Abstract
The relative contribution of dietary arsenic (As) to aggregate daily exposure has not been well-characterized, especially in relation to the current EPA maximum contaminant level (MCL) of 10 p.p.b. for As in drinking water. Our objectives were to: (1) model exposure to inorganic and total As among non-seafood eaters using subject-specific data, (2) compare the contribution of food, drinking and cooking water to estimated aggregate exposure in households with variable background tap water As levels, and (3) describe the upper distribution of potential dose at different thresholds of tap water As. Dietary As intake was modeled in regional study populations and NHANES 2003–2004 using dietary records in conjunction with published food As residue data. Water As was measured in the regional studies. Among subjects exposed to tap water As >10 p.p.b., aggregate inorganic exposure was 24.5–26.1 μg/day, with approximately 30% of intake from food. Among subjects living in homes with tap water As ≤10, 5 or 3 p.p.b., aggregate inorganic As exposure was 8.6–11.8 μg/day, with 54–85% of intake from food. Median inorganic As potential dose was 0.42–0.50 μg/kg BW/day in subjects exposed to tap water As >10 p.p.b. and less than half that among subjects exposed to tap water As ≤10 p.p.b. The majority of inorganic and total As exposure is attributable to diet in subjects with tap water As <MCL. Further research is needed to determine the potential toxicity and need for regulation of As in foods.
Keywords: arsenic, diet, water, exposure, potential dose
INTRODUCTION
Ingestion of arsenic (As) poses a significant public health risk to millions of people in the US. Classified as a Group 1 human carcinogen,1 chronic exposure to even relatively low levels of inorganic As in drinking water (i.e., 10–50 μg/l) has been associated with increased rates of urinary2 and lung cancers and increased mortality from cardiovascular disease,3–5 kidney disease,3,4 and diabetes.4 In 2001, the US Environmental Protection Agency (EPA) reduced the maximum contaminant level (MCL) for inorganic As in municipal drinking water from 50 p.p.b. to 10 p.p.b. (μg/l), but the adequacy of the 10 p.p.b. limit in protection of public health is still being debated.6 This question is further complicated by the lack of health-based As standards for daily dose. In 2011, the World Health Organization (WHO) withdrew the estimated 1983 provisional tolerable daily intake (PTDI) standard for inorganic As of 2.1 μg/kg body weight/day, stating that the PTDI was “in the region of the BMDL0.5,” the benchmark dose for a 0.5% increased incidence of lung cancer.7 Although a number of studies have suggested that food rather than water provides the majority of exposure to total and inorganic As,8–11 As regulations in the United States pertain only to drinking water.
Most of the monitoring of As in food has focused on total As, based on the belief that As in food is primarily organic and nontoxic.9 However, information on other As species in foods is incomplete. Although fish, shellfish and seaweed do have extremely high concentrations of organic As, inorganic As accounts for the vast majority of total As in non-seafood products.9,12 A recent study found that dietary inorganic As intake was a better predictor of urinary As biomarkers than water As intake, especially in populations that have lower levels of As in their tap water.13 There have also been questions raised regarding differences in the bioavailability of and potential risks associated with ingestion of inorganic As in foods as compared with water,9,14 and addressing these questions in future research was a key recommendation of the WHO.15 In an animal model, absorption of As was found to be dependent on the As species ingested.16 A recent human study concluded that bioavailability of As from ingestion of foods and beverages was approximately 90–95% over 6 days.17
In the analyses presented here, we explored the overall contribution of dietary inorganic and total As intake to aggregate modeled exposure via ingestion in multiple study populations and at different background concentrations of tap water As. The objectives were to: (1) model exposure to inorganic and total As from food and water among non-seafood eaters, based on subject-specific dietary records; (2) compare the contribution of food, drinking and cooking water to estimated aggregate As exposure among subjects living in households with tap water As concentrations above and below the current MCL, and below proposed thresholds of 5 p.p.b. and 3 p.p.b.; and (3) explore the upper percentiles of potential dose stratified by tap water As concentration, particularly in relation to the previous PTDI for inorganic As.
METHODS
These analyses include data from three different population studies: The National Human Exposure Assessment Survey (NHEXAS), the Binational Arsenic Exposures Study (BAsES), and the 2003–04 National Health and Nutrition Examination Survey (NHANES).
Study Populations
NHEXAS was a population-based probability sample of the total population of Arizona, conducted between 1995 and 1997, designed to evaluate water, food and other environmental and biological samples for multiple contaminants.18–20 An additional survey of 25 census tracts along the Arizona–Mexico border (Arizona Border Survey) was conducted between 1997 and 1998 using the same protocols. Participants were asked to complete a 24-h diet diary consisting of a checklist of US Food and Drug Administration Total Diet Study (TDS)-coded foods plus additional food items commonly consumed by Mexican-Americans,21 and were asked the number of servings and serving sizes consumed. NHEXAS also obtained water samples from all water sources used for drinking and/or cooking. A total of 246 participants, 163 from the original NHEXAS and 83 from the Arizona Border Survey, completed diet diaries and provided water samples. These populations are described together in the analyses presented here and referred to as NHEXAS-AZ.
The methods used in BAsES have been described in detail elsewhere.22 Conducted between 2006 and 2007, BAsES was a cross-sectional survey of communities in northern Mexico and Arizona, selected to represent a range of exposures based on As concentration of the groundwater.23 Only the Arizona population was considered in the analyses presented here. Arizona households were selected via random-digit dialing, and adults aged >18 years who had lived in their homes for at least 1 year were eligible to participate. During a household visit, trained study personnel administered a single 24-h dietary recall using food models to prompt portion size recall. Participants were asked to describe all foods and beverages consumed over the previous 24 h, including method of preparation and quantity consumed. Dietary data were entered into the Minnesota Nutrition Data System-Research Version (NDS-R) computer-based software system for analysis. Water samples were obtained from all sources used for drinking and/or cooking; information on frequency of use of each source was obtained from questionnaires. The study included a total of 223 Arizona participants with complete dietary recall data.
The 2003–04 National Health and Nutrition Examination Survey (NHANES) used a complex, stratified, four-stage probability cluster design to select a population-proportional sample of the civilian US population, oversampling certain population subgroups to ensure representation (EPA, 2003). Detailed dietary interviews (day 1), physical exams and sample collections were conducted in Mobile Examination Centers for persons ≥6 years of age. The one-third subsample of this population who participated in laboratory assessment of urinary As (n 2420) were included in these analyses.24 Subjects reported the total amount of plain water that they drank the previous day, but no samples of drinking or cooking water were collected in NHANES.
All participants in the original studies provided informed consent according to the requirements of either The University of Arizona Human Subjects Protection Program or the Centers for Disease Control/National Center for Health Statistics Ethics Review Board. The University of Arizona Human Subjects Protection Program granted this secondary data analysis exempt status.
