Abstract
Objective
The adipocyte fatty acid-binding protein, aP2, has important effects on insulin resistance, lipid metabolism, and atherosclerosis. Its expression in macrophages enhances early foam cell formation and atherosclerosis in vivo. This study was designed to determine whether aP2 deficiency has a similar effect in the setting of advanced atherosclerosis and severe hypercholesterolemia.
Methods and Results
Mice deficient in aP2 and apolipoprotein E (aP2−/−apoE−/− mice) and apolipoprotein E-deficient control mice (apoE−/− mice) were fed a Western diet for 14 weeks. No significant differences in fasting serum levels of cholesterol, triglycerides, or free fatty acids were found between groups for each sex. Compared with apoE−/− control mice, male and female aP2−/−apoE−/− mice had significant reductions in mean atherosclerotic lesion size in the proximal aorta, en face aorta, and innominate/right carotid artery. Feeding the Western diet in the apoE-deficient background did not cause a significant reduction in insulin sensitivity in vivo, as determined by steady-state serum glucose levels and insulin tolerance testing.
Conclusions
These data demonstrate an important role for aP2 expression in the advanced stages of atherosclerotic lesion formation. Thus, aP2 provides an important physiological link between different features of the metabolic syndrome and is a potential target for therapy of atherosclerosis.
Keywords: fatty acid–binding protein, aP2, atherosclerosis, mice, insulin resistance
Cytoplasmic fatty acid–binding proteins (FABPs) consist of a family of 14- to 15-kDa proteins capable of binding hydrophobic ligands with high affinity.1 The adipocyte FABP, aP2 (also known as adipocyte lipid–binding protein or FABP4), is a member of the FABP family. Putative functions of FABPs include shuttling of fatty acid ligands in the cytoplasm to various intracellular compartments, modulation of intracellular lipid metabolism, and regulation of gene expression.2 The expression of aP2 is regulated during development and by fatty acids, peroxisome proliferator–activated receptor-γ agonists, and insulin.3–5 Deficiency of aP2 protects against the development of hyperinsulinemia and insulin resistance in genetic and diet-induced obesity in mice.6,7 Thus, it represents an important link in the pathogenesis of diabetes mellitus and obesity.
Interestingly, aP2 is also expressed in macrophages, and its expression is modulated by peroxisome proliferator–activated receptor-γ agonists and oxidized LDLs.8–10 We have recently shown that aP2 deficiency protects apoE-deficient (apoE−/−) mice fed a low-fat diet from atherosclerosis. Furthermore, macrophage-specific aP2 deficiency protects against atherosclerosis in apoE−/− mice, independent of any effects on lipid or glucose metabolism.8 Macrophage aP2 deficiency also has major effects on inflammatory cytokine production and cholesterol loading in macrophages.8
ApoE−/− mice develop severe hypercholesterolemia and spontaneous atherosclerosis, but not obesity or insulin resistance, on a normal chow diet.11,12 In our previous study, aP2 deficiency greatly reduced early foam cell formation, but it was unclear whether this effect would be persistent in severe hypercholesterolemia and advanced atherosclerosis. Feeding apoE−/− mice a high-fat Western-type diet accelerates atherosclerosis.13 In C57BL/6 mice, a high-fat diet also promotes the development of features of type 2 diabetes mellitus, including obesity, hyperglycemia, and hyperinsulinemia.14 However, it is not clear whether a high-fat diet induces significant hyperglycemia and insulin resistance in apoE−/− mice. In the present study, we sought to determine whether aP2 could modulate insulin resistance and/or atherogenesis in apoE−/− mice on a high-fat diet. We show that male and female mice deficient in aP2 and apoE (aP2−/−apoE−/− mice) and mice deficient only in apoE (aP2+/+apoE−/− control mice) fed a Western diet for 14 weeks do not develop significant obesity or insulin resistance, as assessed by insulin tolerance testing, and have no significant differences in serum cholesterol or triglycerides. However, aP2 deficiency clearly reduces atherosclerosis in male and female mice in this model of severe hypercholesterolemia.
Methods
Animal Procedures
The aP2−/−apoE−/− and aP2+/+apoE−/− mice on a C57BL/6 background used for this experiment were generated as previously described.8 The mice were kept on a 12-hour light cycle and were fed a high-fat Western diet (21% fat, 0.15% cholesterol, no cholate; diet No. TD88137, Harland Teklad) ad libitum beginning at 4 weeks of age for 14 weeks. Animal care and experimental procedures were performed under approval from the Animal Care Committees of Vanderbilt and Harvard Universities.
