Abstract
OBJECTIVES
Refluxing perforators contribute to venous ulceration. We sought to describe patient characteristics and procedural factors that (1) impact rates of incompetent perforator vein (IPV) thrombosis with ultrasound-guided sclerotherapy (UGS) and (2) impact the healing of venous ulcers (CEAP 6) without axial reflux.
METHODS
Retrospective review of UGS of IPV injections from 1/2010–11/2012 identified 73 treated venous ulcers in 62 patients. Patients had no other superficial/axial reflux and were treated with standard wound care and compression. Ultrasound was used to screen for refluxing perforators near ulcer(s), and these were injected with sodium tetradecyl sulfate or polidocanol foam and assessed for thrombosis at 2 weeks. Demographic data, comorbidities, treatment details and outcomes were analyzed. Univariate and multivariable modeling was performed to determine covariates predicting IPV thrombosis and ulcer healing.
RESULTS
62 patients with active ulcers for an average of 28 months with compression therapy prior to perforator treatment had an average age of 57.1 years, were 55% male, 36% had a history of DVT and 30% had deep venous reflux. 32 patients (52%) healed ulcers, while 30 patients (48%) had non-healed ulcer(s) in mean follow-up of 30.2 months. Ulcers were treated with 189 injections, with average thrombosis rate of 54%. Of 73 ulcers, 43 ulcers healed (59%), and 30 ulcers did not heal (41%). Patients that healed ulcers had an IPV thrombosis rate of 69 % vs. 38% in patients who did not heal (P<.001). Multivariate models demonstrated male gender and warfarin use negatively predicted thrombosis of IPVs (P=.03, P=.01). Multivariate model for ulcer healing found complete IPV thrombosis was a positive predictor (P=.02), while large initial ulcer area was a negative predictor (P=.08). Increased age was associated with fewer ulcer recurrences (P=.05). Hypertension and increased follow-up time predicted increased ulcer recurrences (P=.04, P=.02). Calf vein thrombosis occurred after 3% (6/189) of injections.
CONCLUSIONS
Thrombosis of IPVs with UGS increases venous ulcer healing in a difficult patient population. Complete closure of all IPVs in an ulcerated limb was the only predictor of ulcer healing. Men and patients on warfarin have decreased rates of IPV thrombosis with UGS.
INTRODUCTION
Incompetent perforator veins (IPV) have long been associated with venous disease and ulceration. Perforator veins in and around the ankles are particularly vulnerable to incompetence, and venous hypertension in this area creates edema, skin discoloration, and ulceration. 1 Compression is the mainstay of treatment for venous incompetence and reflux. However, even in compliant patients there is a high chance of recurrent ulceration and symptoms due to failure to correct the underlying pathology. 2, 3 Milic et al found a 24% recurrence rate at 1-year in those compliant with compression vs. 53% recurrence rate in those without (p<.05). 4 This study buttresses the plethora of literature demonstrating that compression therapy decreases but does not prevent ulcer recurrence. 5–8 Due to poor ulcer healing with compression alone, other treatment strategies aim to treat the mechanisms of venous incompetence and reduce venous hypertension.
Although open perforator ligation (Linton procedure) 9 and subfascial endoscopic perforator surgery (SEPS) 10, 11 have been proven to improve ulcer healing 12, 13, they are both associated with high morbidity. A paradigm shift toward ablative therapy has occurred with increased technical success and fewer complications. 14
Ultrasound-guided sclerotherapy (UGS) has recently been advocated to treat incompetent perforator veins associated with venous ulcers. Masuda et al demonstrated good technical results with low complication rates using UGS for treatment of IPV. 15 Although factors affecting overall venous ulcer healing and recurrence have been previously described 2, 5, 16–19, published studies of specific modalities so far have focused primarily on improved subjective venous clinical scores rather than direct healing rates of venous ulcers. Without a randomized comparison between UGS and direct catheter based ablation techniques, uncertainty persists regarding the best type of IPV treatment. In addition, limited data on the predictors of successful UGS of IPV and its impact on ulcer healing are available. The purpose of this report is to describe patient characteristics and peri-procedural factors which impact rates of IPV closure using UGS and how this impacts healing of venous ulcers.
