Abstract
Objective
Aortic sources of peripheral and visceral embolization remain challenging to treat. The safety of stent graft coverage continues to be debated. This study reports the outcomes of stent coverage of these complex lesions.
Methods
Hospital records were retrospectively reviewed for patients undergoing aortic stenting between 2006 and 2013 for visceral and peripheral embolic disease. Renal function, method of coverage, and mortality after stent grafting were reviewed.
Results
Twenty-five cases of embolizing aortic lesions treated with an endovascular approach were identified. The mean age was 65 ± 13 years (range, 45–87 years), and 64% were female. Sixteen (64%) patients presented with peripheral embolic events, six with concomitant renal embolization. Five patients presented with abdominal or flank pain, and two were discovered incidentally. Three patients had undergone an endovascular procedure for other indications within the preceding 6 months of presentation. Nineteen patients had existing chronic kidney disease (stage II or higher), but only three had stage IV disease. Of the eight patients tested, four had a diagnosed hypercoagulable state. Eight of the patients had lesions identified in multiple aortic segments, and aortic aneurysm disease was present in 24%. Coverage of both abdominal and thoracic sources occurred in eight patients, whereas 17 had only one segment covered. Minimal intraluminal catheter and wire manipulation was paired with the use of intravascular ultrasound in an effort to reduce embolization and contrast use. Intravascular ultrasound was used in the majority of cases and transesophageal echo in 28% of patients. Two patients with stage IV kidney disease became dialysis-dependent within 3 months of the procedure. No other patients had an increase in their postoperative or predischarge serum creatinine levels. No embolic events were precipitated during the procedure, nor were there any recurrent embolic events detected on follow-up. The 1-year mortality rate was 25%.
Conclusions
Endovascular coverage of atheroembolic sources in the aorta is feasible and is safe and effective in properly selected patients. It does not appear to worsen renal function when performed with the use of specific technical strategies.
Embolizing thoracoabdominal aortic lesions are a significant source of morbidity and mortality and have long presented vascular surgeons with a treatment dilemma.1–4 It is estimated that 20% of embolic events are secondary to noncardiac arterial embolization, with the most common source being the aorta.1 Half of these lesions are related to aneurysmal disease; the other half, historically termed cryptogenic, has frequently been localized to the aortic wall. Diagnosis has been enhanced by imaging techniques such as computed tomographic (CT) angiography and transesophageal echocardiography (TEE) in addition to intravascular ultrasonography. The 2-year mortality and recurrence rates for patients with noncardiac arterial embolization to the viscera and lower extremities in a report by Kvilekval et al1 was 17% and 15%, respectively. Subjects with supradiaphragmatic sources were disproportionately affected, with mortality and recurrence rates of 60% each.
Given the unfavorable natural history of such lesions, endovascular therapies have been used to treat different aortic sources of embolization. However, their use has been tempered by the perceived risk of iatrogenic embolization, contrast nephropathy, and overall comorbidities of the patient population leading to clinical deterioration. We report a series of 25 patients treated with stent grafting of embolizing aortic lesions and propose a treatment algorithm for this challenging pathology.
METHODS
This study was approved by the Institutional Review Board of the University of Pittsburgh.
Patient population
Consecutive individuals who underwent endovascular aortic stenting for visceral and/or peripheral embolic disease between 2006 and 2013 were identified in a prospectively maintained institutional database. Baseline clinical and demographic data including presenting symptoms, preoperative glomerular filtration rate, preoperative imaging studies, and any endovascular procedures within 6 months of presentation were identified. Patients diagnosed with a spontaneous aortic thrombus underwent hypercoagulable screening. Spontaneous aortic thrombus was defined as thrombus within the lumen of the aorta without an underlying associated plaque, dissection, or aneurysmal degeneration.
Outcomes
All operative reports were reviewed for technical details regarding method of arterial access, type and location of stent graft(s) used, intraoperative use of TEE, or intravascular ultrasound and method of arterial closure. The primary outcomes assessed included postoperative renal function, recurrent embolization, amputation, and death.
Technique
All interventions were performed in a fixed imaging suite with the use of fluoroscopy, and most underwent intra-arterial contrast-enhanced imaging. No contrast was used in four patients. Intravascular ultrasound (IVUS Visions catheter, 8.2F × 90 cm; Volcano Corp, San Diego, Calif) and/or TEE was used to identify the area of pathology. In patients with multiple areas of atherosclerotic aortic plaque, only mobile components were covered. Catheter and wire manipulations and exchanges were minimized. Spinal drains were not used. Percutaneous access sites were closed with the use of closure devices that included Angioseal (St. Jude Medical, St. Paul, Minn), Prostar XL (Abbott Vascular, Abbott Park, Ill), or a combination of both.
Device selection and length of coverage was based on a combination of intraoperative TEE or IVUS examination and preoperative CT scan measurements. Stent graft selection as well as access and closure techniques were at the discretion of the operator. The shortest graft length necessary to cover the offending lesion was chosen to minimize coverage of the adjacent aorta. Four anatomic segments of the aorta were identified when describing regions covered with stent grafts: arch (defined as the aorta spanning the great vessel origins), descending thoracic (aorta extending from just distal to the left subclavian artery to the diaphragm), visceral (the aorta spanning the celiac, superior mesenteric artery [SMA], and renal artery origins), and infrarenal (the aorta distal to the origin of the renal arteries and proximal to the iliac arteries). Grafts used included the Gore Excluder (W. L. Gore & Associates, Flagstaff, Ariz) bifurcated modular and aortic extension cuffs, AneuRx (Medtronic, Minneapolis, Minn) cuffs, Gore TAG endografts, Wallstents and Wallgrafts (Boston Scientific, Inc, Natick, Mass), iCast stents (Atrium Medical Corporation, Hudson, NH), and a Zenith (Cook Medical, Bloomington, Ind) extender limb. Systemic heparinization (100 U/kg) was given in all cases, and grafts were inserted with the use of standard techniques, except in one case of direct aortic access. Wires, catheters, and sheaths were all identified, and manipulations were all monitored by means of fluoroscopy. Balloon angioplasty of the fixation segments of the endografts was sparingly used except in cases of modular bifurcated grafts placed in the infrarenal aorta for aneurysmal disease.