Dietary As Modeling
The 24-h dietary records were evaluated by the Arizona Diet, Behavior and Quality of Life Assessment Lab at the Arizona Cancer Center for inorganic and total As content. Missing food quantities were assumed to be standard serving sizes.25 Dietary As was modeled based on Schoof et al.,26 who used a market basket survey approach to determine inorganic and total As content of 40 food commodities that were assumed to represent approximately 90% of dietary inorganic As intake in the United States and Canada. This approach involved collecting four samples of each food commodity from supermarkets in two Texas towns and using local tap water for preparation. Inorganic and total As for these food items were determined using inductively coupled plasma-mass spectrometry (ICP-MS), with a limit of detection (LOD) of 2 ng/g for inorganic As and 3.6 ng/g for total As.26 The protocol for assigning As values to the food records involved calculating mean concentrations based on food groups for food items that were not in the list of 40 commodities measured. For example, for unlisted fruits reported in the dietary recalls, a mean concentration was calculated from all of the measured fruits (i.e., raw apples, bananas, grapes, oranges, peaches and watermelons). Furthermore, for mixed food items, such as macaroni and cheese, As values were assigned based on the estimated proportion of key ingredients used in standard recipes.13,27,28
Water As Estimates
In NHEXAS-AZ and BAsES-AZ, water samples were collected from all sources of water that subjects reported using for drinking or cooking and then analyzed in the laboratory for total As. Water samples from NHEXAS-AZ were analyzed in duplicate for total As by EPA contract laboratories using ICP-MS, with a LOD of 0.20 μg/l.20,29 BAsES-AZ water samples were analyzed by the Hazard Identification Core at the University of Arizona, supported by the Superfund Basic Research Program Grant from the National Institute of Environmental Health Sciences. ICP-MS analysis was used, with a LOD of 0.10 μg/l for total As.
In NHEXAS-AZ and BAsES-AZ, subjects reported which water sources were used for drinking, cooking or both, the frequency of use by source and the quantity of drinking water consumed. To estimate average daily exposure to As from drinking water, the quantity consumed per source was multiplied by the concentration of As in the sample. Missing drinking water volumes were replaced with mean volumes for specific age groups calculated for each population.13,23 The quantity of water used in cooking was estimated for those food items that were cooked at home and required the addition of water for preparation (i.e., pasta, grains, beans, powdered mixes, coffee, tea, soups, etc.). The number of grams of water in food items prepared at home was determined from the Nutritional Data System for Research (NDSR) output for specific foods in the diet records as a proportion of the total grams consumed.25 Exposure to As in cooking water was then calculated as the sum of the total grams of water added in food preparation times the mean As concentration of all water sources used for cooking.
No water samples were collected in the 2003–04 NHANES, but daily drinking water intake was reported and the quantity of water used in cooking was estimated using the NDSR data output methods described above. To estimate drinking and cooking water As concentrations in NHANES, a constant was used—the EPA average for tap water in the United States of 2.4 μg/l based on a study of 3834 households.30 Exposure to As in drinking and cooking water was then calculated as the product of the volume consumed (l/day) and the constant (2.4 μg/l).
Statistical Methods
Stata 11.2 (StataCorp, College Station, TX, USA) was used for statistical analyses, and a P-value <0.05 was considered statistically significant for all statistical tests. For analysis of NHANES data, survey weights for the one-third subsample of the population with urinary As data were used.
Subjects who had consumed fish or other seafood in the past 24 h were identified from dietary records and excluded from these analyses because of the overwhelming contribution of seafood to total As intake and uncertain contribution to inorganic As intake. Descriptive statistics and two-sample t-tests were used to compare population characteristics by ethnic group. In NHEXASAZ and BAsES-AZ, ethnic groups represented were Hispanic white (predominantly Mexican-American) and non-Hispanic white. In analysis of the NHANES population, only non-Hispanic whites and Mexican-Americans are included in the comparison of population characteristics by ethnic group, but all ethnic groups are represented in analyses of the total population. As concentration and exposure variables were assessed for normality and log(10) transformations applied to normalize right-skewed distributions. Geometric means (GM) and 95% confidence intervals (CI) are reported. Wilcoxon rank-sum test was used to compare differences in As exposure variables by ethnic group and by household tap water As concentration above versus below 10, 5 and 3 p.p.b.. Two-sample t-tests were used to compare log-transformed mean As in food, drinking water and cooking water between populations. Total and inorganic As potential dose from food and water were calculated by dividing the sum of each individual's exposures by his/her body weight (BW), and the median, 75th, 90th and 95th percentiles were compared at different tap water As concentration thresholds for the NHEXAS-AZ and BAsES-AZ study populations.
RESULTS
Population characteristics for the total population and by ethnic group within each study population are shown in Table 1. The combined NHEXAS-AZ population was 64% female and 45% Mexican-American. BAsES-AZ was 55% female and 23% Mexican-American and 2003–04 NHANES was 52% female, 9% Mexican-American and 71% non-Hispanic white (other ethnic groups not shown). The mean age was >40 years in each study. Children aged <18 years were not eligible for inclusion in the BAsES-AZ study, but the other studies included subjects aged ≥6 years. Approximately 14% of subjects in each of these study populations reported seafood consumption during the previous 24 h. In the NHANES population, subjects who consumed seafood were significantly older than those who did not, but no other population characteristics differed among subjects who did and did not report seafood in their diet records. Subjects who reported eating seafood were excluded from all the analyses presented here.
Table 1.
Population characteristics of subjects with dietary data, stratified by ethnic group (includes seafood and non-seafood eaters).
| NHEXAS-AZ |
BAsES-AZ |
NHANES 2003–04a |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Non-Hispanic | Hispanic | Total | Non-Hispanic | Hispanic | Total | Non-Hispanic | Mexican-American | Total | |
| Subjects, N (%) | 135 (54.9) | 111 (45.1) | 246 | 171 (76.7) | 52 (23.3) | 223 | (71.0) | (8.7) | 2420 |
| Female, N (%) | 81 (60.0) | 76 (68.5) | 157 (63.8) | 95 (55.6) | 28 (53.9) | 123 (55.2) | (52.4) | (49.8) | (52.0) |
| Mean age, years | 43.1 | 44.8 | 43.8 | 57.7 | 48.6 | 55.6 | 41.6 | 30.2 | 40.4 |
| Age range, years | 6–83 | 10–78 | 6–83 | 20–87 | 19–80 | 19–87 | 6–85 | 6–85 | 6–85 |
| Mean body weight, kg | 68.2 | 72.9 | 70.3 | 79.8 | 88.3 | 81.6 | 76.7 | 70.6 | 75.3 |
| Mean BMI | 24.4 | 27.5 | 25.8 | 28.5 | 31.3 | 29.1 | 27.0 | 26.8 | 27.0 |
| Current smokers, N (%) | 28 (20.7) | 14 (13.3) | 42 (17.1) | 30 (17.5) | 3 (5.8) | 33 (14.8) | (27.3) | (17.3) | (25.6) |
| Consumed seafood, N (%) | 18 (13.1) | 17 (14.8) | 35 (13.9) | 24 (14.0) | 7 (13.5) | 31 (13.9) | (12.5) | (8.9) | (13.8) |
Population-proportional percentages and means.