METHODS
Institutional Review Board approval was obtained for both prospective and retrospective reviews of a clinical database of patients with active venous ulcer(s) treated at the University of Pittsburgh Medical Center. Research was supported in part through a 2009 Young Investigator’s Grant from the American College of Phlebology. Statistical analysis was supported by the National Institutes of Health through Grant Numbers UL1-RR-024153 and UL1-TR-000005.
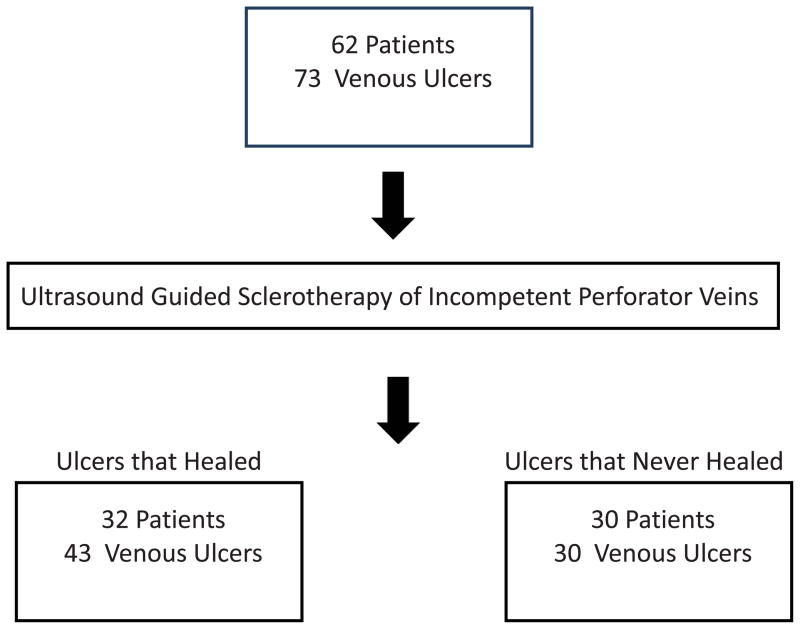
Patients were identified both from a prospectively maintained database of patients with venous ulcers and through a query for ultrasound-guided injections in our electronic medical record system. Those with venous ulcers (CEAP 6, active ulcer) 20 who underwent UGS of IPV(s) from 1/2010 to 11/2012 were included in the analysis. All patients received standard of care compression and wound therapy throughout the study treatment period. (Figure 1)
Figure 1.
Study Design
Initial Evaluation
Patients underwent a complete history and physical and comprehensive venous duplex ultrasound (US) assessment of surface varicosities as well as the deep, superficial, and perforator veins. All veins were assessed for dilation, reflux, presence of acute or chronic thrombus and geographic relationship to the ulcer. Refluxing perforators of at least 3.5 millimeters in diameter and in immediate proximity to the ulcer or directly feeding varicosities in the vicinity of the ulcer were considered pathologic. All patients were scored with CEAP classification. 20 Patients were assessed in this manner at the time of initial presentation to the practice, but due to variations in practice patterns and/or patient preference, patients may not have had treatment of perforator disease until it was demonstrated that compressive therapy alone was not successful in healing venous wounds.
Ulcer Management
All patients underwent compressive therapy, usually with Unna’s Boot(s) or short-stretch bandages. Superficial debridement was performed on venous ulcers at the discretion of the provider. Patients with saphenous vein reflux of > 1 second and diameter ≥5 mm were treated with radiofrequency or laser ablation. If patients had persistent ulceration and refluxing perforators > 3.5 mm after saphenous ablation, they were then included in the study.
