Abstract
Methionine is an essential amino acid that is metabolized mainly by the liver where it is converted to S-adenosylmethionine (SAMe) by the enzyme methionine adenosyltransferase. Although all mammalian cells synthesize SAMe, the liver is where the bulk of SAMe is generated as it is the organ where about 50% of all dietary methionine is metabolized. SAMe is mainly needed for methylation of a large variety of substrates (DNA, proteins, lipids and many other small molecules) and polyamine synthesis, so if the concentration of SAMe falls below a certain level or rises too much the normal function of the liver will be also affected. There are physiological conditions that can affect the hepatic content of SAMe. Consequently, to control these fluctuations, the rate at which the liver both synthesizes and catabolizes SAMe is tightly regulated. In mice, failure to do this can lead to fatty liver disease and to the development of hepatocellular carcinoma (HCC). Therefore, maintaining SAMe homeostasis may be a therapeutic target in nonalcoholic steatohepatitis, alcoholic- and non-alcoholic liver cirrhosis, and for the chemoprevention of HCC formation.
Keywords: Methionine, Steatosis, Non-alcoholic steatohepatitis (NASH), Livercirrhosis, Hepatocellular carcinoma
ENZYMES INVOLVED IN S-ADENOSYLMETHIONINE SYNTHESIS AND CATABOLISM
Methionine is an essential sulfur-containing gluconeogenic amino acid that is converted to S-adenosylmethionine (SAMe, abbreviated also as AdoMet or SAM) by the enzyme methionine adenosyltransferase (MAT) using ATP as co-substrate (Figure 1) (reviewed in 1). SAMe’s methyl group is then transferred to a legion of different substrates, such as DNA, RNA, proteins, phosphatidylethanolamine (PE), glycine, and guanidinoacetate. These reactions receive the general name of transmethylation reactions, and are catalyzed by specific methyltransferases (MTs). Over 200 proteins in the human genome have been identified as known or putative SAMe-dependent MTs.1 S-adenosylhomocysteine (SAH), an inhibitor of most MTs, is generated as a byproduct of all transmethylation reactions and is hydrolyzed to form homocysteine and adenosine by a reversible enzyme known as SAH hydrolase (AHCY). There are two fates for homocysteine: to be remethylated to regenerate methionine, or enter the transsulfuration pathway to be converted to cysteine and α-ketobutyrate.
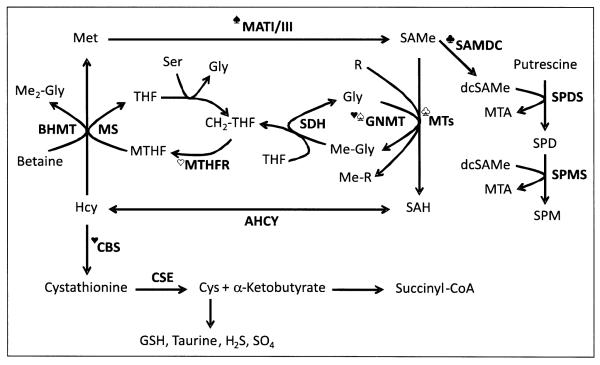
Figure 1.
