonstrated much increase in responding over the four eight-trial blocks.
Figure 7.
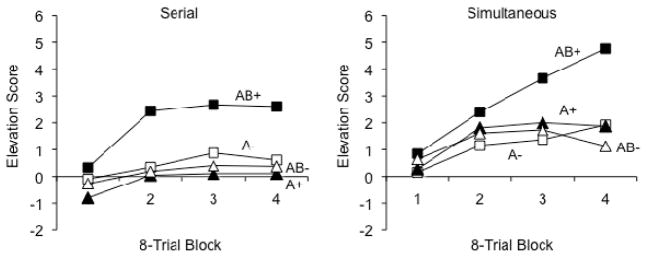
Mean responding over trial blocks for the serially-trained (Serial) and Simultaneously trained (Simultaneous) groups in Experiment 4. In either panel, the AB+ and A− trials correspond to the feature-positive group whereas the AB− and A+ trials correspond to the feature-negative group.
These impressions were confirmed by a Temporal Arrangement (Ser or Sim) x Discrimination Type (FP or FN) x Trial type (reinforced or nonreinforced) x CS Counterbalancing (noise or light) x Trial Block ANOVA on the elevation scores. The ANOVA revealed a significant effect of Trial Type, F (1, 24) = 61.50, MSE = 0.69, and a Trial Type x Trial Block interaction, F (3, 72) = 7.25, MSE = 0.65; overall, the rats responded more on reinforced trials. There was also a main effect of Discrimination Type, F (1, 24) = 14.16, MSE = 3.87, and crucially, a significant Discrimination Type x Trial Type interaction, F (1, 24) = 68.92, MSE = 0.69. The latter indicates that there was better discriminative responding in the groups that had the FP procedure. There were no interactions involving the Temporal Arrangement factor, ps > .10, although the main effect of Temporal Arrangement was significant, F (1, 24) = 19.56, MSE = 3.87; Group Sim generally responded more than Group Ser. The Trial Type x Discrimination Type x Temporal Arrangement did not approach significance, F (1, 24) < 1. There was a main effect of Trial Block, F (3, 72) = 20.11, MSE = 1.61, and a Trial Block x Discrimination Type interaction, F (3, 72) = 2.95, MSE = 1.61. The CS Counterbalancing factor interacted with Trial Block, F (3, 72) = 2.86, MSE = 1.61, Trial Type, F (1, 24) = 9.09, MSE = 0.69, and the Discrimination Type x Trial Type interaction, F (1, 24) = 25.93, MSE = 0.69. The interactions involving the counterbalancing factor resulted from the fact that the noise generally controlled more responding than the light. However, there was clear evidence of a feature-positive effect given both temporal arrangements in this experiment. Planned comparisons on the data collapsed over Trial Block and CS counterbalancing confirmed that in both the Ser and Sim arrangements, responding of the FP group on AB+ trials was significantly higher than responding on any other type of trial (A− in FP and A+ and AB− in FN), highest probability F (1,14) = 8.04, MSE = 4.05.
Pre-CS responding was analyzed with a parallel ANOVA. This revealed only a main effect of Trial Block, F (3, 72) = 5.95, MSE = 0.65, and of Trial Type, F (1, 24) = 13.38, MSE = 0.25, and a Trial Type x Temporal Arrangement x Trial Block interaction, F (3, 72) = 2.83. MSE = 0.34. The Trial Block effect was related to an overall decrease in pre-CS responding during conditioning (means of 1.6, 1.2, 1.1, and 1.1, on the four Trial Blocks). The other effects were small and evidently inconsequential; for example, the mean responding on reinforced and nonreinforced trials was 1.1 and 1.4, respectively.
Discussion
The results of this experiment confirm that a feature-positive effect can occur in the present method. They also suggest that presenting a 10-s B after the onset of a 20-s A (the procedure used by Bouton & Hendrix, 2011, Experiment 4) makes no difference to the result. Such a possibility was worth evaluating, because in the serial case, the longer positive stimulus (A) in the FN discrimination could have acquired weaker conditioning than the shorter positive stimulus (B) in the FP discrimination merely because longer CSs may generally evoke less conditioned responding (e.g., Bouton & Sunsay, 2003).