The mean concentration of inorganic and total As in different media and the estimated exposure to As via ingestion are shown for non-seafood eaters in each study population, stratified and unstratified by ethnic group (Table 2). As concentrations in both cooking and drinking water were significantly higher in BAsES-AZ than in NHEXAS-AZ (both P<0.001) due to the community-level selection criteria used in the BAsES study design. Overall, total As exposure modeled from food was 4–6 times greater than inorganic As exposure from food, and inorganic As exposure from food was 1–5 times greater than exposure from drinking water and 2–6 times greater than exposure from cooking water. Total As exposure from food averaged 47 μg/day in NHEXAS-AZ, 26 μg/day in BAsES-AZ and 41 μg/day in NHANES, and geometric mean inorganic As exposure from food was 7.2 μg/ day in NHEXAS-AZ, 5.8 μg/day in BAsES-AZ and 8.0 μg/day in NHANES. In NHEXAS-AZ, both total and inorganic As exposure from food were significantly lower among Hispanic than among non-Hispanic whites (both P=0.003). Opposite results were found in the NHANES population, in which total As exposure from food was significantly higher among Mexican-Americans than among non-Hispanic whites (P=0.036). Hispanics in both NHEXAS-AZ and BAsES-AZ had significantly lower estimated drinking water As exposure than non-Hispanics (both P<0.05). In NHANES, estimated exposure to cooking water As, but not drinking water, was lower among Mexican-Americans (P=0.002).
Table 2.
Geometric mean (GM) and 95% confidence interval (CI) for inorganic and total As concentration (μg/l) and exposure (μg/day) among non-seafood eaters, stratified by study population and ethnic group.
|
NHEXAS-AZ
|
BAsES-AZ
|
NHANES 2003–04a
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Non-Hispanic (n = 117) | Hispanic (n = 94) | Total (n = 211) | Non-Hispanic (n = 147) | Hispanic (n = 45) | Total (n = 192) | Non-Hispanic (n = 897) | Mexican-American (n = 527) | Total (n = 2114) | |
| Total As concentration, GM (μg/l) | |||||||||
| Drinking water | 1.24 | 0.70 | 0.96* | 3.81 | 2.98 | 3.60 | 2.4 | 2.4 | 2.4 |
| 95% CI | 0.85–1.80 | 0.48–1.03 | 0.48–1.01 | 2.97–4.89 | 2.18–4.09 | 2.94–4.41 | |||
| Cooking water | 4.40 | 4.50 | 4.44 | 7.44 | 4.68 | 6.67* | 2.4 | 2.4 | 2.4 |
| 95% CI | 3.67–5.27 | 3.80–5.33 | 3.92–5.03 | 5.85–9.45 | 3.84–5.70 | 5.52–8.07 | |||
| As exposure, GM (μg/day) | |||||||||
| Drinking water | 2.03 | 0.95 | 1.44* | 5.00 | 3.27 | 4.52* | 2.59 | 2.13 | 2.57 |
| 95% CI | 1.38–2.98 | 0.64–1.40 | 1.09–1.90 | 3.62–6.91 | 2.27–4.70 | 3.48–5.88 | 2.21–2.97 | 1.84–2.43 | 2.29–2.86 |
| Cooking water | 2.03 | 1.50 | 1.77 | 2.61 | 1.92 | 2.43 | 1.43 | 0.97 | 1.28# |
| 95% CI | 1.56–2.64 | 1.14–1.97 | 1.46–2.14 | 1.91–3.57 | 1.25–2.95 | 1.88–3.15 | 1.32–1.55 | 0.81–1.13 | 1.20–1.36 |
| Food, inorganic | 8.60 | 5.73 | 7.18** | 5.78 | 5.77 | 5.78 | 7.90 | 9.98 | 7.96 |
| 95% CI | 7.6–9.7 | 4.6–7.1 | 6.4–8.1 | 5.2–6.4 | 4.5–7.4 | 5.2–6.4 | 7.0–8.8 | 4.8–15.1 | 7.3–8.7 |
| Food, total | 56.25 | 37.42 | 46.91** | 25.40 | 27.99 | 25.99 | 38.22 | 42.75 | 40.89* |
| 95% CI | 48.9–64.7 | 30.4–16.1 | 41.5–53.1 | 22.8–28.3 | 22.5–34.8 | 23.6–28.6 | 36.0–40.5 | 39.7–45.8 | 38.1–43.7 |
Wilcoxon Rank-Sum Test used to compare differences by ethnicity.
P < 0.05
P ≤ 0.01
P ≤ 0.001 for comparison of means by ethnic group.
Population proportional estimates with water As concentration based on national average.
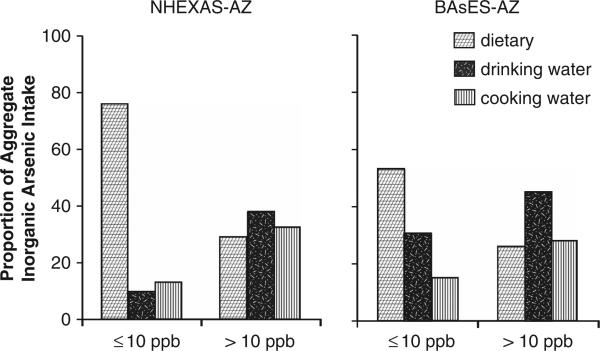
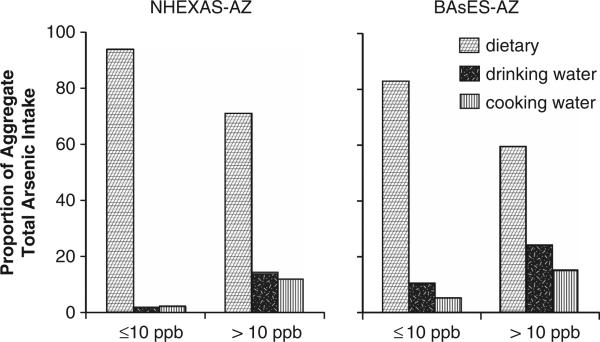
The quantity and relative proportion of inorganic and total As ingested via food, drinking water and cooking water are shown in Table 3, stratified by household tap water As concentration above and below 10 p.p.b., below 5 p.p.b. and below 3 p.p.b. for NHEXASAZ and BAsES-AZ. (As discussed in the methods, all subjects in NHANES were assumed to live in homes with tap water As equal to the EPA national average of 2.4 p.p.b.) Mean aggregate inorganic As exposure among subjects living in homes with tap water As ≤10 p.p.b., 5 p.p.b. and 3 p.p.b. was similar in all of the studies, approximately 9–12 μg/day, and >54% of this exposure was from food. Among subjects with household tap water As >10 p.p.b., aggregate inorganic As exposure was 26.1 μg/day and 24.5 μg/day (NHEXAS-AZ and BAsES-AZ, respectively), with less than a third of the aggregate intake contributed by food (see also Figure 1). Mean aggregate total As exposure ranged between 31.2 and 70 μg/day and was markedly higher among NHEXAS-AZ participants. Again there was variation in total As exposure by background tap water level. In subjects living in households with tap water As ≥10 p.p.b., mean aggregate total As ingested was 46–56 μg/day in NHEXAS-AZ and 30–35 μg/day in BAsES-AZ, and over 95% and 84%, respectively, was from food. In households with tap water As >10 p.p.b., aggregate total As exposure was 70.7 μg/day in NHEXAS-AZ and 45.7 μg/day in BAsES, and food consumption accounted for 74% and 60% of the total, respectively (Figure 2). In households with tap water As >MCL, drinking water As contributed 14% (NHEXAS-AZ) and 24% (BAsES) of total As exposure, and cooking water, 12% and 15%, respectively. Aggregate total As exposure in NHANES was estimated at 45 μg/day, similar to NHEXAS-AZ subjects exposed to tap water As <MCL.