Serum Measurements and Insulin Tolerance Testing
Mice were fasted overnight (12 hours), and blood samples were collected by retro-orbital venous plexus puncture with the animals under isoflurane (IsoVet, Schering-Plough) anesthesia. Serum was separated by centrifugation, and 1 mmol/L phenylmethylsulfonyl fluoride was added (Sigma Chemical Co). Serum total cholesterol and triglyceride levels were determined by using a Raichem cholesterol reagent kit and a Sigma GPO-Trinder triglyceride reagent kit adapted for microtiter plate assay, as previously described.15 Fasting serum glucose was determined by a commercially available colorimetric assay (Schiapparelli Biosystems). Plasma glycerol and free fatty acids were measured by using commercially available colorimetric assays (Sigma and Wako Pure Chemical Industries). Lipoprotein analysis by fast protein liquid chromatography was performed on fasting serum samples, as previously described.16 Insulin tolerance tests (0.5 IU/kg body wt) were performed on conscious mice after a 6-hour fast, as previously described.17 Blood glucose concentrations during insulin tolerance testing were determined with whole blood by using Gluco-analyzer blood glucose strips (Medisense).
Quantification of Arterial Lesions, Histology, and Immunocytochemistry
Mice were anesthetized with isoflurane (IsoVet, Schering-Plough), euthanized by cervical dislocation, and bled via cardiac puncture. Perfusion-fixation and preparation of the aortas were performed as described previously.18 For arterial lesion quantification in the innominate/right carotid and en face aorta, aortas were pinned out as previously described in an en face preparation adapted from the method of Paigen et al.19,20 Images of Sudan-IV–stained aortas and innominate/right carotid arteries en face were captured with a Sony DXC-960 MD color video camera, and quantitative image analysis was performed by the use of Imaging System KS 300 (Release 2.0, Kontron Electronik GmbH). Thick (10-μm) cryosections of the proximal aorta were stained with oil red O and counterstained with hematoxylin, as described.16,21 For qualitative assessment of atherosclerotic lesion morphology, representative 5-μm serial cryosections from each group were stained with modified Masson's trichrome and modified Russell-Movat pentachrome methods. For detection of macrophages and aP2 protein in arterial lesions, 5-μm serial cryosections of the proximal aorta were incubated with either a polyclonal rabbit antiserum against mouse aP2 (gift from D. Bernlohr, University of Minnesota, Minneapolis) or rat antibody against mouse macrophage marker (MOMA-2, Accurate), as previously described.16,22 Photomicroscopy was performed on a Zeiss Axioskop microscope with Plan-Fluar objectives.
Statistical Analysis
Initial analyses were performed with the Student t test. A value of P<0.05 was considered significant. Data that did not fit the constraints of this parametric test were analyzed by the Mann-Whitney test where indicated in the text, with P<0.06 considered significant.
Results
Four-week-old male aP2−/−apoE−/− mice (n=8, experimental group) and aP2+/+apoE−/− mice (n=13, control group) were fed a Western diet for 14 weeks. Similarly, 4-week-old female aP2−/−apoE−/− mice (n=19, experimental group) and aP2+/+apoE−/− mice (n=14, control group) were fed a Western diet for 14 weeks. After 14 weeks on the Western diet, fasting total serum cholesterol and triglyceride levels were markedly elevated in the aP2−/− and aP2+/+ groups, without significant differences between experimental and control groups for each sex (Table). Likewise, distribution of cholesterol in the serum lipoproteins showed a large peak in the VLDL fractions and a reduced HDL peak that was due to apoE deficiency, without significant differences between the aP2−/−apoE−/− and aP2+/+apoE−/− groups (Figure 1). After 14 weeks on the Western diet, serum glucose levels after an overnight (12-hour) fast were within the normoglycemic range in all mouse groups.23,24 Glucose levels were modestly higher in females than in males, but there was no significant difference between the aP2−/−apoE−/− and aP2+/+apoE−/− groups (173±63 and 170±37 mg/dL [mean±SD], respectively; P=0.72). Mean serum glucose values in the male aP2−/−apoE−/− and aP2+/+apoE−/− groups were similar (118±90 and 132±48 mg/dL [mean±SD], respectively; P=0.66).