RESULTS
Preoperative details
Over the 7-year study period, a total of 25 patients with embolizing aortic lesions who required treatment were identified. More than half of these patients were women (Table I). A majority of patients had hypertension and tobacco use as risk factors. Eight patients had a documented hypercoagulable workup performed during the perioperative period, and only four were diagnosed with a hypercoagulable state. Fifty-two percent of the patients were receiving full perioperative anticoagulation by means of an unfractionated heparin drip. All were receiving antiplatelet therapy with aspirin and statin medication. Three patients had undergone an endovascular procedure (two angiograms for concern of embolic disease and one left heart catheterization) within 6 months of presentation. Only in the patient who had undergone a heart catheterization was it considered to be the precipitating factor of the embolic event. None of the patients had atrial fibrillation or potential intracardiac sources seen on perioperative transthoracic echocardiography, which was performed in 13 of 20 patients. Interestingly, 12 patients presented with stage 3 or more advanced chronic kidney disease.
Table I.
Patient demographics and baseline characteristics
| Variable | Data |
|---|---|
| No. of patients | 25 |
| Mean age, years (range) | 64 (45–87) |
| Female | 16 |
| CKD stage | |
| I | 6 |
| II | 7 |
| III | 9 |
| IV | 3 |
| Diabetes | 7 |
| Hypertension | 19 |
| Dyslipidemia | 13 |
| History of smoking | 19 |
| History of cancer | 5 |
| Hypercoagulable | 4a |
| Recent endo intervention (within 3 months) | 3 |
CKD, Chronic kidney disease.
Eight of 25 patients had hypercoagulable workup results available.
Clinical presentation
Two-thirds of the patients (17/25) presented with peripheral symptoms, 12 with foot pain and blue toes. Five patients presented with acute lower extremity ischemia. Six patients had progressive renal insufficiency presumed to be secondary to emboli, four with documented cortical renal infarcts on CT. Abdominal pain was the presenting symptom for five patients with clinically significant visceral emboli. One patient had concurrent visceral emboli to the SMA, spleen, and kidney, ultimately requiring SMA thrombectomy during coverage of the embolizing aortic lesion. Another patient had bilateral renal and splenic infarcts on CT imaging, whereas another had liver, splenic, and right renal artery emboli. Three patients had incidentally discovered aortic thrombus. One of these patients was undergoing a CT chest scan for shortness of breath and had a large amount of free-floating thoracic aortic thrombus. The other patient was having a CT scan for evaluation of chronic abdominal pain and had a large burden of free-floating thoracic aortic thrombus in addition to evidence of old splenic and renal infarcts. One patient had a known aortic abdominal aneurysm and was discovered to have a large free-floating thrombus in the descending thoracic aorta seen on preoperative CT scanning. This was addressed at the same time as the infrarenal aneurysm.
Anatomic distribution and types of lesions
All patients except for one had preoperative CT scans as part of the diagnostic workup to localize the embolic source. The exception had a TEE that identified the suspected culprit lesion and had advanced renal insufficiency and thus contrast imaging was avoided. Twelve patients had lesions limited to the thoracic aorta, whereas eight had lesions in more than one segment. Five patients had involvement of only the abdominal aorta. Six patients had aneurysmal disease all in the abdominal aorta, whereas no thoracic aneurysmal disease was identified. Two of these were thought to be a potential source of embolization to the lower extremities (Table II). Spontaneous aortic thrombus without any evidence of associated plaque or calcification throughout the aorta was noted in nine patients. All were large floating thrombus with minimal attachment to the wall. The caliber of these aortas was normal. Two patients had an area of an old healed dissection with an associated ulcerated plaque. Six patients had focal atherosclerotic aortic plaque associated with ulceration and thrombus. Eight patients had atheromatous disease in multiple segments associated with mobile plaques.
Table II.
Patient characteristics, presentation, region of aortic disease, operative details, and mortality (patients 1–13)
| Patient No. |
Age, years |
Sex | Presentation | Region(s) of aortic disease |
AAA disease |
Additional operative imaging |
Stent graft | Postoperative complications |
Mortality |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 65 | M | Blue toe syndrome | Arch thoracic infrarenal | <5.5 cm | IVUS | TAG | None | Alive |
| 2 | 78 | F | Blue toe syndrome | Thoracic | ≥5.5 cm | IVUS | TAG Excluder bifurcated graft | ESRD on dialysis | Death at 201 days (pneumonia) |
| 3 | 44 | F | Abdominal pain | Thoracic | <5.5 cm | TEE | Excluder Ao extender | PE | Alive |
| 4 | 84 | F | Blue toe syndrome | Thoracic supra/para | None | IVUS | Excluder Ao extender ×3 | None | Alive |
| 5 | 56 | F | Abdominal pain | Arch | None | TEE | Excluder Ao extender | None | Alive |
| 6 | 77 | M | Blue toe syndrome | Thoracic supra/para infrarenal | None | IVUS | Excluder Ao extenders (thoracic) ×3 AneuRx cuffs (abd) ×2 |
None | Alive |
| 7 | 70 | F | Blue toe syndrome | Thoracic infrarenal | <5.5 cm | IVUS | Excluder Ao extender Excluder bifurcated graft | ESRD at 3 months | Alive |
| 8 | 79 | M | Blue toe syndrome | Thoracic | <5.5 cm | IVUS | TAG | None | Alive |
| 9 | 50 | M | Ischemic lower extremity | Thoracic | None | TEE | TAG | Fasciotomy wound infection | Alive |
| 10 | 69 | M | Blue toe syndrome | Thoracic supra/para infrarenal | None | IVUS | Excluder Ao extender (thoracic) Excluder Ao extender (pararenal) ×2 Wallstent (abd) | None | Alive |
| 11 | 74 | F | Ischemic lower extremities | Infrarenal | None | None | Wallgraft | BKA for gangrene | Death at 115 days (unknown) |
| 12 | 54 | F | Blue toe syndrome | Arch infrarenal | None | TEE | Excluder Ao extender (thoracic) iCast (abd) ×2 | None | Alive |
| 13 | 59 | F | Blue toe syndrome | Infrarenal | None | none | Excluder iliac extender ×2 | None | Alive |
Abd, Abdominal; AKI, acute kidney injury; AML, acute myeloid leukemia; Ao, aorta; BKA, below-knee amputation; GFR, glomerular filtration rate; IVUS, intravascular ultrasound; LGIB, lower gastrointestinal bleed; PE, pulmonary embolism; TEE, transesophageal echocardiogram.