Hepatic S-adenosylmethionine (SAMe) metabolism. Methionine (Met) is an essential sulfur-containing amino acid that is converted to SAMe by the enzyme methionine adenosyltransferase (MAT) using ATP as co-substrate. Two main MAT isoforms, MATI and MATIII, are expressed in the liver. MATIII is activated by methionine. SAMe’s methyl group is transferred to a large variety of substrates, such as DNA, RNA, proteins, phosphatidylethanolamine, glycine (Gly), and guanidinoacetate. These reactions receive the general name of transmethylation reactions, and are catalyzed by specific methyltransferases (MTs). S-adenosylhomocysteine (SAH), the byproduct of att transmethylation reactions, is an inhibitor of MTs. SAH is hydrolyzed to form homocysteine (Hcy) and adenosine by a reversible enzyme known as SAH hydrolase (AHCY). Hcy may be remethylated to regenerate Met, or enter the transsulfuration pathway to be converted first to cystathionine, by a condensation reaction with homoserine, and then to cysteine (Cys) and α-ketobutyrate. Whereas Cys is transformed into a variety of sulfur containing molecules, such as glutathione (GSH), taurine, sulfate (SO,4 and hydrogen sulfide (H2S), α-ketobutyrate is transformed to succinyl-CoA and metabolized in the mitochondria. Cystathionine β-synthase (CBS), the enzyme that catalyzes the synthesis of cystathionine, is activated by SAMe. Methionine is regenerated from Hcy via two routes; the methionine synthase (MS) pathway, which uses 5-methyttetrahydrofolate (MTHF) as methyl donor, and the BHMT route, that uses betaine (trimethylglycine) as methyl donor. The synthesis of MTHF is catalyzed by the enzyme MTHF reductase (MTHFR), which is inhibited by SAMe. In its turn, MTHF inhibits GNMT, the enzyme that converts Gly to sarcosine (methylglycine, Me-Gly). GNMT is activated by SAMe. The regeneration of Gly from sarcosine is catalyzed by the enzyme sarcosine dehydrogenase (SDH).
Another major role of SAMe is to donate its propylamino group for polyamine synthesis. Here, SAMe is first decarboxylated by the enzyme SAMe decarboxylase (SAMDC) to form decarboxylated SAMe (dcSAMe). Then, this compound donates its propylamine group to putrescine to form spermidine (SPD), and to SPD to form spermine (SPM). This pathway is regulated by putrescine, which activates SAMDC. Methytthioadenosine (MTA), a byproduct of SPD and SPM synthesis, is used to regenerate methionine (not shown). Ser, serine; SPDS, SPD synthase; SPMS, SPM synthase; THF, tetrahydrofolate; CH2-THF, methylene tetrahydrofolate; R, methyl accepting substrate; Me-R, methylated product; Me2-Gly, dimethylglycine.  , activated by methionine;
, activated by methionine;  , activated by putrescine;
, activated by putrescine;  , activated by SAMe;
, activated by SAMe; 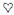 , inhibited by SAMe;
, inhibited by SAMe; 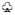 , inhibited by MTHF;
, inhibited by MTHF; 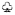 , inhibited by SAH. Metabolites and enzymes are indicated by normal and bold letters, respectively.
, inhibited by SAH. Metabolites and enzymes are indicated by normal and bold letters, respectively.
Methionine can be regenerated from homocysteine via methionine synthase (MS), an enzyme that requires vitamin B12 as a cofactor and uses 5-methyl tetrahydrofolate (5-MTHF, vitamin B9) as methyl donor, or using betaine-homocysteine methyltransferase (BHMT), an enzyme that uses betaine (trimethylglycine), which is synthesized from choline by the enzyme choline oxidase, as methyl donor (Figure 1) (reviewed in 1). Tetrahydrofolate (THF) is generated as a byproduct of MS and is converted to methylene-THF (CH2-THF) by the enzyme CH2-THF synthase, which uses serine as donor of the methylene group, or by the enzyme sarcosine dehydrogenase (SDH), which uses sarcosine (methylglycine) as the donor of the methylene group, and then again to 5-MTHF by the enzyme MTHF-reductase (MTHFR). Alternatively, CH2-THF may be used for the synthesis of deoxy-thymidine monophosphate by the enzyme thymidine synthase, a critical reaction for DNA synthesis that generates dihydrofolate (DHF) as a byproduct, which is converted to THF and reenters the folate cycle (Figure 1). MS and BHMT differ in their kinetic and regulatory properties. BHMT has the highest and MS the lowest Km for homocysteine.