Ultrasound-Guided Sclerotherapy Technique
Prior to injection, the location(s) of the perforator(s) were described in the ultrasound report, and this detailed documentation was used as a reference for follow-up comparison. Ultrasound-guided sclerotherapy injections were performed by Board-certified vascular surgeons with the aid of a registered vascular technologist using a General Electric Logiq 9 or E9 machine (General Electric Medical Systems, Milwaukee, WI). Foam was prepared using the Tessari method with a 4:1 air: sclerosant mixture. 21 Prior to May 2010, 1% sodium tetradecyl sulfate (STS) was used. After May 2010, either 1% or 3% polidocanol (POL) sclerosing agent was utilized at the discretion of the provider. Under direct ultrasound visualization, a 23-gauge needle was inserted into the varicosities fed by the IPV. Foam was prepared and immediately instilled into the cannulated vein under direct ultrasound visualization. The skin surrounding the ulcer and injection site was massaged to move the foam into the perforator as well as into adjacent varicosities or venous plexi. When foam sclerosant filled the incompetent perforating vein, pressure was held at the junction of the IPV and the deep vein for at least 2 minutes with an ultrasound probe. The injected perforating vein and surrounding varicosities were subsequently imaged to ensure sclerosis. A goal amount of 10 cc of foam or less was used per injection session to limit the amount of air instilled. Patients with multiple perforators could have several injections sessions scheduled to limit foam. Following the injection, deep veins were imaged to ensure that they were clear of foam and compressible. Patients were all injected supine, compression was applied and they were allowed to ambulate immediately after. Compression therapy was applied immediately post-procedure and left in place for at least 24 hours before the patient’s standard wound care and compression therapy were resumed. Patients with Unna’s boots had the dressing reapplied immediately after the UGS procedure. Patients on anticoagulation did not have this therapy held during the injections. All patients underwent an UGS injection in at least one IPV during the study period, and some had multiple injections in an IPV or multiple IPVs.
Follow-up
Patients with continuous Unna’s Boot compression therapy were seen weekly after treatment as per standard of clinical care. Patients on other wound care and compression therapies were seen at 2, 4, and 6 weeks post-procedure, and then every 6 weeks thereafter. At 2 weeks, a duplex ultrasound by the same technologist used during the injection was performed to evaluate for thrombosis of the injected perforator, rule out deep venous thrombosis and assess for new IPVs. Repeat US to assess for new perforators or recanalization of treated IPVs was performed for a decline or stagnation in wound healing progress or other clinical indication.
Venous ulcer(s) were measured at each visit and recorded in a flow sheet imbedded in the electronic medical record. The area of the ulcer was calculated to determine the rate of healing. The dates of complete ulcer healing and last known follow-up were recorded for each patient. After complete ulcer healing, patients were told to continue compression and return for any new symptoms. Phone contact was initiated on patients absent from the clinic for 6 months or more to ensure that no new ulcers had developed.
Definitions and Classifications of Covariates
Outcomes
Primary outcomes included 1) incidence of thrombosis after UGSs of IPV(s), 2) success of complete closure of all PIVs in an ulcerated limb, and 3) healing status, a binary outcome defined by the presence or absence of a venous ulcer at last known follow-up. Secondary continuous outcome focused on ulcer recurrence. Recurrence was identified as ulcer healing and then opening in the same anatomic area at any point during the study period.
Statistics
All statistical analysis was conducted by the Clinical and Translational Science Institute at the University of Pittsburgh Medical Center using R version 2.15.1 (R Foundation for Statistical Computing, Vienna, Austria) and Stata version 11.2 (StataCorp. 2009. Stata Statistical Software: Release 11. College Station, TX: StataCorp LP).