Intraoperative details
Arterial access was obtained through unilateral or bilateral common femoral artery access approaches (eight purely percutaneous and the remainder with at least one femoral cutdown). One device was deployed through an aortic exposure in a patient who had a concurrent SMA embolectomy. All procedures except for one were performed under general anesthesia. Although all patients had covered stent grafts used, one patient had a bare metal stent placed to expand a significantly narrowed distal aorta and another had a self-expanding bare metal stent placed to trap residual chronic thrombus left in the iliac artery after thrombectomy was performed.
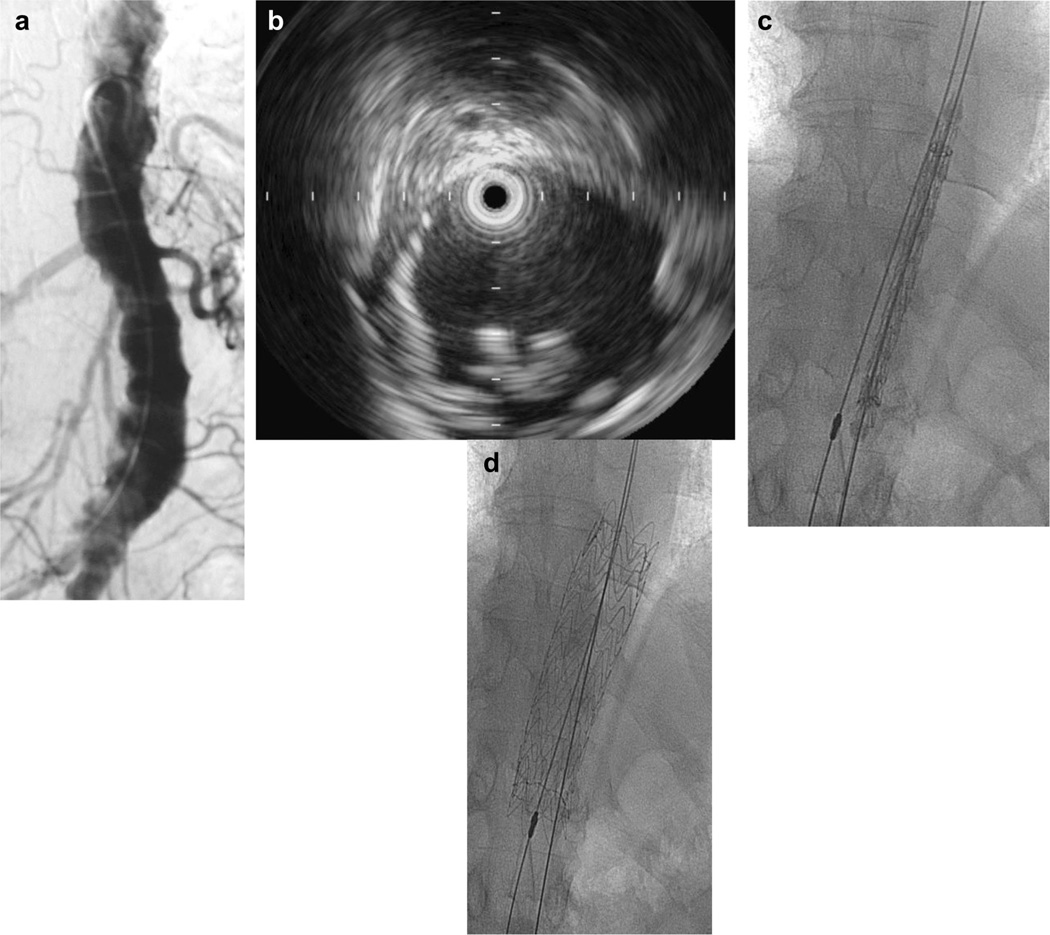
In an effort to identify the most plausible offending lesion in a diffusely diseased aorta and to minimize use of contrast and power injections around vulnerable thrombus, TEE, IVUS, or both were used in 17 patients. In four patients, the entire procedure was performed under IVUS guidance without any contrast use. Fig 1, a illustrates luminal irregularity representative of atheroembolic plaque. Axial imaging with CT scanning was performed in all except for one patient and was used to determine whether the underlying aortic lesion was atheromatous plaque or focal thrombus. In aortas with multiple involved segments, imaging with IVUS was used to determine which segments of plaque were mobile and required coverage, as shown in Fig 1, b. The exact volume of contrast used is not available for all cases. In two recent cases in which IVUS was used to localize the culprit lesion, a total of 30 mL of contrast was used during each procedure. IVUS was the sole determinant of exact intraoperative localization in 10 patients and TEE in one. In patients 4, 6, and 10, there were areas of thrombus in the paravisceral aorta that did not appear mobile on intraoperative IVUS and were therefore not addressed either with endovascular or open techniques (Table II).
Fig 1.
a, Aorta with multiple segments of disease. b, Intravascular ultrasound (IVUS) imaging of mobile atheroma. c and d, IVUS localization and stent graft coverage of lesion.