Table 3.
Geometric mean (%) inorganic and total As intake (μg/day) from food, drinking water and water used in cooking and food preparation among subjects who did not report eating seafood, stratified by tap water As concentration ≤3, ≤5, ≤10 and >10 p.p.b.
| NHEXAS-AZ |
BAsES-AZ |
NHANES 2003–04a |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Tap water As (p.p.b.) |
Tap water As (p.p.b.) |
Tap water As (p.p.b.) |
|||||||
| ≤3 | ≤5 | ≤10 | >10 | ≤3 | ≤5 | ≤10 | >10 | 2.4 | |
| N | 51 | 128 | 176 | 35 | 31 | 73 | 138 | 54 | 2114 |
| Dietary inorganic As | 8.12 (84.8%) | 6.79 (79.0%) | 7.09 (75.4%) | 7.97 (30.5%) | 7.17 (74.0%) | 5.95 (64.0%) | 5.55 (53.6%) | 6.41 (26.2%) | 7.96 (67.4%) |
| Drinking water As | 0.74 (7.7%) | 0.83 (9.7%) | 0.97 (10.3%) | 9.78 (37.4%) | 1.58 (16.3%) | 2.16 (23.3%) | 3.19 (30.8%) | 11.08 (45.3%) | 2.57 (21.8%) |
| Cooking water As | 0.72 (7.5%) | 0.97 (11.3%) | 1.34 (14.3%) | 8.38 (32.1%) | 0.94 (9.7%) | 1.18 (12.7%) | 1.61 (15.6%) | 6.99 (28.6%) | 1.28 (10.8%) |
| Aggregate inorganic As exposure | 9.58 | 8.59 | 9.40 | 26.13 | 9.69 | 9.29 | 10.35 | 24.48 | 11.81 |
| Dietary total As | 54.53 (97.4%) | 44.22 (96.2%) | 46.20 (95.2%) | 52.58 (74.3%) | 32.77 (92.9%) | 27.87 (89.3%) | 25.38 (84.1%) | 27.60 (60.4%) | 40.89 (91.4%) |
| Drinking water As | 0.74 (1.3%) | 0.83 (1.8%) | 0.97 (2.0%) | 9.78 (13.8%) | 1.58 (4.5%) | 2.16 (6.9%) | 3.19 (10.6%) | 11.08 (24.3%) | 2.57 (5.7%) |
| Cooking water As | 0.72 (1.3%) | 0.97 (2.1%) | 1.34 (2.8%) | 8.38 (11.8%) | 0.94 (2.7%) | 1.18 (3.8%) | 1.61 (5.3%) | 6.99 (15.3%) | 1.28 (2.9%) |
| Aggregate total As exposure | 55.99 | 46.02 | 48.51 | 70.74 | 35.29 | 31.21 | 30.18 | 45.67 | 44.74 |
Population proportional estimates with water As concentration based on national average.
Figure 1.
Proportion of aggregate inorganic As intake among non-seafood eaters that was attributable to food and water used for drinking and cooking, stratified by household tap water As concentrations ≤10 p.p.b. versus >10 p.p.b. (MCL), in two study populations, NHEXAS-AZ and BAsES.
Figure 2.
Proportion of aggregate total As intake intake among non-seafood eaters that was attributable to food and water used for drinking and cooking, stratified by household tap water As concentrations ≤10 p.p.b. versus >10 p.p.b. (MCL), in two study populations, NHEXAS-AZ and BAsES.
The minimum and maximum potential dose of inorganic As among non-seafood eaters in these populations was 0.01–2.8 μg/kg BW/day in NHEXAS-AZ, 0.03–38.9 μg/kg BW/day in BAsES and 0.004–1.00 μg/kg BW/day in NHANES, while total As potential dose was 0.03–5.25, 0.12–39.95 and 0.02–4.63 μg/kg BW/day, respectively. Table 4 shows the median and upper 75th, 90th and 95th percentiles of the distributions for potential daily dose of inorganic and total As, stratified by background household tap water As concentrations of 3, 5 and 10 p.p.b., based on an average daily intake of food, drinking water and water used for food and beverage preparation. The median inorganic As potential dose among subjects living in households with tap water >MCL was 0.42 μg/kg BW/day in BAsES-AZ and 0.50 μg/kg BW/day in NHEXAS-AZ, and less than half that among subjects living in households with tap water As<MCL. The 95th percentile for inorganic As dose was <0.70 μg/kg BW/day among subjects in all three populations living in households with ≤10, 5 and 3 p.p.b. tap water As. Among subjects with tap water As >MCL, the 95th percentile was 2.08 μg/kg BW/day in NHEXAS-AZ and 22.34 μg/kg BW/day in BAsES. The potential dose of inorganic As exceeded the 1983 WHO PTDI (2.1 μg/kg BW/day) for inorganic As in 2.9% of subjects in NHEXAS-AZ and 14.8% in BAsES-AZ, all of whom lived in homes with tap water As >MCL.
Table 4.