As noted above, the feature-positive effect is consistent with elemental models of compound conditioning (e.g., Rescorla & Wagner, 1972), but is not predicted by Pearce’s configural model (1987, 1994). The results of this experiment might provide some further insight into its nature. With the small amount of conditioning used here, the main result was that high responding developed rapidly to AB+ in the FP groups; none of the other trial types evoked as much responding. The pattern extends, and is entirely consistent with, the findings of Experiments 1 – 3. First, there was apparently little generalization of responding from AB+ to A in the FP groups (as in Experiment 3). Second, there was less responding to A+ in the FN groups than AB+ in the FP groups. That result presumably occurred because of substantial generalization of extinction from AB− back to A (Experiments 1 and 2). Although it is in principle possible that comparatively little responding to A+ was also due to weaker conditioning to A+ than with AB+ (Rescorla & Wagner, 1972), Experiment 3 uncovered no evidence of that effect when the A+ and AB+ were conditioned in separate groups without the intermixed AB− (or A−) trials included in the present experiment. Thus, the form of the present feature-positive effect followed directly from what the previous experiments indicate regarding the generalization of conditioning (Experiment 3) and extinction (Experiments 1 and 2) from AB to A and A to AB.
General Discussion
The present experiments provide evidence that generalization between compounds and elements can often be asymmetrical. In Experiment 1, there was better generalization of extinction from a compound (AB) to an element (A) than there was from an element (A) to a compound (AB). That result was replicated in Experiment 2, which also extended the finding to a conditioning procedure that involved three times the number of original conditioning trials with A and AB. Experiment 3 then demonstrated a second asymmetry: There was less generalization of conditioning (as opposed to extinction) from a compound (AB) to an element (A) than from an element (A) to a compound (AB). The results of Experiment 4 then suggested that the two asymmetries documented in Experiments 1 – 3 can both contribute to the feature-positive effect. Specifically, less generalization of excitation from AB+ to A− (in the FP procedure) than from A+ to AB (in the FN procedure) can make the FP discrimination easier, as will less generalization of extinction from A− to AB (in the FP procedure) than from AB− to A (in FN). The two asymmetries evident in Experiments 1, 2, and 3 were both present in the feature-positive effect observed here.
The generalization patterns summarized above are also consistent with results that have been obtained in temporal discrimination learning (Bouton & García-Gutiérrez, 2006; Bouton & Hendrix, 2011; see also Todd, 2010; Kyd et al., 2007). Recall that the temporal element hypothesis (Bouton & Hendrix, 2011; see also, e.g., Desmond & Moore, 1988) represents the passage of time as a hypothetical series of cascading stimulus elements. On this view, a longer interval would involve exposure to at least one more element than would a shorter interval (e.g., as in AB and A, respectively). When we have studied generalization of extinction over different ITIs, extinction appears to generalize from a long ITI to a short ITI better than it does from a short ITI to a long ITI (Bouton & Garcia-Gutierrez, 2006). The results of Experiments 1 and 2, where extinction generalized better from AB to A than from A to AB, are analogous to that finding. In addition, in studies of temporal discrimination learning, we and others have found better learning when a long interval is associated with reinforcement and a short interval is not (a long+/short− discrimination) than the reverse, i.e., where the short interval is associated with reinforcement and the long interval is not (the short+/long− discrimination, Bouton & Garcia-Gutierrez, 2006; Bouton & Hendrix, 2011; Kyd et al., 2007; Todd, et al., 2010). The results of Experiment 4, where rats were more successful learning AB+/A− than A+/AB− (the feature-positive effect), are in turn analogous to that finding. The parallel between the compound-conditioning and temporal-discrimination results may be uniquely consistent with the temporal elements view (Bouton & Hendrix, 2011). It suggests that at least some examples of temporal learning might follow the rules of associative learning.