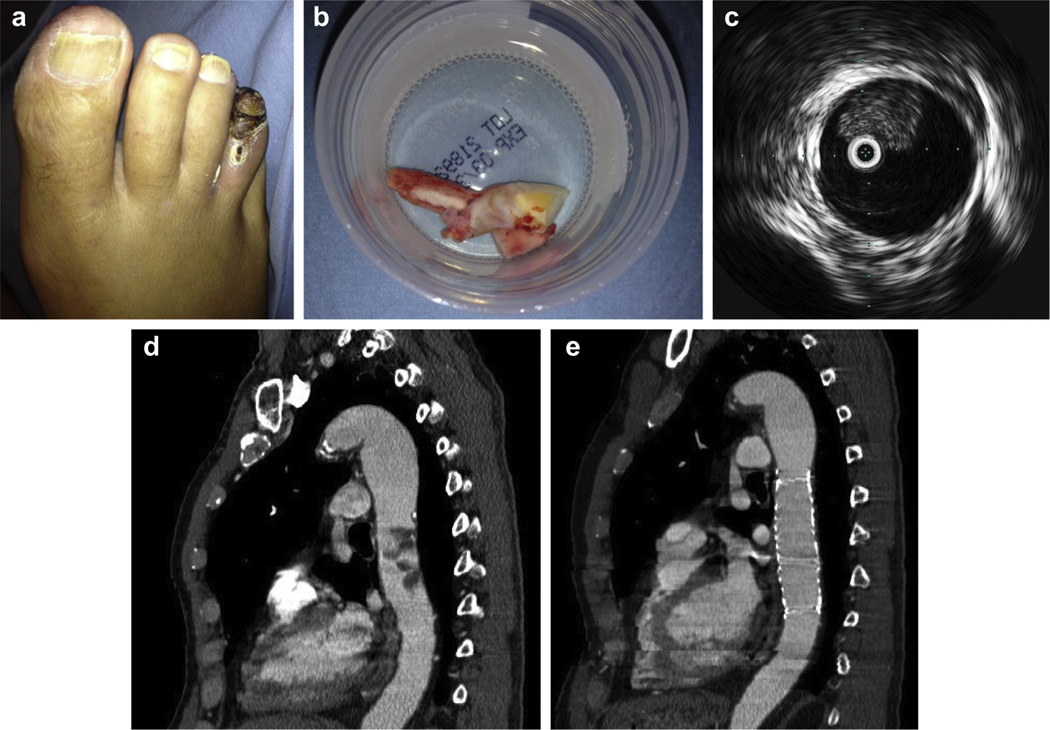
In three patients, coverage of the abdominal aneurysmal disease was provided concurrently with a modular bifurcated device concurrently because this was also identified as a potential source of emboli and/or met size criteria for repair. Intraoperative thrombectomy of iliac arterial emboli was performed in one case, as was thrombectomy of an SMA embolus in another. Fig 2, a–e, illustrates typical embolic findings in a patient’s toe, preoperative and postoperative imaging of a thoracic embolizing lesion, as well as intraoperative IVUS imaging and retrieval of a chronic organized iliac embolus. This particular patient had ischemic rest pain related to distal digital emboli from a thrombus localized to both the thoracic aorta and iliac arteries. In patients who had clinically significant renal dysfunction, the medical management strategies included hydration and avoidance of nephrotoxic agents. In patients with large areas of splenic infarction, trivalent vaccines for encapsulated organisms were administered.
Fig 2.
a, Toe gangrene as presenting symptom. b, Chronic organized thrombus retrieved fromiliac arteries. c, Intravascular ultrasound (IVUS) image of eccentric thrombus in the descending thoracic aorta. d, Preoperative computed tomography (CT) shows descending thoracic aortic thrombus. e, Postoperative CT with stent graft coverage of embolizing lesion.
Postoperative outcomes and follow-up
Overall mean hospital length of stay for patients after the procedure was 7 days (range, 1–38 days). There were no operative deaths. No patient had any evidence of postoperative iatrogenic embolic episodes precipitated by the procedure. Periprocedural complications were primarily related to the initial episode of embolization. One procedure-related complication was a groin wound seroma that was treated with open drainage 1 month after surgery. No patient had spinal cord ischemia. The patient with a symptomatic SMA embolism had a concurrent thrombectomy of the SMA and resolution of the intestinal ischemia. The patient with a partial inferior mesenteric artery thrombosis required an initial laparoscopic evaluation and eventual sigmoid colectomy 2 months after endograft coverage of the infrarenal aorta. Other procedures that were performed in the poststenting period included a below-the-knee amputation and lower extremity wound complications related to initial embolization. No clinical signs of recurrent embolization were clinically evident after stent graft coverage throughout the follow-up period. Of the five patients who died during follow-up, three died of pulmonary pathology (pneumonia, pulmonary fibrosis), one had a hematologic malignancy (acute myeloid leukemia), and the other was of unknown etiology (Table III).
Table III.
Patient characteristics, presentation, region of aortic disease, operative details, and mortality (patients 14–25)
| Patient No. |
Age, years |
Sex | Presentation | Region(s) of aortic disease |
AAA disease |
Additional operative imaging |
Stent graft | Postoperative complications |
Mortality |
|---|---|---|---|---|---|---|---|---|---|
| 14 | 49 | M | Ischemic lower extremity | Infrarenal | None | None | Zenith extender limb | Sigmoid colectomy for ischemic bowel | Death at 359 days (AML) |
| 15 | 62 | F | Blue toe syndrome | Arch thoracic infrarenal | None | IVUS | TAG (proximal) ×2 Excluder Ao extender ×2 | None | Alive |
| 16 | 87 | M | Incidental finding | Thoracic infrarenal | <5.5 cm | None | TAG Excluder bifurcated graft | None | Alive |
| 17 | 69 | F | Left flank pain | Thoracic | None | TEE | TAG | None | Death at 111 days (pulmonary fibrosis) |
| 18 | 60 | M | Incidental finding | Thoracic | None | IVUS | TAG | Epistaxis, LGIB | Alive |
| 19 | 55 | M | Blue toe syndrome | Thoracic infrarenal | None | IVUS | TAG Protégé (iliac) | Groin wound seroma | Alive |
| 20 | 56 | F | Ischemic lower extremity | Infrarenal | None | None | Excluder iliac extender | None | Alive |
| 21 | 73 | F | Blue toe syndrome | Infrarenal | None | None | Excluder iliac extender | None | Alive |
| 22 | 65 | F | Incidental finding | Thoracic | None | TEE | TAG | None | Alive |
| 23 | 64 | F | Ischemic lower extremity | Thoracic | None | TEE | TAG | ESRD, dialysis | Death at 75 days (bladder necrosis) |
| 24 | 53 | F | Abdominal pain | Thoracic | None | None | TAG | None | Alive |
| 25 | 52 | F | Abdominal pain | Thoracic | None | None | Zenith TX2 | None | Alive |
Abd, Abdominal; AKI, acute kidney injury; AML, acute myeloid leukemia; Ao, aorta; BKA, below-knee amputation; GFR, glomerular filtration rate; IVUS, intravascular ultrasound; LGIB, lower gastrointestinal bleed; PE, pulmonary embolism; TEE, transesophageal echocardiogram.