The synthesis of cysteine includes two steps:
The condensation of homocysteine with homoserine via cystathionine β-synthase (CBS), a B6-dependent enzyme, to form cystathionine and
The break down of cystathionine by cystathionine γ-lyase (CSE) to form cysteine and α-ketobutyrate (reviewed in 1). Finally, the enzyme α-ketoacid dehydrogenase converts α-ketobutyrate to propionyl-CoA, which is further metabolized to succinyl-CoA feeding the Krebs cycle, and cysteine is ultimately transformed into a variety of sulfur containing molecules, such as glutathione (GSH), taurine, sulfate and hydrogen sulfide (H2S) (Figure 1).
Another major role of SAMe is to donate its propylamino group for polyamine synthesis (Figure 1) (reviewed in 1). Here, SAMe is first decarboxylated by the enzyme SAMe decarboxylase to form S-adenosylmethioninamine also known as decarboxylated SAMe (dcSAMe). Then, this compound donates its propylamine group to putrescine to form spermidine (SPD), and to SPD to form spermine (SPM). This pathway is regulated by putrescine, which lowers the Km for SAMe of SAMe decarboxylase activating polyamine synthesis. Methylthioadenosine (MTA) is generated as a byproduct of SPD and SPM synthesis, a molecule that is used to regenerate methionine through an intricate set of biochemical reactions known as the methionine salvation pathway.
Although all mammalian cells and tissues convert methionine to homocysteine via SAMe, the liver is where the bulk of SAMe is generated as it is the organ where about 50% of all dietary methionine is metabolized and where up to 85% of all transmethylation reactions take place.2,3 Mammals express two genes, MAT1A and MAT2A, which encode for two homologous MAT catalytic subunits, α1 and α2. MAT1A is mainly expressed in the liver, where the α1 subunit is found forming two MAT isoenzymes, a homodimer (MA TIII) and a homotetramer (MATI). MAT2A is widely expressed, and its α2 subunit is found forming a complex with a β-regulatory subunit that is the product of MAT2B. The α2/β complex is known as MATII. MAT isoenzymes differ in their kinetic and regulatory properties. MATIII has highest, MATI intermediate, and MATII the lowest Km for methionine. SAMe strongly inhibits MATII, minimally inhibits MATI, and stimulates MATIII. The β-regulatory subunit renders MATII more susceptible to feedback inhibition by SAMe (reviewed in 1). Consistent with this tissue distribution and kinetics, individuals deficient in hepatic MATI/III due to mutations of the MAT1A gene have persistent hypermethioninemia.4 A similar situation is observed in MAT1A-knockout mice.5 In these mice, the hepatic expression of MAT2A is markedly increased to compensate for the loss of MATI/III; however, since MATII does not have the same high catalytic capacity to metabolize methionine as that of MATI/III, MAT1A-knockout mice have elevated blood levels of methionine and reduced hepatic SAMe and GSH content.5 The increase of MAT2A expression observed in MAT1A-deficient mice agrees with the observation that hepatic MAT2A expression is inhibited by SAMe.5
The three reactions that contribute most to the hepatic transmethylation flux are glycine methylation by glycine N-methyltransferase (GNMT) to form sarcosine, methylation of PE by PE N-methyltransferase (PEMT) to form phosphatidylcholine (PC), and methylation of guanidinoacetate by guanidinoacetate N-methyltransferase (GAMT) to form creatine.6 The expression of these three MTs, GNMT, PEMT, and GAMT, is particularly abundant in the liver (reviewed in 1). GAMT-deficient humans have elevated levels of plasma guanidinoacetate but normal content of methionine and SAMe, whereas plasma sarcosine is elevated.6 Similarly, PEMT-knockout mice have normal plasma levels of methionine and SAMe and normal hepatic SAMe content, as well as elevated sarcosine.6 Although sarcosine is a poor biomarker because its rapid turnover, these results suggest that the reduction in total transmethylation flux caused by the absence of GAMT or PEMT is compensated by GNMT. Consistent with this, GNMT-deficient individuals have elevated serum methionine and SAMe,6 and GNMT-knockout mice have elevated levels of methionine and SAMe both in serum and in the liver.7
Of the three genes that metabolize homocysteine, CBS, BHMT and MS, the first two are expressed mainly in the liver, whereas MS expression is ubiquitous (reviewed in 1). Related to this, deletion of MS in mice causes embryonic lethality, whereas CBS- and BHMT- knockout mice are viable. CBS-deficient individuals have elevated serum methionine and homocysteine, and CBS deletion in mice associates with elevated homocysteine, SAH, and SAMe, indicating that BHMT and MS cannot compensate the reduction in the catabolism of homocysteine via the transsulfuration pathway caused by CBS deletion (reviewed in 1). BHMT-knockout mice have also increased levels of homocysteine and SAH, as well as reduced SAM; and deletion of MTHFR in mice, which synthesizes the 5-MTHF that is necessary for the regeneration of methionine via MS, causes also the elevation of homocysteine and SAH, as well as the reduction of SAMe (reviewed in 1).