The results also seem to challenge several associative learning models, however. As we have noted, none of the asymmetries evident in the generalization of extinction (Experiments 1 and 2), conditioning (Experiment 3), or the feature-positive effect (Experiment 4) appear consistent with the Pearce configural model (Pearce, 1987, 1994), which predicts symmetrical, rather than asymmetrical, generalization between compounds and elements. On the other hand, elemental models (e.g., Harris, 2006; Rescorla & Wagner, 1972; Wagner, 1981, 2003, 2008) are also challenged. While they do predict an asymmetry in the generalization of conditioning observed in Experiment 3, and broadly predict the feature-positive effect (Experiment 4), they predict an asymmetry that is opposite to the one obtained in Experiments 1 and 2, where there was more generalization, rather than less generalization, of extinction from AB to A than A to AB. Thus, neither configural nor elemental approaches to conditioning appear to handle the overall pattern of results reported here.
Is there a way to reconcile the models with the results? One way to reconcile elemental models with the findings of Experiments 1 and 2 is the mechanism mentioned in the Introduction of Experiment 2: Blocking to Stimulus B could have been incomplete after intermixing A+ and AB+ trials during acquisition. Although tripling the amount of A+/AB+ acquisition training (which should have enhanced the degree of blocking, Rescorla & Wagner, 1972) did not change the generalization results (Experiment 2), any excitation that still remained to CS B after conditioning would have (1.) created responding to AB after extinction of A, and (2.) enhanced associative loss to Stimulus A after extinction of AB (e.g., Rescorla, 2000). It is worth noting that the literature on blocking suggests that the blocking effect is often incomplete; that is, B often does acquire some associative strength after blocking treatments (for example, see the experiments reviewed by Mackintosh, 1978). A second possibility is that intense stimuli might elicit more responding than less intense stimuli (cf. Hull, 1949). If a compound is more intense than an element alone, the rats might respond more to AB after A is extinguished (but not necessarily more to A after AB is extinguished), as in Experiments 1 and 2. Such a mechanism could also contribute to greater generalization decrement (less responding) when A is tested after AB+ training than when AB is tested after A+ training (Experiment 3; Brandon et al., 2000; Gonzalez et al., 2003). And it could be involved in the feature-positive effect (Experiment 4), where the easier feature-positive discrimination likewise requires more responding to AB than to A. One challenge to this possibility is that the current experiments found no evidence of more responding to AB than A during extinction (Experiments 1 and 2), during tests that immediately preceded extinction (Experiments 1 and 2), or when different groups received conditioning with AB+ or A+ (Experiment 3). However, there was some evidence of stronger responding to AB than A during acquisition in Experiments 1 and 2. It should be noted that it is not clear whether the potential role for stimulus intensity would apply as readily when A and AB are short and long temporal intervals (e.g., as in Bouton & Hendrix, 2011) instead of being composed of visual and auditory cues.
The designs of Experiments 1 and 2 also bear some resemblance to experiments on recovery from overshadowing (e.g., Dickinson, Shanks, & Evenden, 1984; Williams, Sagness, & McPhee, 1994). In that phenomenon, conditioning with AB+ is followed by A− trials before tests of B. In human participants in particular, an increase in responding to B can be observed, as if B has been liberated from overshadowing. One explanation of this effect is that responding to B after AB+ is ordinarily suppressed in performance by an A-US association. Extinction of A during the A− trials would weaken the manifest strength of that association and unmask an existing B-US association (Stout & Miller, 2007). Alternatively, the organism might first associate A and B during the AB+ trials. The presentation of A− alone in the next phase might therefore activate a representation of B; the activation of B along with no US could cause new excitatory learning with B (Dickinson & Burke, 1996; van Hamme & Wasserman, 1994). Either possibility could yield greater responding to AB than to A after the extinction of A in the present designs. Thus, the results of Experiments 1 and 2 might be within reach of these theoretical approaches.