Potential dose (μg/kg/day) of inorganic and total As based on dietary and water As intakea among non-seafood eaters, stratified by tap water As concentration in NHEXAS-AZ, BAsES-AZ and NHANES 2003–04.
| NHEXAS-AZ |
BAsES-AZ |
NHANES 2003–04a |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Tap water As (p.p.b.) |
Tap water As (p.p.b.) |
Tap water As (p.p.b.) |
|||||||
| ≤3 | ≤5 | ≤10 | >10 | ≤3 | ≤5 | ≤10 | >10 | 2.4 | |
| Subjects, N | 51 | 128 | 176 | 35 | 31 | 73 | 138 | 54 | 2114 |
| Inorganic As dose | |||||||||
| Median | 0.19 | 0.17 | 0.20 | 0.50 | 0.14 | 0.13 | 0.17 | 0.42 | 0.13 |
| 75th percentile | 0.31 | 0.32 | 0.35 | 0.90 | 0.24 | 0.22 | 0.25 | 1.04 | 0.20 |
| 90th percentile | 0.48 | 0.48 | 0.48 | 1.81 | 0.42 | 0.42 | 0.42 | 7.69 | 0.31 |
| 95th percentile | 0.61 | 0.56 | 0.60 | 2.08 | 0.67 | 0.69 | 0.66 | 22.34 | 0.39 |
| % >2.1 μg/kg/dayb | 0 | 0 | 0 | 2.86 | 0 | 0 | 0 | 14.81 | 0 |
| Total As dose | |||||||||
| Median | 1.17 | 0.82 | 0.94 | 1.34 | 0.53 | 0.38 | 0.41 | 0.84 | 0.49 |
| 75th percentile | 1.72 | 1.57 | 1.57 | 1.84 | 0.75 | 0.62 | 0.60 | 1.41 | 0.81 |
| 90th percentile | 1.96 | 1.96 | 1.96 | 2.75 | 1.12 | 1.45 | 0.98 | 7.80 | 1.22 |
| 95th percentile | 2.98 | 2.80 | 2.75 | 3.99 | 2.67 | 2.42 | 1.78 | 22.52 | 1.64 |
| % >2.1 μg/kg/dayb | 7.84 | 7.81 | 7.96 | 17.14 | 0.68 | 8.22 | 4.34 | 22.22 | 2.60 |
As concentration in drinking and cooking water in NHEXAS/ABS and BAsES based on measured concentrations in water samples; national average tap water As was used to calculate exposure and dose in NHANES.27
2.1 μg/kg/day was the provisional tolerable daily intake (PTDI) for inorganic As proposed by WHO in 1983 and withdrawn in 2011.7
DISCUSSION
Currently, there are few standards for acceptable levels of As in food products.31 Because of limited and highly variable data on As content of food, as well as uncertainties regarding the toxicity of various As species, it is unclear whether the current standards for water alone are adequate for protection of public health. In this study, we evaluated the potential contribution of As from consumption of food, drinking water and water used in food preparation to estimate aggregate inorganic and total As exposure at different background levels of tap water As concentration: above and below 10 p.p.b. (the MCL), 5 p.p.b. and 3 p.p.b. We estimated dietary As exposure and potential daily dose in the NHEXAS-AZ, BAsES-AZ and 2003–04 NHANES study populations based on detailed 24-h dietary recall records and national databases on As levels in foods. In the two regional studies, multiple source water samples were collected and analyzed for total As. Subjects who recorded having eaten any seafood were excluded from all analyses.
The estimated aggregate exposure in these study populations for inorganic As (geometric mean) ranged from 6 μg/day to 8 μg/day, and from 26 μg/day to 47 μg/day for total As exposure. Among subjects with household tap water As <MCL, the majority of exposure to both inorganic and total As was attributable to diet. Among subjects living in homes with tap water As >MCL, 37–45% of aggregate exposure to inorganic As was attributable to drinking water and another 29–32% to water used for food preparation. Inorganic As exposure in these subjects was 2–3 times higher as compared with subjects living in homes with tap water As <MCL. In NHEXAS-AZ, approximately 3% and, in BAsES, approximately 15% of non-seafood eaters living in homes with tap water >MCL exceeded the 1983 PTDI for inorganic As.
Several studies have estimated dietary As exposure in the US population. In the TDS conducted by the Food and Drug Administration between 1991 and 1996, average daily total As intake (including from seafood) was estimated to be 2–95 μg/day for different sex and age groups in the population.32,33 Making the assumption that 100% of the As in foods other than seafood is inorganic and that 10% of total As in seafood is inorganic, Tao and Bolger32 concluded from the TDS data that dietary intake of inorganic As comprises <16% of the provisional tolerable weekly intake for all age groups. Egan et al.33 instead evaluated dietary total As intake as a percentage of the PTDI and concluded that average intake in population subgroups varied between 11% and 88% of PTDI.32,33 In another study based on 1986–1991 TDS data, the potential dose of total As from food was estimated between 0.44 μg/kg BW/day and 0.51 μg/kg BW/day for adults.34 This estimate is comparable to the median total As dose from both food and water calculated for BAsES-AZ and NHANES 2003–04 (0.048 μg/kg BW/day and 0.49 μg/kg BW/day, respectively). Yost et al.9 estimated dietary intake of inorganic As to be between 8 μg/day and 14 μg/day, 21–40% of dietary total As intake.9
Most of what is known about As content of foods is from evaluation of total As in commonly consumed foods collected in market basket surveys.26,35 Total As comprises inorganic As (arsenate and arsenite), organic As, including arsenobetaine and arsenocholine, methylated As compounds, such as monomethylarsonic acid (MMA) and dimethylarsinic acid (DMA), and arsenosugars. Existing data on As species in food are limited and show high variability.9,36–46 Fish and other seafood contain high concentrations of total As, composed largely of arsenobetaine, but some seafood also contains sizeable concentrations of inorganic and/or methylated As species and arsenosugars.15,47,48
The sources and toxicity of As species in foods are uncertain and may depend on a number of different factors. These include the type or species of food, growing conditions, exposure to arsenical pesticides, methods used in processing and preparation,15,49 etc. When inorganic As is ingested, metabolism occurs in the liver in what is generally considered a detoxification process.50 Arsenate and arsenite undergo reduction and oxidative methylation via one carbon metabolism before excretion. During metabolism, arsenate is reduced to arsenite, methylated to MMA+5, reduced to MMA+3, methylated a second time to DMA+5 and subsequently reduced, in part, to DMA+3. The trivalent methylated compounds are short-lived and difficult to measure, but in vitro studies have demonstrated that MMA+3 has greater cytotoxicity than inorganic As+3 and may actually be the predominant toxic As species.51–53
In 2011, the United Nations Joint Food and Agriculture Organization/World Health Organization Expert Committee published a report in which they conclude that the 1983 PTDI standard for inorganic As is no longer appropriate and that the current body of information suggests that there is no threshold below which As is non-toxic.7 Data on As species present in foods other than seafood are scarce,7,32 yet conclusions regarding population risk depend on this information. In our study, we modeled inorganic and total As intake separately and specifically excluded seafood eaters from our estimates. Our results clearly indicate that a considerable amount of non-seafood dietary As comprises species other than arsenate and arsenite.