Deletion of MAT1A, GNMT, CBS, BHMT or MTHFR in mice causes fatty liver disease (reviewed in 1). Additionally, MAT1A- and GNMT-deficient mice spontaneously develop HCC (reviewed in 1). PEMT-knockout mice fed a normal diet have been reported to have normal or moderate fatty liver, but when fed a choline-deficient diet, develop fatty liver within days due to loss of membrane integrity caused by a reduced PC/PE ratio (reviewed in 1). These mice models reveal a critical requirement for methionine metabolism in maintaining normal liver lipid homeostasis and for hepatic function.
Recently, the role of SAMe in very low density lipoprotein (VLDL) assembly and lipogenesis has been investigated. Deletion of MAT1A was found to disrupt VLDL assembly, leading to the synthesis of small, lipid-poor VLDL particles, and to a decrease in the secretion oftriglycerides (TG).8 Importantly, these abnormalities were corrected after SAMe treatment. Additionally, acute knockdown of MAT1A resulted in activation of SREBP-1a, which enhanced lipogenesis.9 Several abnormal pathways have also been identified in MAT1A-knockout mice that can contribute to HCC formation (reviewed in 1):
A fall in apurinic/apyrimidinic endonuclease activity, which leads to a reduction in DNA base excision repair, genome instability, and malignant transformation.
An increase in LKB1 activity, which induces the activation of AMPK, an increase in cytoplasmic HuR and enhanced hepatocyte proliferation.
A reduction of prohibitin 1 content, which leads to impaired mitochondrial function, liver injury, and multifocal HCC.
A decrease in dual-specificity phosphatases 1, which leads to uncontrolled ERK activation, a feature of HCC.
Expansion of tumorigenic oval stem cells.
Increased ubiquitin-conjugating enzyme 9 protein expression leading to increased protein sumoylation, which also occur in HCC.10
Conversely, deletion of GNMT was found to accelerate the flux from PE to PC as well as from PC to diglycerides and TG, probably as a way to compensate for the increase in the PC/PE ratio, leading to the accumulation of hepatic fat and steatosis (8, JMM, MLMC and SCL, unpublished). In parallel, increased hepatic SAMe in this mouse model was found to induce hypermethylation and silencing of several tumor suppressor genes, such as RASSFl and SOCS2, and the activation of oncogenic pathways, such as Ras and JAKIST AT.7 These results demonstrate, that in mice a normal hepatic SAMe content is necessary to maintain liver health and prevent injury and HCC.