Table 1. Body Weight, Serum Lipids, FFAs, and Glucose for aP2+/+apoE−/− and aP2−/− apoE −/− Mice on Western Diet.
| Group (Sex) | Body Weight, g | Glycerol, mM | FFAs, mM | Cholesterol, mg/dL | Triglycerides, mg/dL | Glucose, mg/dL |
|---|---|---|---|---|---|---|
| aP2+/+apoE−/− (male) | 31.8±4.7 | 1.30±0.40 | 1.96±0.46 | 865±175 | 218±99 | 132±48 |
| aP2−/−apoE−/− (male) | 35.6±4.0* | 1.25±0.43 | 1.96±0.52 | 857±103 | 183±58 | 118±90 |
| aP2+/+apoE−/− (female) | 24.7±1.6 | 0.81±0.28 | 2.14±0.45 | 636±93 | 177±76 | 170±37 |
| aP2−/−apoE−/− (female) | 26.8±3.7* | 0.77±0.19 | 2.10±0.29 | 632±54 | 140±57 | 173±63 |
Total serum cholesterol, triglycerides, free fatty acids (FFAs), glycerol, and glucose were drawn after overnight (12 hours) fast. Values are mean±SD.
P<0.05 compared with controls. For all other values P was not significant between experimental and control groups for each sex.
Figure 1.

Lipoprotein distribution in male and female aP2−/−apoE−/− and aP2+/+apoE−/− mice. Data are an average (n=4 for each group) percent distribution of total cholesterol for each group. Fractions 14 to 17 contain VLDL; fractions 18 to 24, IDL/LDL; and fractions 25 to 29, HDL. Fractions 30 to 40 are non-lipoprotein-associated proteins.
To further evaluate the effect of the Western diet on insulin resistance, we performed insulin tolerance tests on 16-week-old male and female mice from each group. Fasting insulin sensitivity did not differ between the male aP2−/−apoE−/− (n=8) and aP2+/+apoE−/− (n=13) groups (Figure 2A). Likewise, there was no significant difference in insulin sensitivity between the female aP2−/−apoE−/− (n=13) and aP2+/+ apoE−/− (n=5) groups (Figure 2B). Mean body weight was slightly greater in the male aP2−/−apoE−/− mice than in the male aP2+/+apoE−/− mice (35.6±4.0 versus 31.8±4.7 g [mean±SD], respectively; P=0.03). A similar pattern was also seen in the body weight of female aP2−/−apoE−/− mice compared with female control mice (26.8±3.7 versus 24.7±1.6 g [mean±SD], respectively; P=0.01). Plasma free fatty acid levels were nearly identical between the male aP2−/−apoE−/− (n=8) and aP2+/+apoE−/− (n=13) groups (1.96±0.52 and 1.96±0.46 mmol/L [mean±SD], respectively; P=0.98) and between the female aP2−/−apoE−/− (n=13) and aP2+/+ apoE−/− (n=5) groups (2.10±0.29 and 2.14±0.45 mmol/L [mean±SD], respectively; P=0.68). Likewise, plasma glycerol levels were similar between male aP2−/−apoE−/− and aP2+/+apoE−/− mice (1.25±0.43 and 1.30±0.40 mmol/L [mean±SD], respectively; P=0.75) as well as between female aP2−/−apoE−/− and aP2+/+apoE−/− mice (0.77±0.19 and 0.81 ±0.28, mmol/L [mean±SD], respectively; P=0.98).
Figure 2.

Insulin tolerance tests in male (A) and female (B) aP2−/−apoE−/− and aP2+/+apoE−/− mice. Insulin tolerance tests were performed with 0.5 U/kg human insulin injected intraperitoneally per animal. Data are mean±SEM. P=NS between groups for all time points in both panels.
We next analyzed the extent of atherosclerosis in all groups at 18 weeks of age. After 14 weeks on the Western diet, there was a significant 29% reduction in mean lesion area of the proximal aorta of the male aP2−/−apoE−/− (n=8) compared with the male aP2+/+apoE−/− (n=13) mice (248 425 ±19 269 versus 350 258±19 375 μm2 per section [mean±SEM], respectively; P=0.002; Figure 3A). Similarly, there was a significant (17%) reduction in mean lesion area of female aP2−/−apoE−/− (n=19) compared with aP2+/+apoE−/− control (n=14) mice (370 136±13 666 versus 447 566±24 411 μm2 per section [mean±SEM], respectively; P=0.006; Figure 3A).
Figure 3.