Patients were followed with clinical examination at regular intervals and CT scanning at 1 month after surgery if renal function was not prohibitive (Fig 2, e). There were no instances of clinically significant endoleak or graft migration. Patients were treated with the use of anticoagulation after surgery with warfarin or therapeutic enoxaparin if they had a documented underlying hypercoagulable state. Fourteen of 25 patients continued to receive long-term anticoagulation in addition to stent graft placement. Not all of these patients had diagnosed hypercoagulable states, and a few were receiving anticoagulation for other indications (such as atrial fibrillation). All patients were receiving antiplatelet therapy with aspirin and/or clopidogrel bisulfate.
Renal function
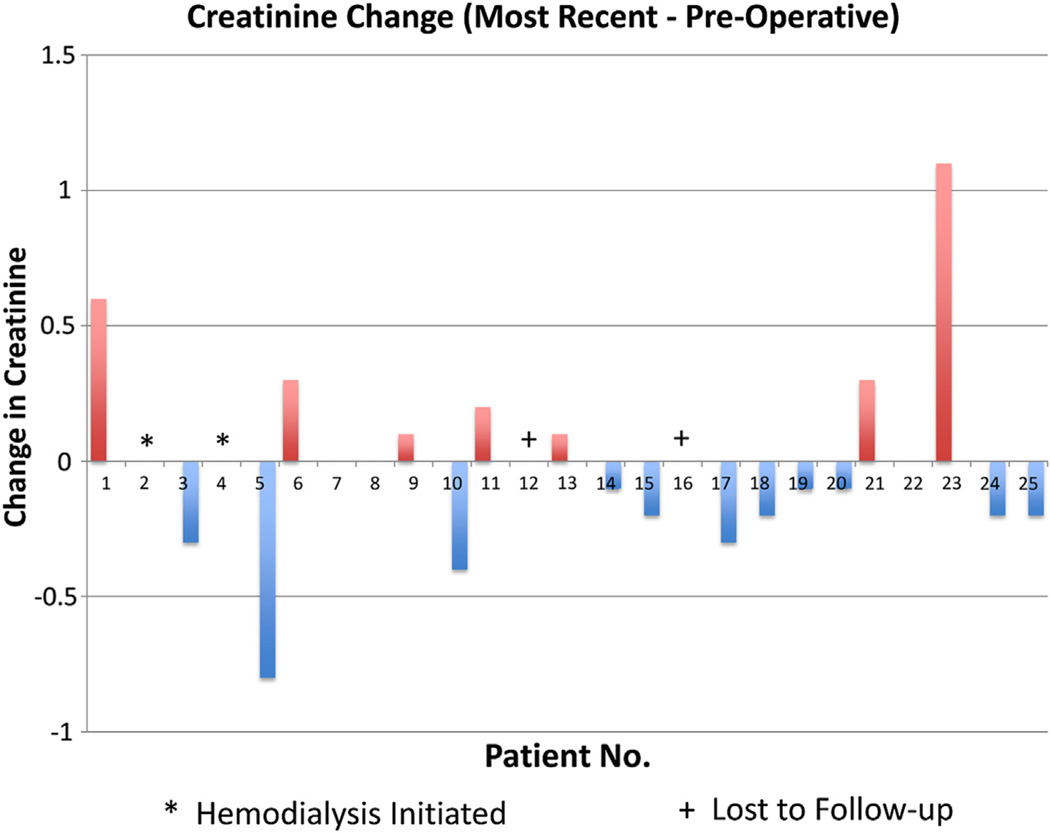
Three patients had stage IV chronic kidney disease; two of these patients became dialysis-dependent within 3 months of the procedure. Although continued embolization may have been possible in one patient, the other patient became dialysis-dependent 3 months after surgery during a hospitalization for respiratory compromise and sepsis. It is unlikely that ongoing embolization was a cause of this patient’s decline in renal function because interval labs showed stable renal function until the acute decline during the hospitalization for sepsis. There was no clinical evidence suggestive of cholesterol embolization (ie, urinalysis showing urine eosinophils or casts), although the patient did not have contrast-enhanced imaging to prove that there were no infarcts. As mentioned previously, 36% of patients had stage III and 28% had stage II chronic kidney disease. Despite this large proportion of patients with renal dysfunction, none had a decline in postoperative predischarge serum creatinine levels (mean postoperative day 4.9). Two patients were lost to long-term follow-up. At last follow-up, only one third of these patients had a mild decline in estimated glomerular filtration rate measured at a mean of 520 days (median of 356 days with a range of 21–1524 days after the procedure) (Table IV; Fig 3).
Table IV.