The relevance of these findings to human health is obvious, since patients with liver cirrhosis have decreased hepatic MAT and PEMT activity.11 A marked reduction of hepatic MAT1A expression as well as of other main genes involved in methionine and SAMe metabolism, such as GNMT, PEMT, and MS, is often observed both in human liver cirrhosis and HCC.12
REGULATION OF HEPATIC SAME CONTENT
Due to the importance of SAMe as a regulator of multiple hepatic functions, the hepatic content of SAMe must be maintained constant independently of the daily intake of methionine, or of the ingest of betaine and choline, the only two dietary compounds that can supply the methyl group for methionine synthesis, or of the endogenous synthesis of 5-MTHF. Thus, when the hepatic content ofmethionine is high, this amino acid is rapidly converted to SAH due to the concerted action of MATIII and GNMT, which are activated by methionine and SAMe respectively, and then to homocysteine by AHCY (Figure 1). Moreover, SAMe activates CBS and inhibits MTHFR, and consequently the synthesis of 5-MTHF, the substrate ofMS and an inhibitor of GNMT. This way, SAMe controls the flow of homocysteine into the transsulfuration and remethylation pathways, so that when it is high homocysteine is channeled through the transsulfuration pathway, to form cysteine and α-ketobutyrate, and when it is low is used to form methionine and regenerate SAMe.
In addition to regulating the activity of the main hepatic enzymes involved in methionine catabolism and regeneration, SAMe stimulates the expression of MAT1A and inhibits the expression of MAT2A and BHMT.13,14 Finally, sarcosine, the byproduct of GNMT, is rapidly reconverted to glycine by SDH, a flavoprotein that catalyzes the oxidative demethylation of sarcosine to glycine. In this reaction THF is converted to CH2-THF as a byproduct. This way, when the hepatic content of methionine is high, the methyl group of SAMe is not wasted by what is a futile cycle carrying out opposing reactions at high rates with no net substrate flow in any direction, but saved as CH2-THF to be used to regenerate methionine when the content of SAMe is reduced and the inhibition by this molecule ofMTHFR is released.
In summary, the available evidence indicates that the liver tightly regulates SAMe levels as part of metabolic homeostasis. SAMe is mainly needed for methylation reactions and polyamine synthesis, so if the concentration falls below this level, the normal methylation of DNA, proteins, PE, guanidinoacetate and many other molecules, as well as mUltiple detoxification reactions, and the synthesis of polyamines may not be able to continue. Conversely, if the level of SAMe rises too much, hypermethylation of these same molecules and excess biosynthesis of polyamines may occur and the normal function of the liver will be also affected. There are natural conditions that can affect the hepatic content of SAMe. For example, eating a protein rich meal will increase and liver regeneration will decrease hepatic SAMe content. To control these fluctuations, the rate at which hepatocytes both synthesize and catabolize SAMe is mainly regulated by the enzymes MATI, MATIII, and GNMT. MATIII reduces the level of blood methionine when it is too high by increasing the rate of SAMe synthesis, and GNMT turns this excess of SAMe into SAH, which is converted to homocysteine by the enzyme AHCY. Then, SAMe reduces the hepatic level of homocysteine by activating the rate at which this sulfur amino acid is converted to cystathionine by the enzyme CBS. Subsequently, cystathionine is converted to cysteine and other sulfur containing molecules, such as GSH, taurine or sulfate, and α-ketobutyrate is oxidized in the mitochondria.
MTHFR increases the level of hepatic methionine by doing roughly the opposite to CBS. When the concentration of blood methionine and hepatic SAMe falls too much, the inhibition that SAMe exerts on the enzyme MTHFR is released increasing the synthesis of 5-MTHF, which is utilized by the enzyme MS for the synthesis of methionine followed by its conversion to SAMe catalyzed mainly by the enzyme MATI, because MATIII has higher Km that MATI. In addition to this, 5-MTHF inhibits GNMT activity and therefore controls SAMe availability for other methylation reactions, such as for the synthesis of PC or creatine.
SAME IN LIVER DISEASE TREATMENT
Results obtained with a variety of genetically modified mouse models indicate that SAMe plays an important function in the hepatic accumulation of fat and hepatocyte proliferation, and that a chronic hepatic imbalance in SAMe synthesis or catabolism leads to fatty liver disease and the development of HCC (reviewed in 1). Therefore, maintaining SAMe homeostasis may be a therapeutic target in nonalcoholic steatohepatitis (NASH), alcoholic- and non-alcoholic liver cirrhosis. In this respect, it is important to note that SAMe treatment has been shown to increase survival in patients with alcoholic liver cirrhosis15 and studies are underway to examine its efficacy in the treatment of NASH (personal communication).