Quantification of atherosclerotic lesion area in the proximal aorta (A), en face aorta (B), and innominate/right carotid artery (C) in male and female aP2−/−apoE−/− and aP2+/+apoE−/− mice. Data are average mean lesion area for each group, with error bars indicating SEM. Probability values are as indicated. Student t test was used for data in panels A and B, and the Mann-Whitney test was used for data in panel C.
Analysis of the en face aorta demonstrated even more dramatic reductions in the extent of atherosclerosis between the experimental and control groups (Figure 3B). There was a significant (48%) reduction in mean lesion area of the en face aorta of the male aP2−/−apoE−/− mice compared with the male aP2+/+apoE−/− mice (0.88±0.29% versus 1.69±0.23% [mean±SEM], respectively; P=0.04), and there was a 39% reduction in the female aP2−/−apoE−/− mice compared with control mice (1.19±0.13% versus 1.97±0.37% [mean±SEM], respectively; P=0.03). A subset of mice from the male and female control and experimental groups was also analyzed for the extent of atherosclerosis in the innominate and proximal right carotid arteries. Innominate/right carotid artery atherosclerosis by en face lesion analysis showed a mean 78% reduction in male aP2−/−apoE−/− (n=5) compared with male aP2+/+apoE−/− (n=7) mice (0.55±0.16% versus 2.47±0.48% [mean±SEM], respectively; P<0.06 by Mann-Whitney test; Figure 3C). There was a mean 91% reduction in innominate/right carotid artery atherosclerosis in female aP2−/−apoE−/− (n=5) compared with control (n=8) mice (0.08±0.07% versus 0.83±0.30% [mean±SEM], respectively; P<0.06 by Mann-Whitney test).
Immunocytochemistry of the proximal aortic lesions revealed complicated lesion morphology for the male and female aP2−/−apoE−/− and aP2+/+apoE−/− groups. All groups had macrophages located predominantly on the luminal surfaces of the lesions, as shown by staining with anti-macrophage antibody, MOMA-2 (Figure 4). For the male and female groups, staining for aP2 colocalized to areas of macrophage accumulation in the aP2+/+apoE−/− sections but was absent in the aP2−/−apoE−/− sections (data not shown). Histological staining with Masson's trichrome and Movat pentachrome methods showed substantial collagen and ground substance accumulation within the lesions of the male and female aP2−/−apoE−/− and aP2+/+apoE−/− groups, without significant qualitative differences in lesion morphology between groups for each sex. Sections from each group stained with Masson's trichrome are shown in Figure 5. As expected, the lesions in both of the female groups were generally larger and more advanced than those in the males.
Figure 4.
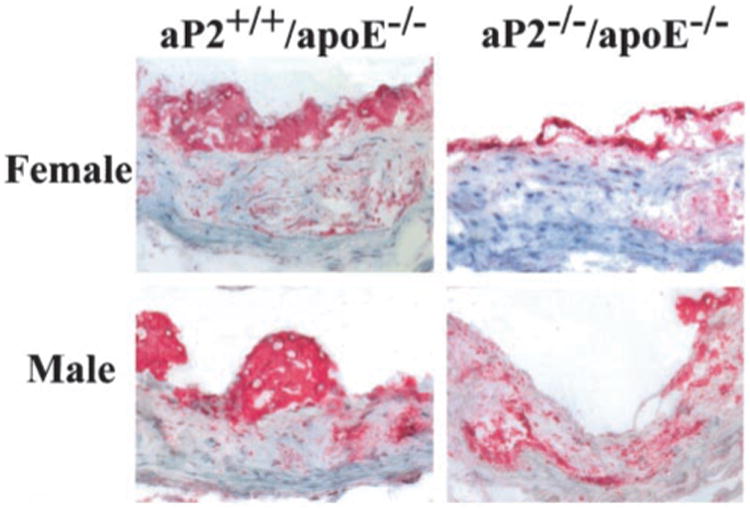
Immunocytochemical detection of macrophages in the proximal aorta of male and female aP2−/−apoE−/− and aP2+/+apoE−/− mice. Macrophages are stained with antibody to mouse macrophage clone, MOMA-2.
Figure 5.