Analyses were initiated by running exploratory univariate models using clinically relevant variables. For each primary and secondary outcome, each demographic, comorbidity and procedural predictor was entered by itself in a linear (for continuous outcomes) or binomial (for dichotomous outcomes) mixed effects model, which accounts for the repeated measures of multiple ulcers in individual patients. The coefficient (continuous outcomes) or odds ratio (dichotomous outcomes) was calculated with the p-value < 0.05 considered significant. Within each outcome, any variable that achieved p < 0.20 significance in univariate modeling was selected as a candidate for multivariable modeling. Multivariable modeling was conducted as a backward stepwise regression, excluding the least significant variable until only variables with p < 0.10 remained. Procedural predictors with partial collinearity with the outcome were not tested or included. Linear or binomial mixed effects models were used to account for multiple ulcers from the same patient by means of incorporating a random effects term and robust standard errors.
RESULTS
Analysis of UGS injections of IPVs from 1/2010 to 11/2012 identified 73 venous ulcers in 62 patients who had compression and standard wound care for an average of 28 months prior to perforator ablation. Follow-up duplex ultrasound was performed in 98% of patients. Of 62 patients, 32 patients healed ulcers (52%), while 30 had at least 1 non-healed ulcer at last follow-up visit (Table I). Of 73 ulcers, 43 ulcers healed (Group H), while 30 ulcers recurred or never healed (Group NH) for a healing rate of 59% at last follow-up. Mean initial ulcer size was 3.56 cm2 in Group H vs. 15.15 cm2 in Group NH (p=.10). Median initial ulcer size was 1.61 cm2 in Group H vs. 4.40 cm2 in Group NH.
Table I.
Demographic variables in patients undergoing UGS of IPV
| Variable | N=62 |
|---|---|
| Male (%) | 55% |
| Mean age (years) | 57.1 |
| BMI>30kg/m2 (%) | 53% |
| Deep venous reflux (%) | 31% |
| Previous deep venous thrombosis (%) | 36% |
| History of smoking, current smoking (%) | 53% |
| Diabetes mellitus (%) | 13% |
| Hypercoagulable state (%) | 5% |
| Hyperlipidemia (%) | 47% |
| Hypertension (%) | 71% |
| COPD (%) | 5% |
| Taking aspirin (%) | 36% |
| Taking Coumadin (%) | 21% |
| Taking both aspirin and Coumadin (%) | 8% |
| Taking diuretic (%) | 39% |
Perforator Injection Results
189 injections were performed. An average of 10.2 cc foam was used per session. Polidocanol was used in 74% of injections, with 86% of these using 3%. There were no differences in STS 1% vs. 1% or 3% POL injection thrombosis rates or complications. There was a 54% overall IPV closure rate. Thrombosis occurred in 69% of injections for Group H vs. 38% of the injections for Group NH (p < 0.001). At the end of follow-up, 92% of Group H ulcers had closure of all IPV in the affected limb (complete closure) vs. 68% of Group NH ulcers (p=.02). The average number of unsuccessful injections before first successful injection for group H was 0.28 vs. group NH was 0.52 (p=.29). Group H ulcers averaged 2.3 injections per ulcer vs. 3.1 in Group NH (p=.13).
Forty-eight ulcers had thrombosed IPVs and 25 ulcers had IPVs fail to thrombose after first UGS (66% closure rate for first injection). There were 116 subsequent UGS treatments of IPVs, 52 of which were successful (45%), (p=.12 compared to initial injections). In Group H, 36 out of 54 subsequent injections were successful (67%), compared to group NH, where 16 of 62 subsequent injections were successful (26%) (p<.001). Of healed ulcers, 23% required a single perforator injection.
Post-procedure DVTs were seen in 3% of injections (6/189) in 6 patients (10%): 2 in Group H and 4 in Group NH (p=.35). All were short occlusion posterior tibial vein thromboses. In these patients, 33% were already on warfarin for various reasons and the remaining 66% were placed on 325 mg/day aspirin, with 100% recanalization of their short-occlusion thromboses on follow-up duplexes. No other injection complications were seen.