Patients with chronic liver disease often have reduced SAMe biosynthesis because of decreased MAT1A expression and inactivation of MAT1A-encoded isoenzymes.16 MAT1A knockout mice have chronic hepatic SAMe deficiency, increased susceptibility to liver injury and develop HCC on a normal diet.5,17 Abnormalities in mUltiple signaling pathways discussed above contribute to the increased malignant degeneration in the setting of chronic SAMe deficiency. These collectively support the idea of using SAMe in the chemoprevention of HCC. Indeed, studies from Feo and coworkers from the mid 1980s already demonstrated in several rodent models of hepatocarcinogenesis that SAMe is effective in preventing HCC.18-21 In these models, the major mechanism of chemoprevention by SAMe was thought to be preventing the hypo methylation of several proto-oncogenes. More recently we examined SAMe’s chemopreventive effect in an orthotopic HCC model where liver cancer cells were directly injected into the liver parenchyma without changing hepatic SAMe level.22 SAMe was able to inhibit establishment of HC, which may be related to SAMe’s ability to selectively kill liver cancer cells as well as SAMe’s anti-angiogenic properties.22 However, SAMe was ineffective in treating already existing HCC as the normal liver was able to up-regulate compensatory mechanisms (such as GNMT expression) to dispose excess SAMe and prevent its accumulation.22 Thus, hepatic SAMe level increased by 10-fold 24 hours after intravenous administration of SAMe but when the treatment was maintained for 24 days, it increased only 30%.22 This level of increase in SAMe may be insufficient to exert proapoptotic and anti-angiogenic effect. However, in human HCC, GNMT is frequently silenced so it remains to be examined what the outcome of SAMe treatment is when GNMT is not expressed.
More than 500 million people in the world are at risk for HCC development.23 Most of them have chronic hepatitis B or C viral infection but the incidence of HCC is expected to rise in the west. Chemoprevention of HCC is an important area that has received very little to no attention (23). Given SAMe’s excellent safety profile, abundance of basic science data and efficacy of SAMe in preventing HCC in experimental models, it is imperative to begin studies examining SAMe’s role in both primary and secondary chemoprevention of human HCC.
ACKNOWLEDGEMENTS
This work was supported by NIH grants R01DK51719 (SC Lu and JM Mato), P30DK48522 (SC Lu), R01AT1576 (SC Lu, ML Martínez-Chantar, and JM Mato), R01AT004896 (SC Lu, ML Martinez-Chantar, and JM Mato), Plan Nacional of I+D SAF 2011-29851 (JM Mato), Departamento de Educación Gobierno Vasco and Etortek 2011 (ML Martínez-Chantar and JM Mato), Sanidad Gobierno Vasco and FIS PI11/01588 (ML Martínez-Chantar).
ABBREVIATIONS
- AHCY
S-adenosylhomocysteine hydrolase.
- BHMT
betaine-homocysteine methyltrasnferase.
- CBS
cystathionine β-synthase.
- CH2-THF
methylene tetrahydrofolate.
- CSE
cystathionine γ-Iyase.
- DHF
dihydrofolate.
- GAMT
guanidinoacetate N-methyltransferase.
- GNMT
glycine N-methyltransferase.
- GSH
glutathione.
- HCC
hepatocellular carcinoma.
- H2S
hydrogen sulfide.
- MAT
methionine adenosyltransferase.
- MS
methionine synthase.
- MT
methyltransferase.
- MTA
methylthioadenosine.
- 5-MTHF
5-methyl tetrahydrofolate.
- MTHFR
methyl tetrahydrofolate reductase.
- NASH
nonalcoholic steatohepatitis.
- PC
phosphatidylcholine.
- PE
phosphatidylethanolamine.
- PEMT
phosphatidylethanolamine N-methyltransferase.
- SAH
S-adenosylhomocysteine.