It might also be possible to reconcile the results with configural theory (e.g., Pearce, 1987, 1994). First, the stimulus intensity mechanism noted above might apply equally well there. In addition, there are precedents for modifying the Pearce (1994) model in ways that might accommodate the present results. To address Experiments 1 and 2, one could follow Pearce and Redhead (1999), who suggested that when stimuli A and AB are paired with a common reinforce during conditioning, the animal might form a common configural unit that is activated by presentation of either A or AB. (According to Pearce, 1994, it is the configural unit, rather than specific stimuli, that is associated with the US.) If A is then extinguished alone, the surprising nonreinforcement and the absence of B might engage a new configural unit that is then associated with extinction (inhibition). This would leave excitation to the first unit intact, explaining why the animal responds to AB after extinction of A. In contrast, during extinction of AB, because A and B are both presented, there is less reason for AB to engage a new configural unit; inhibition would therefore be associated with the original unit. Test presentations of either A or AB would therefore evoke extinction behavior. To account for the results of Experiments 3 and 4, one could follow George and Pearce (in press), who have begun to integrate attentional principles with configural theory. In Experiment 3, after conditioning with AB+, tests of A alone would result in substantial generalization decrement (as in Pearce, 1994). In contrast, as a consequence of new attention principles, conditioning with A+ alone would cause a substantial increase in attention to A, so that adding a new stimulus (B) would have less effect on performance. Finally, to handle Experiment 4, George and Pearce (in press; see also Lotz et al. 2012) have noted that the animal needs to attend to both A and B to solve the difficult feature-negative discrimination (A+/AB−). In contrast, in the easier feature-positive discrimination (AB+/A−), the animal must only attend to B. The latter might decrease the relative similarity of A and AB, and thus produce the feature-positive effect. Together, these ideas suggest that extensions of the Pearce (1994) configural model that (1.) adjust the rules by which configural units are engaged or employed (Experiments 1 and 2; Pearce & Redhead, 1999) and (2.) add attentional processes (Experiments 3 and 4; George & Pearce, in press) might be able to accommodate the present results.
In summary, the results of the present experiments have at least two important implications. First, they suggest that there is a parallel between generalization over long and short temporal intervals (Bouton & García-Gutiérrez, 2006; Bouton & Hendrix, 2011; Todd et. al., 2010) and over explicit stimulus compounds and elements, as the temporal elements hypothesis (Bouton & Hendrix, 2011) predicts. The results thus encourage the view that temporal learning may have more in common with compound conditioning than is often assumed. Second, the results also suggest that generalization between compounds and elements can be asymmetrical. In Experiment 3, there was less generalization of excitation from compound to element than from element to compound. And in Experiments 1 and 2, animals appeared to generalize extinction better from compound to element than from element to compound. Both effects are consistent with, and may play a role in producing, the well-known feature-positive effect.
Acknowledgments
This research was supported by Grant R01 MH064847 from the National Institute of Mental Health. We thank Matthew Campolattaro, Jeremy Fonte, and Andy Weis for their help running experiments, and John Pearce, Travis Todd, and Neil Winterbauer for discussion and comments on the manuscript.