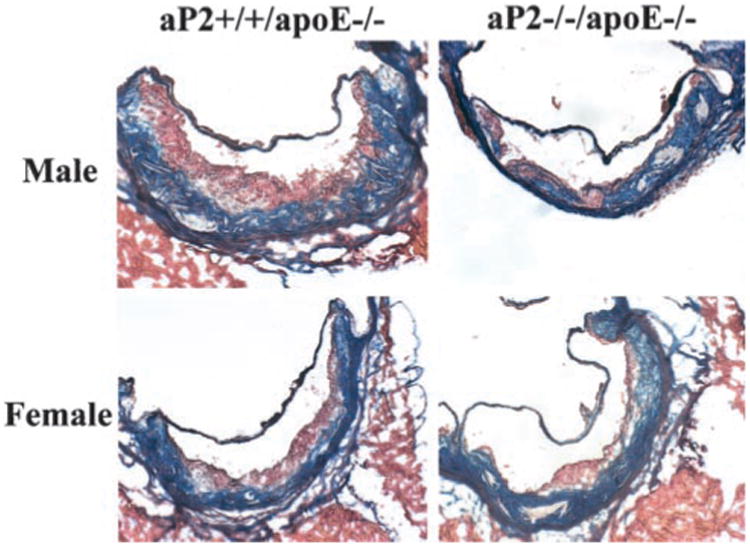
Histological appearance of atherosclerotic lesions in the proximal aorta. Each micrograph is a representative section from each group. Frozen 5-μm sections were fixed and stained with Masson's trichrome and captured with an Olympus PRO-VIS AX70 microscope and digital camera at ×200 magnification.
Discussion
In the present study, we showed that male and female aP2−/−apoE−/− mice had a significant reduction in mean atherosclerotic lesion area in the proximal aorta, en face aorta, and innominate/right carotid artery compared with age-and sex-matched aP2+/+apoE−/− control mice after 14 weeks on the Western diet, despite a similar degree of severe hypercholesterolemia due to the Western diet and apoE deficiency.
Metabolic studies did not show any significant differences between groups among male and female aP2−/−apoE−/− and aP2+/+apoE−/− mice. Male and female groups displayed similar reductions in serum glucose by insulin tolerance testing, without significant insulin resistance. The level of insulin sensitivity by insulin tolerance testing was very similar to that of apoE−/− mice on a low-fat chow diet and considerably higher than that associated with other established mouse models of insulin resistance.8,17,25 Previous studies have demonstrated that a high-fat or Western diet can induce insulin resistance and hyperglycemia in C57BL/6 mice and LDL receptor–deficient mice.14,26,27 However, the effects of a Western diet on the development of insulin resistance in apoE−/− mice have not been well studied. Interestingly, we did not observe alterations in insulin sensitivity in apoE−/− mice on a Western diet. The lack of significant insulin resistance and hyperglycemia may be explained by the fact that these animals did not develop significant obesity. The effect of aP2 deficiency on insulin resistance and hyperglycemia becomes apparent only in mice with significant obesity (body weight >40 g) and is not observed in lean mice.6,7 Thus, there is a threshold of obesity and insulin resistance that must be achieved to observe the protective effects of aP2 deficiency on glucose and lipid metabolism. Consistent with this, no significant metabolic differences were observed between aP2−/−apoE−/− and aP2+/+ apoE−/− mice in the present study.
The male and female aP2−/−apoE−/− mice gained more weight on the Western diet than did the aP2+/+apoE−/− control mice. The observation of higher body weight in aP2−/− mice compared with control mice has been previously described in aP2−/− mouse models on a high-fat diet and aP2−/− mouse models with genetic obesity.6,7 The weight difference between experimental and control groups was statistically significant, with a 12% higher mean body weight in male aP2−/−apoE−/− mice and an 8.5% higher mean body weight in female aP2−/−apoE−/− mice compared with control mice. Despite the higher mean body weight, the male and female aP2−/−apoE−/− mice showed no significant biochemical differences compared with control mice and yet had significantly reduced atherosclerosis.
Our previous study demonstrated that aP2 deficiency was protective against atherosclerosis in apoE−/− mice on a chow diet, independent of its effects on glucose and lipid metabolism.8 However, in that study there appeared to be an attenuation of the protective effect in female mice in the proximal aorta, where the lesions were the most advanced. Therefore, we wanted to determine whether the protective effect of aP2 deficiency would persist in the setting of advanced lesion formation. With the hypercholesterolemic effect of the Western diet, the size and complexity of the resulting lesions were markedly greater than those in our previous study, yet the protection of aP2 deficiency remained apparent in all 3 sites studied: the proximal aorta, innominate/right carotid artery, and en face aorta. This pattern was seen in male and female mice, adding further evidence that the effect of aP2 on atherogenesis is not sex specific. It has been recently reported that after 12 weeks on the Western diet, male aP2−/−apoE−/− mice had a 90% reduction in atherosclerosis in a cross-sectional analysis of the proximal innominate artery and a 99% reduction in the distal innominate artery.28 However, in that study, atherosclerotic burden in other regions of the arterial system was not quantitatively evaluated, no female mice were studied, and no metabolic analyses were performed. The present study demonstrated a similar degree of reduction in atherosclerosis in the en face analysis of the innominate/right carotid artery of 78% and 91%, respectively, in male and female aP2−/−apoE−/− mice compared with control mice.