- SAMe
S-adenosylmethionine.
- SDH
sarcosine dehydrogenase.
- SPM
spermine.
- SPMS
spermine synthase.
- SPD
spermidine.
- SPDS
spermidine synthase.
- TG
triglycerides.
- THF
tetrahydrofolate.
- VLDL
very low density lipoproteins.
REFERENCES
- 1.Petrossian TC, Clarke SG. Uncovering the human methyltransferasome. Mol Cell Proteomics. 2011;10:M110.000976. doi: 10.1074/mcp.M110.000976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Finkelstein JD. Methionine metabolism in mammals. J Nutr Biochem. 1990;1:228–37. doi: 10.1016/0955-2863(90)90070-2. [DOI] [PubMed] [Google Scholar]
- 3.Mudd SH, Poole JR. Labile methyl balances for normal humans on various dietary regimens. Metabolism. 1975;24:721–35. doi: 10.1016/0026-0495(75)90040-2. [DOI] [PubMed] [Google Scholar]
- 4.Fernández-Irigoyen J, Santamaría E, Chien YH, Hwu WL, Korman SH, Faghfoury H, Schulze A, et al. Enzymatic activity of methionine adenosyltransferase variants identified in patients with persistent hypermethioninemia. Mol Genet Metab. 2010;101:172–7. doi: 10.1016/j.ymgme.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 5.Lu SC, Alvarez L, Huang ZZ, Chen L, An W, Corrales FJ, Avila MA, et al. Methionine adenosyltransferase 1A knockout mice are predisposed to liver injury and exhibit increased expression of genes involved in proliferation. Proc Natl Acad Sci USA. 2001;98:5560–5. doi: 10.1073/pnas.091016398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mudd SH, Brosnan JT, Brosnan ME, Jacobs RL, Stabler SP, Allen RH, Vance DE, et al. Methyl balance and transmethylation fluxes in humans. Am J Clin Nutr. 2007;85:19–25. doi: 10.1093/ajcn/85.1.19. [DOI] [PubMed] [Google Scholar]
- 7.Martínez-Chantar ML, Vázquez-Chantada M, Ariz U, Martínez N, Varela M, Luka Z, Capdevila A, et al. Loss of the glycine N-methyltransferase gene leads to steatosis and hepatocellular carcinoma in mice. Hepatology. 2008;47:1191–9. doi: 10.1002/hep.22159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cano A, Buqué X, Martínez-Uña M, Aurrekoetxea I, Menor A, García-Rodríguez JL, Lu SC, et al. Methionine adenosyltransferase 1A gene deletion disrupts hepatic very low-density lipoprotein assembly in mice. Hepatology. 2011;54:1975–86. doi: 10.1002/hep.24607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walker AK, Jacobs RL, Watts JL, Rottiers V, Jiang K, Finnegan OM, Shioda T, et al. A conserved SREBP-1/phosphatidylcholine feedback circuit regulates lipogenesis in metazoans. Cell. 2011;147:840–52. doi: 10.1016/j.cell.2011.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tomasi ML, Tomasi I, Ramani K, Pascale RM, Xu J, Giordano P, Mato JM, et al. S-adenosylmethionine regulates ubiquitin-conjugating enzyme 9 protein expression and sumoylation in human cancers. Hepatology. 2012;56:982–93. doi: 10.1002/hep.25701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duce AM, Ortíz P, Cabrero C, Mato JM. S-adenosyl-L-methionine synthetase and phospholipid methyltransferase are inhibited in human cirrhosis. Hepatology. 1988;8:65–8. doi: 10.1002/hep.1840080113. [DOI] [PubMed] [Google Scholar]
- 12.Avila MA, Berasain C, Torres L, Martín-Duce A, Corrales FJ, Yang H, Prieto J, et al. Reduced mRNA abundance of the main enzymes involved in methionine metabolism in human liver cirrhosis and hepatocellular carcinoma. J Hepatal. 2000;33:907–14. doi: 10.1016/s0168-8278(00)80122-1. [DOI] [PubMed] [Google Scholar]