Predictors of IPV Thrombosis (Tables II and III)
Table II.
Univariate and Multivariable Binary Logistic Regression Analysis to Predict Thrombosis of Last UGS Injections of IPVs
| Univariate | Multivariable | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Odds Ratio (per unit) | p-value | 95% CI lower | 95% CI upper | Odds Ratio (per unit) | p-value | 95% CI lower | 95% CI upper |
| Male Gender | 0.61 | 0.52 | 0.13 | 2.75 | ||||
| Age (years) | 0.98 | 0.35 | 0.93 | 1.03 | ||||
| Maximum BMI (kg/m2) | 0.73 | 0.20 | 0.46 | 1.17 | ||||
| Maximum BMI > 30 (kg/m2) | 0.27 | 0.11 | 0.05 | 1.36 | ||||
| Maximum Weight (kg) | 0.96 | 0.16 | 0.91 | 1.01 | ||||
| Deep vein reflux | 1.21 | 0.80 | 0.26 | 5.56 | ||||
| Previous deep vein thrombosis | 0.98 | 0.98 | 0.23 | 4.24 | ||||
| History of smoking, current smoking | 2.36 | 0.28 | 0.50 | 11.1 | ||||
| Hyperlipidemia | 0.36 | 0.15 | 0.09 | 1.45 | ||||
| Hypertension | 0.39 | 0.36 | 0.05 | 2.91 | ||||
| Taking Coumadin | 0.13 | 0.51 | 0.0003 | 55.6 | ||||
| Deep vein thrombosis after UGS IPV | 0.06 | 0.31 | 0.0003 | 13.5 | ||||
| Number of UGS IPV | 0.65 | 0.004 | 0.48 | 0.87 | 0.65 | 0.004 | 0.48 | 0.87 |
| Initial follow up to last follow up (days) | 1.0001 | 0.74 | 0.9995 | 1.00 | ||||
| Initial Ulcer Area (cm2) | 1.02 | 0.66 | 0.95 | 1.08 | ||||
Table III.
Univariate and Multivariable Linear Regression Analysis to Predict Percentage of Thrombosis of UGS Injections of IPV
| Univariate | Multivariable | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Net effect (per unit) | p-value | 95% CI lower | 95% CI upper | Net effect (per unit) | p- value | 95% CI lower | 95% CI upper |
| Male Gender | −0.18 | 0.04 | −0.35 | −0.01 | −0.21 | 0.03 | −0.37 | −0.02 |
| Age (years) | −0.003 | 0.41 | −0.009 | 0.004 | ||||
| Maximum BMI (kg/m2) | −0.01 | 0.01 | −0.03 | −0.002 | ||||
| Maximum BMI > 30 (kg/m2) | −0.19 | 0.02 | −0.36 | −0.03 | ||||
| Maximum Weight (kg) | −0.002 | 0.01 | −0.003 | −0.0005 | ||||
| Deep vein reflux | −0.04 | 0.63 | −0.23 | 0.14 | ||||
| Previous deep vein thrombosis | −0.08 | 0.35 | −0.26 | 0.09 | ||||
| History of smoking, current smoking | 0.10 | 0.26 | −0.07 | 0.27 | ||||
| Hyperlipidemia | −0.09 | 0.33 | −0.26 | 0.09 | ||||
| Hypertension | −0.10 | 0.30 | −0.29 | 0.09 | ||||
| Taking Coumadin | −0.18 | 0.06 | −0.36 | 0.005 | −0.21 | 0.01 | −0.38 | −0.05 |
| Deep vein thrombosis after UGS IPV | −0.22 | 0.14 | −0.50 | 0.07 | −0.25 | 0.06 | −0.53 | 0.02 |
| Initial follow up to last follow up (days) | −0.00003 | 0.46 | −0.0001 | 0.00005 | ||||
| Initial Ulcer Area (cm2) | −0.002 | 0.34 | −0.005 | 0.002 | ||||
Univariate analysis revealed increased number of UGS injections negatively predicted successful thrombosis of IPVs. For every additional UGS injection, we expect to see a 35% decrease in the odds of an eventual successful thrombosis of IPVs. (OR .65, CI .48-.87, p=.004)
Increased weight (p=.01), increased BMI (p=.01), BMI>30 kg/m2 (p=.02), and male gender (p=.04) all negatively predicted thrombosis after UGS. Male gender highly correlated with increased BMI, and thus, gender association may be a result of men with increased BMI in our study population.