A number of recent studies have raised concerns about excess intake of As in foods, such as rice, apple juice, grape juice and chicken, and the absence of standards. In our study populations, subjects in the upper percentiles of dietary inorganic As intake tended to report consumption of rice, chicken, mushrooms, cereals, nuts and seeds, wine and granola or cookie bars in their diet records. The extent to which excess As in these foods is attributable to naturally occurring As in agricultural soils, historic or current use of arsenical pesticides, high As content of water used for food preparation, or from other sources is unknown, as is the amount and distribution of As species in varieties of foods grown and/or prepared in different geographic regions. As an example, some of the market rice grown in the southeastern United States has been subject to historical application of arsenical pesticides (to cotton) and contains markedly higher As concentrations than rice grown in California.54,55 Parts of California, however, have higher levels of naturally occurring As in tap water, and use of high As cooking water may markedly increase the As content of the food as it is consumed.16,54,55
We acknowledge a number of limitations to this study. The cross-sectional population studies used in these analyses employed different study designs, dietary assessment instruments, water sampling methods and laboratory protocols. Furthermore, there are uncertainties and potential inaccuracies in the dietary modeling methods used. We relied on individual level 24-h diet recall data for NHANES and BAsES-AZ and structured diet diaries for NHEXAS-AZ to obtain information on the types and amounts of foods and beverages consumed over the course of 1 day, and modeled inorganic and total As intake based on published data on only 40 food commodities.26 Dietary recall data are subject to significant biases, including misreporting errors and under-reporting of caloric intake,56 which could lead to an underestimate of exposure to As from food. Dietary data collected over one 24-h period may not be representative of consumption over a longer period of time. Yet, despite these limitations, the estimates of dietary and aggregate As intake were relatively consistent within our study and with published US population estimates.32,33 In a previously published analysis of NHEXAS-AZ, total dietary As was measured from 24-h duplicate food samples and modeled from diet diaries using several different residue databases. There were large discrepancies in exposure estimates, depending upon the residue database used in modeling, underscoring a clear need for additional As measurement of food samples.57
In the analyses presented here, subject-specific aggregate exposure and potential dose via ingestion were assessed among non-seafood eaters in multiple study populations in the United States. We report that the majority of inorganic As exposure is attributable to diet in subjects living in households with tap water As ≤10 p.p.b. In subjects exposed to tap water As >10 p.p.b., drinking and cooking water together contribute at least 70% of aggregate inorganic As exposure. Although the MCL for municipal water is 10 p.p.b., homes with private wells may still exceed this limit. Potential dose exceeded the WHO 1983 PTDI of 2.1 μg/kg BW/day only in subjects who were living in households with tap water As >MCL.
Parsing the proportion of As exposure attributable to food, drinking water and cooking water provides a tool for assessing risk and developing an approach to regulation. The results of this study suggest that in order to reduce inorganic As exposure in the United States, future regulatory policy should focus more on reduction of As in food than in water. However, due to gaps in present knowledge, the health risks associated with exposure to the various As species cannot be fully ascertained, and further research on the presence and toxicity of speciated As compounds in foods is critically needed.
ACKNOWLEDGEMENTS
This study was completed in partial fulfillment of the requirements of the PhD program in Epidemiology at the University of Arizona, Mel and Enid Zuckerman College of Public Health. Funding for this research was provided by the US EPA Star Grant No. R83399201-0, The University of Arizona Specialized Program of Research Excellence (SPORE) (NIH/NCI CA95060), the Superfund Basic Research Program Grant (NIH ES-04940) and the Southwest Environmental Health Sciences Center Grant (NIH ES-006694).
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- 1.IARC Arsenic in drinking water. International Agency for Research on Cancer (IARC) Monographs. 2004;84:229. Retrieved from http://www.inchem.org/documents/iarc/vol84/84-01-arsenic.html. [Google Scholar]
- 2.Chen CL, Chiou HY, Hsu LI, Hsueh YM, Wu MM, Wang YH, et al. Arsenic in drinking water and risk of urinary tract cancer: a follow-up study from northeastern Taiwan. Cancer Epidemiol Biomarkers Prev. 2010;19:101–110. doi: 10.1158/1055-9965.EPI-09-0333. [DOI] [PubMed] [Google Scholar]
- 3.Lewis DR, Southwick JW, Ouellet-Hellstrom R, Rench J, Calderon RL. Drinking water arsenic in Utah: a cohort mortality study. Environ Health Perspect. 1999;107:359–365. doi: 10.1289/ehp.99107359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meliker JR, Wahl RL, Cameron LL, Nriagu JO. Arsenic in drinking water and cerebrovascular disease, diabetes mellitus, and kidney disease in Michigan: a standardized mortality ratio analysis. Environ Health. 2007;6:1–11. doi: 10.1186/1476-069X-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Medrano MA, Boix R, Pastor-Barriuso R, Palau M, Damian J, Ramis R, et al. Arsenic in public water supplies and cardiovascular mortality in Spain. Environ Res. 2010;110:448–454. doi: 10.1016/j.envres.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 6.NRC Arsenic in Drinking Water: 2001 Update. Free Executive Summary. Retrieved from http://www.nap.edu/catalog/10194.html.
- 7.WHO Evaluation of certain contaminants in food. World Health Organ Tech Rep Ser. 2011;959:1–105. Retrieved from World Health Organ Tech Rep Ser. website: http://www.ncbi.nlm.nih.gov/pubmed/21699062. [PubMed] [Google Scholar]
- 8.MacIntosh DL, Spengler JD, Ozkaynak H, Tsai L, Ryan PB. Dietary exposures to selected metals and pesticides. Environ Health Perspect. 1996;104:202–209. doi: 10.1289/ehp.96104202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yost LJ, Schoof RA, Aucoin R. Intake of inorganic arsenic in the North American diet. Hum Ecol Risk Assess. 1998;4:137–152. [Google Scholar]
- 10.Meacher DM, Menzel DB, Dillencourt MD, Bic LF, Schoof RA, Yost LJ, et al. Estimation of multimedia inorganic rsenic intake in the US population. Hum Ecol Risk Assess. 2002;8:1697–1721. [Google Scholar]
- 11.Xue J, Zartarian V, Wang SW, Liu SV, Georgopoulos P. Probabilistic modeling of dietary arsenic exposure and dose and evaluation with 2003–2004 NHANES data. Environ Health Perspect. 2010;118:345–350. doi: 10.1289/ehp.0901205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Slayton TM, Beck BD, Reynolds KA, Chapnick SD, Valberg PA, Yost LJ, et al. Issues in arsenic cancer risk assessment. Environ Health Perspect. 1996;104:1012–1018. doi: 10.1289/ehp.104-1469498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kurzius-Spencer M. PhD thesis. University of Arizona; Tucson, AZ, USA: 2012. Modeling the effects of dietary arsenic and nutrient intake on urinary arsenic biomarkers. [Google Scholar]
- 14.Del Razo LM, Garcia-Vargas GG, Garcia-Salcedo J, Sanmiguel MF, Rivera M, Hernandez MC, et al. Arsenic levels in cooked food and assessment of adult dietary intake of arsenic in the Region Lagunera, Mexico. Food Chem Toxicol. 2002;40:1423–1431. doi: 10.1016/s0278-6915(02)00074-1. [DOI] [PubMed] [Google Scholar]
- 15.WHO Arsenic and Arsenic Compounds. Environmental Health Criteria. 2001:224. Retrieved from http://whqlibdoc.who.int/ehc/WHO_EHC_224.pdf.