References
- Bouton ME, García-Gutiérrez A. Intertrial interval as a contextual stimulus. Behavioural Processes. 2006;71:307–317. doi: 10.1016/j.beproc.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Hendrix M. Intertrial interval as a contextual stimulus: Further analysis of a novel asymmetry in temporal discrimination learning. Journal of Experimental Psychology: Animal Behavior Processes. 2011;37:79–93. doi: 10.1037/a0021214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME, Sunsay C. Importance of trials versus accumulating time across trials in partially-reinforced appetitive conditioning. Journal of Experimental Psychology: Animal Behavior Processes. 2003;29:62–77. [PubMed] [Google Scholar]
- Brandon SE, Vogel EH, Wagner AR. A componential view of configural cues in generalization and discrimination in Pavlovian conditioning. Behavioural Brain Research. 2000;110:67–72. doi: 10.1016/s0166-4328(99)00185-0. [DOI] [PubMed] [Google Scholar]
- Brooks DC, Bouton ME. A retrieval cue for extinction attenuates spontaneous recovery. Journal of Experimental Psychology: Animal Behavior Processes. 1993;19:77–89. doi: 10.1037//0097-7403.19.1.77. [DOI] [PubMed] [Google Scholar]
- Desmond JE, Moore JW. Adaptive timing in neural networks: Test of a neural-network model. Biological Cybernetics. 1988;58:405–415. doi: 10.1007/BF00361347. [DOI] [PubMed] [Google Scholar]
- Dickinson A, Burke J. Within-compound associations mediate the retrospective revaluation of causality judgments. Quarterly Journal of Experimental Psychology. 1996;49B:60–80. doi: 10.1080/713932614. [DOI] [PubMed] [Google Scholar]
- Dickinson A, Shanks D, Evenden J. Judgment of act-outcome contingency: The role of selective attribution. Quarterly Journal of Experimental Psychology. 1984;36A:29–50. [Google Scholar]
- George DN, Pearce JM. A configural theory of attention and associative learning. Learning & Behavior. doi: 10.3758/s13420-012-0078-2. in press. [DOI] [PubMed] [Google Scholar]
- Glautier S. Asymmetry of generalization decrement in causal learning. Quarterly Journal of Experimental Psychology. 2004;57B:315–329. doi: 10.1080/02724990344000169. [DOI] [PubMed] [Google Scholar]
- Gonzalez F, Quinn JJ, Fanselow MS. Differential effects of adding and removing components of a context on the generalization of conditional freezing. Journal of Experimental Psychology: Animal Behavior Processes. 2003;29:78–83. [PubMed] [Google Scholar]
- Harris JA. Elemental representations of stimuli in associative learning. Psychological Review. 2006;113:584–605. doi: 10.1037/0033-295X.113.3.584. [DOI] [PubMed] [Google Scholar]
- Hearst E. Stimulus relationships and feature selection in learning and behavior. In: Hulse SH, Fowler H, Honig WK, editors. Cognitive processes in animal behavior. Hillsdale, NJ: Erlbaum; 1978. pp. 51–88. [Google Scholar]
- Hearst E. Absence of information: Some implications for learning, performance, and representational processes. In: Roitblat HL, Bever TG, Terrace HS, editors. Animal cognition. Hillsdale, NJ: Erlbaum; 1984. pp. 311–332. [Google Scholar]
- Hearst E. Extinction reveals stimulus control: Latent learning of feature-negative discriminations in pigeons. Journal of Experimental Psychology: Animal Behavior Processes. 1987;13:52–64. [PubMed] [Google Scholar]
- Hull CL. Stimulus intensity dynamism (V) and stimulus generalization. Psychological Review. 1949;56:67–76. doi: 10.1037/h0058051. [DOI] [PubMed] [Google Scholar]
- Jenkins HM, Sainsbury RS. Discrimination learning with the distinctive feature on positive or negative trials. In: Mostovsky D, editor. Attention: Contemporary theory and analysis. New York: Appleton-Century-Crofts; 1970. pp. 239–273. [Google Scholar]
- Kyd RJ, Pearce JM, Haselgrove M, Amin E, Aggleton JP. The effects of hippocampal system lesions on a novel temporal discrimination task for rats. Behavioural Brain Research. 2007;187:159–171. doi: 10.1016/j.bbr.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Lotz A, Uengoer M, Koenig S, Pearce JM, Lachnit H. An exploration of the feature-positive effect in adult humans. Learning & Behavior. 2012;40:222–230. doi: 10.3758/s13420-011-0057-z. [DOI] [PubMed] [Google Scholar]