Our previous work with bone marrow transplantation studies in apoE−/− mice has shown that macrophage aP2 expression plays a central role in modulating atherosclerosis in apoE−/− mice.8 The significant reductions in atherosclerosis in the proximal aorta are of particular interest, inasmuch as the beneficial effect of aP2 deficiency was persistent, despite a high lesion burden and morphologically complex lesions. This suggests that the role of aP2 in the macrophage may not be confined to the early stages of atherogenesis but may also involve macrophage effects on other cells within atherosclerotic lesions, thereby modulating later events in atherosclerotic lesion formation. We have shown previously that aP2-deficient macrophages exhibit markedly reduced production of inflammatory cytokines and chemokine tumor necrosis factor-α, monocyte chemoattractant protein-1 (MCP-1), interleukin-1β, and interleukin-6.8 This reduction in cytokine and chemokine production may potentially modulate factors such as macrophage recruitment and infiltration into the artery wall. Consistent with this, a deficiency of chemokine MCP-1 has been shown to reduce atherosclerosis in LDL receptor-deficient mice.29 Conversely, apoE−/− mice transplanted with bone marrow overexpressing a murine MCP-1 transgene have exhibited accelerated atherosclerosis.30 Although we cannot exclude the possibility that the effects of aP2 deficiency on advanced lesion formation observed in the present study are principally due to an early effect on foam cell formation, other molecular mechanisms that promote early foam cell formation, such as macrophage lipoprotein lipase expression, do not persist in the later stages of atherosclerosis.18 This issue certainly merits further study.
In summary, aP2 deficiency is protective in the setting of advanced atherosclerosis induced by a Western diet in apoE−/− mice and does not result in significant changes in insulin resistance or lipid metabolism in this model. Clearly, aP2 plays a role in atherogenesis in its early and late stages, making it a promising therapeutic target in the prevention and treatment of atherosclerosis and obesity-induced insulin resistance. However, it will be important in future studies to use different mouse models to determine whether the differential effects of aP2 on insulin resistance in adipocytes could also have a significant impact on atherosclerosis.
Acknowledgments
This work was supported by National Institutes of Health grants HL-65405-01 and DK-59637-01 (Lipid, Lipoprotein, and Atherosclerosis Core of the Vanderbilt Mouse Metabolic Physiology Center). M.F.L. is an established investigator of the American Heart Association. V.R.B is supported by American Heart Association grant SE 0160160B. J.B.B. is supported by a Diabetes Training Grant from the National Institute of Diabetes and Digestive and Kidney Diseases (T32 DK7061). K.M. is supported by a postdoctoral fellowship from Manpei Suzuki Diabetes Foundation. G.S.H. is a Pew Scholar in Biomedical Sciences. The authors would like to thank Youmin Zhang and TianLi Zhu for their technical expertise in preparing murine vascular tissues for analysis.