- 13.García-Trevijano ER, Latasa MU, Carretero MV, Berasain C, Mato JM, Avila MA. S-adenosylmethionine regulates MAT1A and MAT2A gene expression in cultured rat hepatocytes: a new role for S-adenosylmethionine in the maintenance of the differentiated status of the liver. FASEB J. 2000;14:2511–8. doi: 10.1096/fj.00-0121com. [DOI] [PubMed] [Google Scholar]
- 14.Ou X, Yang H, Ramani K, Ara AI, Chen H, Mato JM, Lu Sc. Inhibition of human betaine-homocysteine methyltransferase expression by S-adenosylmethionine and methylthioadenosine. Biochem J. 2007;401:87–96. doi: 10.1042/BJ20061119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mato JM, Cámara J, Fernández de Paz J, Caballería L, Coll S, Caballero A, García-Suey L, et al. S-adenosylmethionine in alcoholic liver cirrhosis: a randomized, placebo-controlled, double-blind, multicenter clinical trial. J Hepatol. 1999;30:1081–9. doi: 10.1016/s0168-8278(99)80263-3. [DOI] [PubMed] [Google Scholar]
- 16.Lu SC, Mato JM. S-adenosylmethionine in liver health, injury, and cancer. Physiol Rev. 2012;92:1515–42. doi: 10.1152/physrev.00047.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martínez-Chantar ML, Corrales FJ, Martínez-Cruz A, García-Trevijano ER, Huang ZZ, Chen LX, Kanel G, et al. Spontaneous oxidative stress and liver tumors in mice lacking methionine adenosyltransferase 1A. FASEB J. 2002:10.1096/fj.02–0078fje. doi: 10.1096/fj.02-0078fje. [DOI] [PubMed] [Google Scholar]
- 18.Feo F, Garcea R, Daino L, Pascale R, Pirisi L, Frassetto S, Ruggiu ME. Early stimulation of polyamine biosynthesis during promotion by phenobarbital of diethylnitrosamine-induced rat liver carcinogenesis. The effects of variations of the S-adenosyl-L-methionine cellular pool. Carcinogenesis. 1985;6:1713–20. doi: 10.1093/carcin/6.12.1713. [DOI] [PubMed] [Google Scholar]
- 19.Pascale RM, Simile MM, Satta G, Seddaiu MA, Daino L, Pinna G, Vinci MA, et al. Comparative effects of L-methionine, Sadenosyl-L-methionine and 5′-methylthioadenosine on the growth of preneoplastic lesions and DNA methylation in rat liver during the early stages of hepatocarcinogenesis. Anticancer Res. 1991;11:1617–24. [PubMed] [Google Scholar]
- 20.Pascale RM, Marras V, Simile MM, Daino L, Pinna G, Sennati S, Carta M, et al. Chemoprevention of rat liver carcinogenesis by S-adenosyl-L-methionine: A long-term study. Cancer Res. 1992;52:4979–86. [PubMed] [Google Scholar]
- 21.Pascale RM, Simile MM, De Miglio MR, Nufris A, Daino L, Seddaiu MA, Rao PM, et al. Chemoprevention by S-adenosyl-L-methionine of rat liver carcinogenesis initiated by 1,2-dimethylhydrazine and promoted by orotic acid. Carcinogenesis. 1995;16:427–30. doi: 10.1093/carcin/16.2.427. [DOI] [PubMed] [Google Scholar]
- 22.Lu SC, Ramani K, Ou XP, Lin M, Yu V, Ko K, Park R, et al. S-adenosylmethionine in the chemoprevention and treatment of hepatocellular carcinoma in a rat model. Hepatology. 2009;50:462–71. doi: 10.1002/hep.22990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu Sc. Where are we in the chemoprevention of hepatocellular carcinoma? Hepatology. 2010;51:734–6. doi: 10.1002/hep.23497. [DOI] [PubMed] [Google Scholar]