Renal function
| Patient No. | Preoperative Cr/GFR | Predischarge Cr/GFR | Days after surgery | Most recent Cr/GFR | Days after surgery |
|---|---|---|---|---|---|
| 1 | 2.2/30 | 1.8/38 | 1 | 1.6/44 | 1252 |
| 2 | 2.1/23 | 2.1/23 | 4 | HD initiated | 74 |
| 3 | 0.5/>100 | 0.6/>100 | 6 | 0.8/77 | 1524 |
| 4 | 1.6/31 | 1.5/33 | 1 | 1.6/30 | 1709 |
| 5 | 0.7/87 | 0.6/>100 | 2 | 1.5/36 | 37 |
| 6 | 2.0/33 | 1.6/42 | 1 | 1.7/39 | 1104 |
| 7 | 2.5/19 | 1.9/26 | 2 | HD initiated | 750 |
| 8 | 1.9/34 | 1.5/45 | 1 | 1.9/34 | 407 |
| 9 | 1.0/79 | 0.8/>100 | 3 | 0.9/89 | 81 |
| 10 | 1.9/35 | 1.6/43 | 1 | 2.3/28 | 510 |
| 11 | 0.9/61 | 0.6/98 | 17 | 0.7/82 | 21 |
| 12 | 0.9/65 | 0.9/65 | 5 | LTF | |
| 13 | 1.4/39 | 1.4/39 | 4 | 1.3/42 | 676 |
| 14 | 0.7/>100 | 0.7/>100 | 12 | 0.8/102 | 340 |
| 15 | 1.7/31 | 1.8/29 | 3 | 1.9/27 | 371 |
| 16 | 1.3/52 | 1.1/63 | 2 | LTF | |
| 17 | 0.8/71 | 0.8/71 | 1 | 1.1/49 | 111 |
| 18 | 0.9/86 | 0.8/99 | 11 | 1.1/68 | 100 |
| 19 | 0.5/>100 | 0.6/>100 | 6 | 0.6/140 | 48 |
| 20 | 0.8/74 | 0.7/87 | 2 | 0.9/65 | 22 |
| 21 | 1.0/66 | 1.1/59 | 8 | 0.7/99 | 120 |
| 22 | 1.3/41 | 1.2/45 | 1 | 1.3/43 | 133 |
| 23 | 2.1/24 | 1.5/35 | 5 | 1.0/59 | 60 |
| 24 | 0.7/88 | 0.9/65 | 1 | 0.9/65 | 1 |
| 25 | 0.7/88 | 0.9/66 | 2 | 0.9/66 | 2 |
Cr, Creatinine; GFR, glomerular filtration rate; HD, hemodialysis; LTF, lost to follow-up.
Fig 3.
Change in creatinine levels.
Other embolizing lesions
During the same time period of this study, we identified a cohort of nine patients who had embolization from aortic pathology and were not treated with endovascular strategies for either well-attached thrombus or unsuitable location. Four of these patients were treated with therapeutic anticoagulation alone because the location of the lesion was in the paravisceral segment. These patients were considered medically too high risk to undergo open repair with supravisceral clamping. Another two patients with embolizing lesions limited to the thoracic aorta were also considered of prohibitive risk because of active infectious and medical illness to undergo thoracic endograft coverage and were treated with anticoagulation alone. The remaining two patients were treated with open aortic endarterectomy in one and aortic replacement in the other. These patients were young and medically fit and had embolizing lesions localized to the abdominal aorta. Both had no immediate complications. One patient treated medically with diffuse atherosclerotic aorta died of continued embolization to the legs, kidneys, and mesenteric vessels. This patient was treated with antiplatelet therapy because of the more diffuse distribution of atheromatous disease. Unfortunately, most of these patients were lost to follow-up because of geographic limitations. Of the six patients who were treated with anticoagulation alone, two patients without underlying aortic plaque had repeat imaging during hospitalization demonstrating a reduction in the burden of isolated thoracic aortic thrombus.
DISCUSSION
This series represents the largest in the literature examining the use of endovascular stent graft coverage of embolizing aortic lesions caused by a variety of pathologies. This technique can be applied to isolated aortic thrombus or atherosclerotic embolizing lesions with multisegment involvement. Acute complications from the treatment appear to be quite limited, without episodes of iatrogenically induced emboli, which is the most serious theoretical drawback of the technique. The treatment also appears to be effective in eliminating further embolization and tissue loss. Despite a significant portion of patients having chronic renal insufficiency and having a contrast load with preoperative CT imaging, they did not progress to worsening kidney function after endovascular treatment. Technical considerations such as minimizing contrast angiography during endograft placement, use of IVUS or TEE, and minimizing wire/catheter manipulation and balloon angioplasty all play important roles in minimizing kidney injury and morbidity related to further embolic phenomena. Management strategies for embolizing aortic lesions include medical therapy, surgical treatment, and endovascular stent graft exclusion. However, because of the paucity of data and the variety of clinical results, no therapeutic standard exists because the recurrence rate with medical therapy is unknown, open repair is often untenable, and the safety of stent graft coverage continues to be disputed. Our experience provides evidence for the feasibility of stent graft coverage in the treatment of embolizing aortic lesions, with a significant measure of safety and apparent effectiveness.
Debate persists about the safety of endovascular treatment of mobile atherothrombotic lesions of the aorta, primarily because of the concern of inducing further, potentially lethal, visceral or peripheral embolization. Clinical manifestations of embolizing thoracoabdominal aortic lesions are broad and include visceral ischemia, progressive renal insufficiency, acute or chronic critical limb ischemia, and the classic blue toe syndrome. Embolic events can be spontaneous or iatrogenic from surgical or endovascular manipulation. Several percutaneous cardiac intervention case reports and studies that assessed outcomes in patients with aortic atherosclerotic debris substantiate these concerns with relatively high rates of catheter-related emboli.5–8 In a prospective, observational study of 1000 consecutive patients who underwent percutaneous coronary revascularization interventions, visible aortic debris was recovered from the guiding catheter in >50% of patients.5 However, postprocedure embolic events were minimal and had no association with the presence or size of debris particles. Karalis et al8 found that nearly 20% of patients with transesophageal echocardiographically detected atherosclerotic aortic debris who underwent cardiac catheterization or intra-aortic balloon pump placement through a femoral approach had an embolic event after the procedure. Specifically, patients with mobile aortic debris were at the highest risk for catheter-related embolism. Our experience appears to indicate that these concerns, although quite real, are not common during stent graft coverage and may be circumvented by careful technical maneuvers.
Stent graft exclusion in patients with distinct sources of embolization within the thoracoabdominal aorta provides an alternative treatment modality to the traditional surgical management. Before the explosion of endovascular therapies, Baumann et al2 reported on their institutional experience with atheroembolic disease and found that >20% were incited by a recent invasive radiologic study. Atheroembolic renal disease is particularly concerning because its effects are often silent and dismal, resulting in irreversible renal failure.9,10 Historically, open surgical treatment of embolizing aortic lesions has been viewed as the safest treatment modality because of the ability to control and prevent potential further visceral or extremity embolization during aortic manipulation.1,3,11 However, an analysis by Keen et al11 of 100 patients with atheroembolism managed with open surgical techniques demonstrated a significantly high periprocedural mortality rate among patients with a suprarenal aortic source. Because this disease typically portends an overall poor prognosis with associated significant comorbidities, affected patients are often not ideal candidates for open surgery, making an endovascular option quite attractive except for the unusual patient with minimal risk. We have only offered open repair to two select patients who responded well to the treatment without complications.