- 16.Juhasz AL, Smith E, Weber J, Rees M, Rofe A, Kuchel T, et al. In vivo assessment of arsenic bioavailability in rice and its significance for human health risk assessment. Environ Health Perspect. 2006;114:1826–1831. doi: 10.1289/ehp.9322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stanek EJ, 3rd, Calabrese EJ, Barnes RM, Danku JM, Zhou Y, Kostecki PT, et al. Bioavailability of arsenic in soil: pilot study results and design considerations. Hum Exp Toxicol. 2010;29:945–960. doi: 10.1177/0960327110363860. [DOI] [PubMed] [Google Scholar]
- 18.Lebowitz MD, O'Rourke MK, Gordon S, Moschandreas DJ, Buckley T, Nishioka M. Population-based exposure measurements in Arizona: a phase I field study in support of the National Human Exposure Assessment Survey. J Expo Anal Environ Epidemiol. 1995;5:297–325. [PubMed] [Google Scholar]
- 19.Robertson GL, Lebowitz MD, O'Rourke MK, Gordon S, Moschandreas D. The National Human Exposure Assessment Survey (NHEXAS) study in Arizona—introduction and preliminary results. J Expo Anal Environ Epidemiol. 1999;9:427–434. doi: 10.1038/sj.jea.7500053. [DOI] [PubMed] [Google Scholar]
- 20.O'Rourke MK, Van de Water PK, Jin S, Rogan SP, Weiss AD, Gordon SM, et al. Evaluations of primary metals from NHEXAS Arizona: distributions and preliminary exposures. National Human Exposure Assessment Survey. J Expo Anal Environ Epidemiol. 1999a;9:435–445. doi: 10.1038/sj.jea.7500049. [DOI] [PubMed] [Google Scholar]
- 21.Moschandreas DJ, Karuchit S, Berry MR, O'Rourke MK, Lo D, Lebowitz MD, et al. Exposure apportionment: ranking food items by their contribution to dietary exposure. J Expo Anal Environ Epidemiol. 2002;12:233–243. doi: 10.1038/sj.jea.7500230. [DOI] [PubMed] [Google Scholar]
- 22.Roberge J, O'Rourke MK, Meza-Montenegro MM, Gutierrez-Millan LE, Burgess JL, Harris RB. Binational arsenic exposure survey: methodology and estimated arsenic intake from drinking water and urinary arsenic concentrations. Int J Environ Res Public Health. 2012;9:1051–1067. doi: 10.3390/ijerph9041051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roberge J, O'Rourke MK, Meza-Montenegro MM, Gutierrez-Millan LE, Burgess JL, Harris RB. Binational Arsenic Exposure Survey: methodology and exploration of the relationship between estimated arsenic intake from drinking water and urinary arsenic concentrations. Int J Environ Res Public Health. 2012;9:1051–1067. doi: 10.3390/ijerph9041051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.NHANES Documentation, Codebook, and Frequencies. Laboratory Component: Total and Speciated Arsenics. [Nov 30, 2008];National Health and Nutrition Survey 2003-2004 Data Documentation. 2007 from www.cdc.gov/nchs/nhanes/nhanes2003-2004/L06UAS_C.htm.
- 25.NDSR . Nutrition Data System for Research Software Version. Nutrition Coordinating Center (NCC), University of Minnesota; Minneapolis, MN: 2009. Retrieved from http://www.ncc.umn.edu/products/database.html. [Google Scholar]
- 26.Schoof RA, Yost LJ, Eickhoff J, Crecelius EA, Cragin DW, Meacher DM, et al. A market basket survey of inorganic arsenic in food. Food Chem Toxicol. 1999;37:839–846. doi: 10.1016/s0278-6915(99)00073-3. [DOI] [PubMed] [Google Scholar]
- 27.USDA . Food and Nutrient Database for Dietary Studies 1.0—Documentation and User Guide. Agricultural Research Service, Food Surveys Research Group; Beltsville, MD, USA: 2004. Retrieved from http://www.barc.usda.gov/bhnrc/foodsurvey. [Google Scholar]
- 28.USDA . Food and Nutrient Database for Dietary Studies 2.0. Agricultural Research Service, Food Surveys Research Group; Beltsville, MD, USA: 2006. Retrieved from http://www.ars.usda.gov/SP2UserFiles/Place/12355000/pdf/fndds/fndds2_doc.pdf#title. [Google Scholar]
- 29.O'Rourke MK, Rogan SP, Jin S, Robertson GL. Spatial distributions of arsenic exposure and mining communities from NHEXAS Arizona. National Human Exposure Assessment Survey. J Expo Anal Environ Epidemiol. 1999b;9:446–455. doi: 10.1038/sj.jea.7500050. [DOI] [PubMed] [Google Scholar]
- 30.ATSDR Agency for Toxic Substances and Disease Registry Toxicological Profile for Arsenic (Update) 2007 Retrieved from http://www.atsdr.cdc.gov/toxprofiles/tp2.pdf. [PubMed]
- 31.ATSDR ATSDR Case Studies in Environmental Medicine: Arsenic Toxicity. (Course: WBCBDV1576) 2009 Retrieved from http://www.atsdr.cdc.gov/csem/arsenic/docs/arsenic.pdf. [Google Scholar]
- 32.Tao SS, Bolger PM. Dietary arsenic intakes in the United States: FDA Total Diet Study, September 1991–December 1996. Food Addit Contam. 1999;16:465–472. doi: 10.1080/026520399283759. [DOI] [PubMed] [Google Scholar]
- 33.Egan SK, Tao SS, Pennington JA, Bolger PM. US Food and Drug Administration's Total Diet Study: intake of nutritional and toxic elements, 1991–96. Food Addit Contam 2002. 19:103–125. doi: 10.1080/02652030110071354. [DOI] [PubMed] [Google Scholar]
- 34.Gunderson EL. Dietary intakes of pesticides, selected elements, and other chemicals: FDA Total Diet Study, June 1984–April 1986. J AOAC Int. 1995;78:910–921. [PubMed] [Google Scholar]
- 35.FDA [6 Nov 2011];Total Diet Study—Study Design. 2009 from http://www.fda.gov/Food/FoodSafety/FoodContaminantsAdulteration/TotalDietStudy/ucm184232.htm.