References
- 1.Storch J, Thumser AE. The fatty acid transport function of fatty acid-binding proteins. Biochim Biophys Acta. 2000;1486:28–44. doi: 10.1016/s1388-1981(00)00046-9. [DOI] [PubMed] [Google Scholar]
- 2.Coe NR, Bernlohr DA. Physiological properties and functions of intracellular fatty acid-binding proteins. Biochim Biophys Acta. 1998;1391:287–306. doi: 10.1016/s0005-2760(97)00205-1. [DOI] [PubMed] [Google Scholar]
- 3.Distel RJ, Robinson GS, Spiegelman BM. Fatty acid regulation of gene expression: transcriptional and post-transcriptional mechanisms. J Biol Chem. 1992;267:5937–5941. [PubMed] [Google Scholar]
- 4.Chaput E, Saladin R, Silvestre M, Edgar AD. Fenofibrate and rosiglitazone lower serum triglycerides with opposing effects on body weight. Biochem Biophys Res Commun. 2000;271:445–450. doi: 10.1006/bbrc.2000.2647. [DOI] [PubMed] [Google Scholar]
- 5.Melki SA, Abumrad NA. Expression of the adipocyte fatty acid-binding protein in streptozotocin-diabetes: effects of insulin deficiency and supplementation. J Lipid Res. 1993;34:1527–1534. [PubMed] [Google Scholar]
- 6.Hotamisligil GS, Johnson RS, Distel RJ, Ellis R, Papaioannou VE, Spiegelman BM. Uncoupling of obesity from insulin resistance through a targeted mutation in aP2, the adipocyte fatty acid binding protein. Science. 1996;274:1377–1379. doi: 10.1126/science.274.5291.1377. [DOI] [PubMed] [Google Scholar]
- 7.Uysal KT, Scheja L, Wiesbrock SM, Bonner-Weir S, Hotamisligil GS. Improved glucose and lipid metabolism in genetically obese mice lacking aP2. Endocrinology. 2000;141:3388–3396. doi: 10.1210/endo.141.9.7637. [DOI] [PubMed] [Google Scholar]
- 8.Makowski L, Boord JB, Maeda K, Babaev VR, Uysal KT, Morgan MA, Parker RA, Suttles J, Fazio S, Hotamisligil GS, Linton MF. Lack of macrophage fatty-acid-binding protein aP2 protects mice deficient in apolipoprotein E against atherosclerosis. Nat Med. 2001;7:699–705. doi: 10.1038/89076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pelton PD, Zhou L, Demarest KT, Burris TP. PPARγ activation induces the expression of the adipocyte fatty acid binding protein gene in human monocytes. Biochem Biophys Res Commun. 1999;261:456–458. doi: 10.1006/bbrc.1999.1071. [DOI] [PubMed] [Google Scholar]
- 10.Fu Y, Luo N, Lopes-Virella MF. Oxidized LDL induces the expression of ALBP/aP2 mRNA and protein in human THP-1 macrophages. J Lipid Res. 2000;41:2017–2023. [PubMed] [Google Scholar]
- 11.Zhang SH, Reddick RL, Piedrahita JA, Maeda N. Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein E. Science. 1992;258:468–471. doi: 10.1126/science.1411543. [DOI] [PubMed] [Google Scholar]
- 12.Plump AS, Smith JD, Hayek T, Aalto-Setälä K, Walsh A, Verstuyft JG, Rubin EM, Breslow JL. Severe hypercholesterolemia and atherosclerosis in apolipoprotein E-deficient mice created by homologous recombination in ES cells. Cell. 1992;71:343–353. doi: 10.1016/0092-8674(92)90362-g. [DOI] [PubMed] [Google Scholar]
- 13.Nakashima Y, Plump AS, Raines EW, Breslow JL, Ross R. ApoE-deficient mice develop lesions of all phases of atherosclerosis throughout the arterial tree. Arterioscler Thromb. 1994;14:133–140. doi: 10.1161/01.atv.14.1.133. [DOI] [PubMed] [Google Scholar]
- 14.Surwit RS, Kuhn CM, Cochrane C, McCubbin JA, Feinglos MN. Diet-induced type II diabetes in C57BL/6J mice. Diabetes. 1988;37:1163–1167. doi: 10.2337/diab.37.9.1163. [DOI] [PubMed] [Google Scholar]
- 15.Fazio S, Hasty AH, Carter KJ, Murray AB, Price JO, Linton MF. Leukocyte low density lipoprotein receptor (LDL-R) does not contribute to LDL clearance in vivo: bone marrow transplantation studies in the mouse. J Lipid Res. 1997;38:391–400. [PubMed] [Google Scholar]
- 16.Babaev VR, Fazio S, Gleaves LA, Carter KJ, Semenkovich CF, Linton MF. Macrophage lipoprotein lipase promotes foam cell formation and atherosclerosis in vivo. J Clin Invest. 1999;103:1697–1705. doi: 10.1172/JCI6117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS. Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. Nature. 1997;389:610–614. doi: 10.1038/39335. [DOI] [PubMed] [Google Scholar]