The aortic lesions that result in embolization are fairly diverse. Our series includes patients who have both multisegment mobile, embolizing atheromatous pathology in addition to isolated aortic thrombus. The natural history and behavior of these two distinct populations has not been well described in the literature despite sometimes being treated similarly. Debate also exists regarding the role of medical therapy (anticoagulation) in the context of these various lesions. Anticoagulation has been reported to control embolization from aortic clot in select cases even with eventual resolution but not from atheroembolic debris.12–14 As such, anticoagulation is recommended for aortic thrombi, especially if the only alternative is an open surgical approach to the thoracoabdominal aorta with its attendant morbidity and mortality. We believe stent graft coverage may offer another safe alternative to these patients while providing effective protection from embolization in threatening lesions. We have chosen to therapeutically anticoagulate patients who either have a documented hypercoagulable state or what appeared radiographically to be a well-attached isolated aortic thrombus with limited preceding clinical events or small clot burden. Trailing aortic thrombi, or very large mobile lesions on ultrasound examination, were instead covered with a stent graft because the expected clinical sequelae may be significant. This alternative allows for coverage of the offending lesion to avoid further embolic events while workup for hypercoagulability, underlying malignancy, and so forth occurs. Our small cohort of patients with aortic thrombus who underwent medical treatment with anticoagulation alone appeared to fare well overall, and at least two had a significant decrease in their clot on repeat CT scanning. This strategy is not effective in diffuse atherosclerotic aortas, as illustrated in our patient who refused endovascular treatment and died of further embolization.
The management of diffuse atherosclerotic aorta resulting in arterial embolization of plaque debris and cholesterol emboli is more complex because the exact location of the threatening component can be difficult to pinpoint by traditional means and the medical and surgical options are limited. Medical management with antiplatelet therapy and anticoagulation has been largely ineffective.12–14 Open surgical management can be somewhat useful when the disease is localized and the patient can tolerate aortic replacement.1 However, this is rare and is associated with considerable mortality and morbidity rates.1,3,15 However, this may be the only option when the visceral aorta is the culprit and the patient can tolerate the surgical intervention. Our experience suggests that stent graft coverage may be an alternative with several advantages, making it probably the treatment of choice in these situations. IVUS can help significantly in identifying the portion of the atherosclerotic aorta that contains mobile elements and guides limited coverage of those areas with stent grafts. This strategy has been very useful in this difficult subset of patients except for those in whom the visceral aorta is identified as the site of loose debris. Branched devices or chimneys may be attempted in the future in this subset, but the extra manipulation and technical difficulty may limit the potential of a safe conclusion to the procedure.
Stent graft coverage of embolizing aortic lesions has been reviewed in several case reports and single-institution retrospective small series.16–22 Indications for treatment in these prior series included visceral and embolic events, with the majority of cases involving single-segment infrarenal aortic disease. One case report by Zhang et al20 describes the novel approach of staged stenting for concurrent thoracic and infrarenal aortic embolic sources, unlike the patients in our series treated with concurrent supra-diaphragmatic and infrarenal coverage. Success, generally defined as exclusion of the offending lesion with avoidance of iatrogenic or further clinically significant embolization, was widespread, and the overall conclusion gleaned from these reports was that stent graft coverage of embolizing aortic lesions can be effective in patients with appropriate anatomy; however, long-term results are necessary. Unfortunately, because of the rarity of this disease phenomenon, conclusive prospective data supporting endovascular stent graft coverage for embolizing aortic lesions are lacking. Our series is a limited, retrospective, single-institution review of a relatively larger number of patients, with all of its attendant limitations. It suggests that endovascular coverage of these lesions can be performed safely and that further embolization can be expected to cease. Larger prospective studies examining medical, surgical, and endovascular treatment of aortic embolizing lesions would be strongly desirable but are unlikely to occur. A prospective, randomized trial with matched cohorts of patients with similar aortic pathologies would be the most useful approach to further delineate whether stent graft coverage of these lesions is equivalent or superior to medical therapy, given the risk and cost of procedural intervention vs the risk of ongoing embolization with medical therapy alone. Our intention is to not only extend the evidence for endovascular methods in treating embolic aortic disease but to provide additional information regarding technical aspects that could optimize outcomes. Newer, lower-profile devices and rapidly evolving IVUS imaging techniques may obviate the need for contrast imaging in this vulnerable group of patients.
CONCLUSIONS
Endovascular stent graft coverage of atheroembolic sources in the aorta can be feasible, safe, and effective in the properly chosen patient through the use of meticulous operative technique and perioperative treatment.
Footnotes
Author conflict of interest: None.