- Mackintosh NJ. Cognitive or associative theories of conditioning: Implications of an analysis of blocking. In: Hulse SH, Fowler H, Honig WK, editors. Cognitive processes in animal behavior. Hillsdale, NJ: Erlbaum; 1978. pp. 155–175. [Google Scholar]
- Moody EW, Sunsay C, Bouton ME. Priming and trial spacing in extinction: Effects on extinction performance, spontaneous recovery, and reinstatement in appetitive conditioning. The Quarterly Journal of Experimental Psychology. 2006;59:809–829. doi: 10.1080/17470210500299045. [DOI] [PubMed] [Google Scholar]
- Pavlov IP. In: Conditioned reflexes. Anrep GV, translator. London: Oxford University Press; 1927. [Google Scholar]
- Pearce JM. A model for stimulus generalization in Pavlovian conditioning. Psychological Review. 1987;94:161–73. [PubMed] [Google Scholar]
- Pearce JM. Similarity and discrimination: A selective review and a connectionst model. Psychological Review. 1994;101:587–607. doi: 10.1037/0033-295x.101.4.587. [DOI] [PubMed] [Google Scholar]
- Pearce JM, Redhead ES. Enhanced Pavlovian conditioning with a change in appetitive reinforce. Animal Learning & Behavior. 1999;27:369–378. [Google Scholar]
- Rescorla RA. Associative changes in elements and compounds when the other is reinforced. Journal of Experimental Psychology: Animal Behavior Processes. 1999;25:247–255. doi: 10.1037//0097-7403.25.2.247. [DOI] [PubMed] [Google Scholar]
- Rescorla RA. Extinction can be enhanced by a concurrent excitor. Journal of Experimental Psychology: Animal Behavior Processes. 2000;26:251–260. doi: 10.1037//0097-7403.26.3.251. [DOI] [PubMed] [Google Scholar]
- Rescorla RA. Protection from extinction. Learning & Behavior. 2003;31:124–132. doi: 10.3758/bf03195975. [DOI] [PubMed] [Google Scholar]
- Rescorla RA, Wagner AR. A theory of Pavlovian conditioning: Variations in the effectiveness of reinforcement and nonreinforcement. In: Black AH, Prokasy WK, editors. Classical conditioning II: Current research and theory. New York: Appleton-Century-Crofts; 1972. [Google Scholar]
- Stout SC, Miller RR. Sometimes-competing retrieval (SOCR): A formalization of the comparator hypothesis. Psychological Review. 2007;114:759–783. doi: 10.1037/0033-295X.114.3.759. [DOI] [PubMed] [Google Scholar]
- Thorwart A, Lachnit H. Symmetrical generalization decrements: Configural stimulus processing in human contingency learning. Learning & Behavior. 2009;37:107–115. doi: 10.3758/LB.37.1.107. [DOI] [PubMed] [Google Scholar]
- Todd P, Winterbauer NE, Bouton ME. Interstimulus interval as a discriminative cue: Evidence of the generality of a novel asymmetry in temporal discrimination learning. Behavioural Processes. 2010;84:412–420. doi: 10.1016/j.beproc.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hamme LJ, Wasserman EA. Cue competition in causality judgments: The role of nonpresentation of compound stimulus elements. Learning and Motivation. 1994;25:127–151. [Google Scholar]
- Wagner AR. SOP: A model of automatic memory processing in animal behavior. In: Spear NE, Miller RR, editors. Information processing in animals: Memory mechanisms. Hillsdale, NJ: Erlbaum; 1981. pp. 5–47. [Google Scholar]
- Wagner AR. Context-sensitive elemental theory. Quarterly Journal of Experimental Psychology. 2003;56B:7–29. doi: 10.1080/02724990244000133. [DOI] [PubMed] [Google Scholar]
- Wagner AR. Evolution of an elemental theory of Pavlovian conditioning. Learning & Behavior. 2008;36:253–265. doi: 10.3758/lb.36.3.253. [DOI] [PubMed] [Google Scholar]
- Wheeler DS, Amundson JC, Miller RR. Generalization decrement in human contingency learning. Quarterly Journal of Experimental Psychology. 2006;59:1212–1223. doi: 10.1080/17470210600576342. [DOI] [PubMed] [Google Scholar]
- Williams DA, Sagness KE, McPhee JE. Configural and elemental strategies in predictive learning. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1994;20:694–709. [Google Scholar]