- 18.Babaev VR, Patel MB, Semenkovich CF, Fazio S, Linton MF. Macrophage lipoprotein lipase promotes foam cell formation and atherosclerosis in low density lipoprotein receptor-deficient mice. J Biol Chem. 2000;275:26293–26299. doi: 10.1074/jbc.M002423200. [DOI] [PubMed] [Google Scholar]
- 19.Paigen B, Morrow A, Holmes PA, Mitchell D, Williams RA. Quantitative assessment of atherosclerotic lesions in mice. Atherosclerosis. 1987;68:231–240. doi: 10.1016/0021-9150(87)90202-4. [DOI] [PubMed] [Google Scholar]
- 20.Cyrus T, Witztum JL, Rader DJ, Tangirala R, Fazio S, Linton MF, Funk CD. Disruption of the 12/15-lipoxygenase gene diminishes atherosclerosis in apo E-deficient mice. J Clin Invest. 1999;103:1597–1604. doi: 10.1172/JCI5897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fazio S, Babaev VR, Murray AB, Hasty AH, Carter KJ, Gleaves LA, Atkinson JB, Linton MF. Increased atherosclerosis in C57BL/6 mice reconstituted with apolipoprotein E null macrophages. Proc Natl Acad Sci U S A. 1997;94:4647–4652. doi: 10.1073/pnas.94.9.4647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kraal G, Rep M, Janse M. Macrophages in T and B cell compartments and other tissue macrophages recognized by monoclonal antibody MOMA-2. Scand J Immunol. 1987;26:653–661. doi: 10.1111/j.1365-3083.1987.tb02301.x. [DOI] [PubMed] [Google Scholar]
- 23.Everett R, Harrison S. Clinical biochemistry. In: Foster H, Small J, Fox J, editors. The Mouse in Biomedical Research. Vol. 3. Boston, Mass: Academic Press; 1983. pp. 313–326. [Google Scholar]
- 24.Challet E, van Reeth O, Turek FW. Altered circadian responses to light in streptozotocin-induced diabetic mice. Am J Physiol. 1999;277:E232–E237. doi: 10.1152/ajpendo.1999.277.2.E232. [DOI] [PubMed] [Google Scholar]
- 25.Kubota N, Terauchi Y, Miki H, Tamemoto H, Yamauchi T, Komeda K, Satoh S, Nakano R, Ishii C, Sugiyama T, Eto K, Tsubamoto Y, Okuno A, Murakami K, Sekihara H, Hasegawa G, Naito M, Toyoshima Y, Tanaka S, Shiota K, Kitamura T, Fujita T, Ezaki O, Aizawa S, Nagai R, Tobe K, Kimura S, Kadowaki T. PPAR gamma mediates high-fat diet-induced adipocyte hypertrophy and insulin resistance. Mol Cell. 1999;4:597–609. doi: 10.1016/s1097-2765(00)80210-5. [DOI] [PubMed] [Google Scholar]
- 26.Surwit RS, Feinglos MN, Rodin J, Sutherland A, Petro AE, Opara EC, Kuhn CM, Rebuffe-Scrive M. Differential effects of fat and sucrose on the development of obesity and diabetes in C57BL/6J and A/J mice. Metabolism. 1995;44:645–651. doi: 10.1016/0026-0495(95)90123-x. [DOI] [PubMed] [Google Scholar]
- 27.Merat S, Casanada F, Sutphin M, Palinski W, Reaven PD. Western-type diets induce insulin resistance and hyperinsulinemia in LDL receptor– deficient mice but do not increase aortic atherosclerosis compared with normoinsulinemic mice in which similar plasma cholesterol levels are achieved by a fructose-rich diet. Arterioscler Thromb Vasc Biol. 1999;19:1223–1230. doi: 10.1161/01.atv.19.5.1223. [DOI] [PubMed] [Google Scholar]
- 28.Perrella MA, Pellacani A, Layne MD, Patel A, Zhao D, Schreiber BM, Storch J, Feinberg MW, Hsieh CM, Haber E, Lee ME. Absence of adipocyte fatty acid binding protein prevents the development of accelerated atherosclerosis in hypercholesterolemic mice. FASEB J. 2001;15:1774–1776. doi: 10.1096/fj.01-0017fje. [DOI] [PubMed] [Google Scholar]
- 29.Gu L, Okada Y, Clinton SK, Gerard C, Sukhova GK, Libby P, Rollins BJ. Absence of monocyte chemoattractant protein-1 reduces atherosclerosis in low density lipoprotein receptor-deficient mice. Mol Cell. 1998;2:275–281. doi: 10.1016/s1097-2765(00)80139-2. [DOI] [PubMed] [Google Scholar]
- 30.Aiello RJ, Bourassa PA, Lindsey S, Weng W, Natoli E, Rollins BJ, Milos PM. Monocyte chemoattractant protein-1 accelerates atherosclerosis in apolipoprotein E–deficient mice. Arterioscler Thromb Vasc Biol. 1999;19:1518–1525. doi: 10.1161/01.atv.19.6.1518. [DOI] [PubMed] [Google Scholar]