AUTHOR CONTRIBUTIONS
Conception and design: MM, GJ
Analysis and interpretation: MM, GJ, JW
Data collection: JW, GJ, MM, RC
Writing the article: MM, GJ, JW, RC, LM, SL
Critical revision of the article: MM, GJ, JW, RC, LM, SL
Final approval of the article: MM, GJ
Statistical analysis: Not applicable
Obtained funding: Not applicable
Overall responsibility: GJ
REFERENCES
- 1.Kvilekval KH, Yunis JP, Mason RA, Giron F. After the blue toe: prognosis of noncardiac arterial embolization in the lower extremities. J Vasc Surg. 1993;17:328–334. [PubMed] [Google Scholar]
- 2.Baumann DS, McGraw D, Rubin BG, Allen BT, Anderson CB, Sicard GA. An institutional experience with arterial atheroembolism. Ann Vasc Surg. 1994;8:258–265. doi: 10.1007/BF02018173. [DOI] [PubMed] [Google Scholar]
- 3.Bojar RM, Payne DD, Murphy RE, Schwartz SL, Belden JR, Caplan LR, et al. Surgical treatment of systemic atheroembolism from the thoracic aorta. Ann Thorac Surg. 1996;61:1389–1393. doi: 10.1016/0003-4975(96)00084-7. [DOI] [PubMed] [Google Scholar]
- 4.Shames ML, Rubin BG, Sanchez LA, Thompson RW, Sicard GA. Treatment of embolizing arterial lesions with endoluminally placed stent grafts. Ann Vasc Surg. 2002;16:608–612. doi: 10.1007/s10016-001-0278-2. [DOI] [PubMed] [Google Scholar]
- 5.Keeley EC, Grines CL. Scraping of aortic debris by coronary guiding catheters: a prospective evaluation of 1,000 cases. J Am Coll Cardiol. 1998;32:1861–1865. doi: 10.1016/s0735-1097(98)00497-5. [DOI] [PubMed] [Google Scholar]
- 6.Tilley WS, Harston WE, Siami G, Stone WJ. Renal failure due to cholesterol emboli following PTCA. Am Heart J. 1985;110:1301–1302. doi: 10.1016/0002-8703(85)90031-6. [DOI] [PubMed] [Google Scholar]
- 7.Ramirez G, O’Neill WM, Jr, Lambert R, Bloomer HA. Cholesterol embolization: a complication of angiography. Arch Intern Med. 1978;138:1430–1432. [PubMed] [Google Scholar]
- 8.Karalis DG, Quinn V, Victor MF, Ross JJ, Polansky M, Spratt KA, et al. Risk of catheter-related emboli in patients with atherosclerotic debris in the thoracic aorta. Am Heart J. 1996;131:1149–1155. doi: 10.1016/s0002-8703(96)90090-3. [DOI] [PubMed] [Google Scholar]
- 9.Scolari F, Ravani P. Athereoembolic renal disease. Lancet. 2010;375:1650–1660. doi: 10.1016/S0140-6736(09)62073-0. [DOI] [PubMed] [Google Scholar]
- 10.Scolari F, Tardanico R, Zani R, Pola A, Viola BF, Movilli E, et al. Cholesterol crystal embolism: a recognizable cause of renal disease. Am J Kidney Dis. 2000;36:1089–1109. doi: 10.1053/ajkd.2000.19809. [DOI] [PubMed] [Google Scholar]
- 11.Keen RR, McCarthy WJ, Shireman PK, Feinglass J, Pearce WH, Durham JR, et al. Surgical management of atheroembolization. J Vasc Surg. 1995;21:773–780. doi: 10.1016/s0741-5214(05)80008-4. [DOI] [PubMed] [Google Scholar]
- 12.Bowdish ME, Weaver FA, Liebman HA, Rowe VL, Hood DB. Anticoagulation is an effective treatment for aortic mural thrombi. J Vasc Surg. 2002;36:713–719. [PubMed] [Google Scholar]
- 13.Fayad ZY, Semaan E, Fahoum B, Briggs M, Tortolani A, D’Ayala M. Aortic mural thrombus in the normal or minimally atherosclerotic aorta. Ann Vasc Surg. 2013;27:282–290. doi: 10.1016/j.avsg.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 14.Hollier LH, Kazmier FJ, Ochsner J, Bowen JC, Procter CD. “Shaggy” aorta syndrome with atheromatous embolization to visceral vessels. Ann Vasc Surg. 1991;5:439–444. doi: 10.1007/BF02133048. [DOI] [PubMed] [Google Scholar]
- 15.Tunick PA, Nayar AC, Goodkin GM, Mirchandani S, Francescone S, Rosenzweig BP, et al. Effect of treatment on the incidence of stroke and other emboli in 519 patients with severe thoracic aortic plaque. Am J Cardiol. 2002;90:1320–1325. doi: 10.1016/s0002-9149(02)02870-9. [DOI] [PubMed] [Google Scholar]
- 16.Fueglistaler P, Wolff T, Guerke L, Stierli P, Eugster T. Endovascular stent graft for symptomatic mobile thrombus of the thoracic aorta. J Vasc Surg. 2005;42:781–783. doi: 10.1016/j.jvs.2005.05.054. [DOI] [PubMed] [Google Scholar]
- 17.Dougherty MJ, Calligaro KD. Endovascular treatment of embolization of aortic plaque with covered stents. J Vasc Surg. 2002;36:727–731. [PubMed] [Google Scholar]
- 18.Carroccio A, Olin JW, Ellozy SH, Lookstein RA, Valenzuela R, Minor ME, et al. The role of aortic stent grafting in the treatment of atheromatous embolization syndrome: results after a mean of 15 months follow-up. J Vasc Surg. 2004;40:424–429. doi: 10.1016/j.jvs.2004.06.036. [DOI] [PubMed] [Google Scholar]
- 19.Luebke T, Aleksic M, Brunkwall J. Endovascular therapy of a symptomatic mobile thrombus of the thoracic aorta. Eur J Vasc Endovasc Surg. 2008;36:550–552. doi: 10.1016/j.ejvs.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 20.Zhang WW, Abou-Zamzam AM, Hashisho M, Killeen JD, Bianchi C, Teruya TH. Staged endovascular stent grafts for concurrent mobile/ulcerated thrombi of thoracic and abdominal aorta causing recurrent spontaneous distal embolization. J Vasc Surg. 2008;47:193–196. doi: 10.1016/j.jvs.2007.07.050. [DOI] [PubMed] [Google Scholar]
- 21.Matchett WJ, McFarland DR, Eidt JF, Moursi MM. Blue toe syndrome: treatment with intra-arterial stents and review of therapies. J Vasc Interv Radiol. 2000;11:585–592. doi: 10.1016/s1051-0443(07)61610-8. [DOI] [PubMed] [Google Scholar]
- 22.Criado E, Wall P, Lucas P, Gasparis A, Proffit T, Ricotta J. Transesophageal echo-guided endovascular exclusion of thoracic aortic mobile thrombi. J Vasc Surg. 2004;39:238–242. doi: 10.1016/j.jvs.2003.07.017. [DOI] [PubMed] [Google Scholar]