- 36.MacIntosh DL, Williams PL, Hunter DJ, Sampson LA, Morris SC, Willett WC, et al. Evaluation of a food frequency questionnaire-food composition approach for estimating dietary intake of inorganic arsenic and methylmercury. Cancer Epidemiol Biomarkers Prev. 1997;6:1043–1050. [PubMed] [Google Scholar]
- 37.Meharg A, Hartley-Whitaker J. Arsenic uptake and metabolism in arsenic resistant and nonresistant plant species. New Phytologist. 2002;154:29–43. [Google Scholar]
- 38.Diaz OP, Leyton I, Munoz O, Nunez N, Devesa V, Suner MA, et al. Contribution of water, bread, and vegetables (raw and cooked) to dietary intake of inorganic arsenic in a rural village of Northern Chile. J Agric Food Chem. 2004;52:1773–1779. doi: 10.1021/jf035168t. [DOI] [PubMed] [Google Scholar]
- 39.Lasky T, Sun W, Kadry A, Hoffman MK. Mean total arsenic concentrations in chicken 1989-2000 and estimated exposures for consumers of chicken. Environ Health Perspect. 2004;112:18–21. doi: 10.1289/ehp.6407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu WJ, Zhu YG, Hu Y, Williams PN, Gault AG, Meharg AA, et al. Arsenic sequestration in iron plaque, its accumulation and speciation in mature rice plants (Oryza sativa L.). Environ Sci Technol. 2006;40:5730–5736. doi: 10.1021/es060800v. [DOI] [PubMed] [Google Scholar]
- 41.Meharg A, Lombi E, Williams PN, Scheckel KG, Feldmann J, Raab A, et al. Speciation and localization of arsenic in white and brown rice grains. Environ Sci Technol. 2008;42:1051–1057. doi: 10.1021/es702212p. [DOI] [PubMed] [Google Scholar]
- 42.Signes A, Mitra K, Burlo F, Carbonell-Barrachina AA. Contribution of water and cooked rice to an estimation of the dietary intake of inorganic arsenic in a rural village of West Bengal, India. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2008;25:41–50. doi: 10.1080/02652030701385233. [DOI] [PubMed] [Google Scholar]
- 43.Smith E, Juhasz AL, Weber J, Naidu R. Arsenic uptake and speciation in rice plants grown under greenhouse conditions with arsenic contaminated irrigation water. Sci Total Environ. 2008;392:277–283. doi: 10.1016/j.scitotenv.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 44.Zavala YJ, Gerads R, Gorleyok H, Duxbury JM. Arsenic in rice: II. Arsenic speciation in USA grain and implications for human health. Environ Sci Technol. 2008;42:3861–3866. doi: 10.1021/es702748q. [DOI] [PubMed] [Google Scholar]
- 45.Sun GX, Williams PN, Zhu YG, Deacon C, Carey AM, Raab A, et al. Survey of arsenic and its speciation in rice products such as breakfast cereals, rice crackers and Japanese rice condiments. Environ Int. 2009;35:473–475. doi: 10.1016/j.envint.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 46.Gilbert-Diamond D, Cottingham KL, Gruber JF, Punshon T, Sayarath V, Gandolfi AJ, et al. Rice consumption contributes to arsenic exposure in US women. Proc Natl Acad Sci USA. 2011;108:20656–20660. doi: 10.1073/pnas.1109127108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Velez D, Montoro R. Arsenic speciation in manufactured seafood products. J Food Prot. 1998;61:1240–1245. doi: 10.4315/0362-028x-61.9.1240. [DOI] [PubMed] [Google Scholar]
- 48.Lorenzana R, Yeow A, Colman J, Chappell L, Choudhury H. Arsenic in seafood: speciation issues for human health risk assessment. Hum Ecol Risk Assess. 2009;15:185–200. [Google Scholar]
- 49.Garelick H, Jones H, Dybowska A, Valsami-Jones E. Arsenic pollution sources. Rev Environ Contam Toxicol. 2008;197:17–60. doi: 10.1007/978-0-387-79284-2_2. [DOI] [PubMed] [Google Scholar]
- 50.Moore MM, Harrington-Brock K, Doerr CL. Relative genotoxic potency of arsenic and its methylated metabolites. Mutat Res. 1997;386:279–290. doi: 10.1016/s1383-5742(97)00003-3. [DOI] [PubMed] [Google Scholar]
- 51.Styblo M, Del Razo LM, Vega L, Germolec DR, LeCluyse EL, Hamilton GA, et al. Comparative toxicity of trivalent and pentavalent inorganic and methylated arsenicals in rat and human cells. Arch Toxicol. 2000;74:289–299. doi: 10.1007/s002040000134. [DOI] [PubMed] [Google Scholar]
- 52.Hughes MF. Biomarkers of exposure: a case study with inorganic arsenic. Environ Health Perspect. 2006;114:1790–1796. doi: 10.1289/ehp.9058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smith AH, Steinmaus CM. Health effects of arsenic and chromium in drinking water: recent human findings. Annu Rev Public Health. 2009;30:107–122. doi: 10.1146/annurev.publhealth.031308.100143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Williams PN, Price AH, Raab A, Hossain SA, Feldmann J, Meharg AA. Variation in arsenic speciation and concentration in paddy rice related to dietary exposure. Environ Sci Technol. 2005;39:5531–5540. doi: 10.1021/es0502324. [DOI] [PubMed] [Google Scholar]
- 55.Williams PN, Raab A, Feldmann J, Meharg AA. Market basket survey shows elevated levels of As in South Central U.S. processed rice compared to California: consequences for human dietary exposure. Environ Sci Technol. 2007;41:2178–2183. doi: 10.1021/es061489k. [DOI] [PubMed] [Google Scholar]
- 56.Thompson FE, Subar AF. Dietary assessment methodology. In: Coulston AM, Boushey CJ, editors. Nutrition in the Prevention and Treatment of Disease. 2nd edn. Elsevier Academic Press; Amsterdam, the Netherlands: 2008. pp. 3–39. [Google Scholar]
- 57.Kurzius-Spencer M, O'Rourke MK, Hsu CH, Hartz V, Harris RB, Burgess JL. Measured versus modeled dietary arsenic and relation to urinary arsenic excretion and total exposure. J Expo Sci Environ Epidemiol. 2013;23:442–449. doi: 10.1038/jes.2012.120. [DOI] [PMC free article] [PubMed] [Google Scholar]