Multivariable modeling similarly demonstrated that post-procedural DVT (p=.06), male gender (p=.03) and warfarin use (p=.01) negatively predicted IPV thrombosis.
Predictors of Ulcer Healing and Recurrence (Tables IV and V)
Table IV.
Univariate and Multivariable Binary Logistic Regression Analysis to Predict Ultimate Ulcer Healing
| Univariate | Multivariable | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | ODDS RATIO (per unit) | p-value | OR CI lower | OR CI upper | ODDS RATIO (per unit) | p- value | OR CI lower | OR CI upper |
| Male Gender | 0.64 | 0.35 | 0.25 | 1.64 | ||||
| Age (years) | 0.98 | 0.36 | 0.95 | 1.02 | ||||
| Maximum Weight (kg) | 0.998 | 0.63 | 0.99 | 1.0 | ||||
| Deep vein reflux | 1.07 | 0.89 | 0.40 | 2.87 | ||||
| Previous deep vein thrombosis | 0.89 | 0.81 | 0.34 | 2.31 | ||||
| History of smoking, current smoking | 1.31 | 0.58 | 0.52 | 3.35 | ||||
| Hyperlipidemia | 0.75 | 0.55 | 0.29 | 1.92 | ||||
| Hypertension | 0.88 | 0.83 | 0.30 | 2.63 | ||||
| Taking Coumadin | 0.96 | 0.95 | 0.36 | 2.60 | ||||
| Deep vein thrombosis after UGS IPV | 0.32 | 0.20 | 0.05 | 1.86 | ||||
| Last UGS IPV injection a success | 4.50 | 0.02 | 1.23 | 16.5 | 4.87 | 0.02 | 1.28 | 18.47 |
| Number of UGS IPV | 0.82 | 0.10 | 0.65 | 1.03 | ||||
| Initial follow up to last follow up (days) | 1.0002 | 0.46 | 0.9996 | 1.0008 | ||||
| % success UGS IPV | 4.31 | 0.04 | 1.06 | 17.96 | ||||
| Initial Ulcer Area (cm2) | 0.92 | 0.10 | 0.84 | 1.01 | 0.92 | 0.08 | 0.83 | 1.01 |
Table V.
Univariate and Multivariable Linear Regression Analysis to Predict Ulcer Recurrence
| Univariate | Multivariable | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Net effect (per unit) | p-value | 95% CI lower | 95% CI upper | Net effect (per unit) | p-value | 95% CI lower | 95% CI upper |
| Male Gender | 0.50 | 0.13 | −0.15 | 1.15 | ||||
| Age (years) | −0.02 | 0.14 | −0.04 | 0.01 | −0.02 | 0.05 | −0.34 | 0.0004 |
| Maximum Weight (kg) | 0.003 | 0.34 | −0.003 | 0.008 | ||||
| Deep vein reflux | 0.45 | 0.21 | −0.25 | 1.16 | ||||
| Previous deep vein thrombosis | −0.04 | 0.91 | −0.73 | 0.65 | ||||
| History of smoking, current smoking | 0.34 | 0.30 | −0.31 | 1.002 | ||||
| Diabetes mellitus | 0.90 | 0.07 | −0.07 | 1.87 | ||||
| Hyperlipidemia | −0.002 | 0.99 | −0.67 | 0.66 | ||||
| Hypertension | 0.49 | 0.19 | −0.24 | 1.21 | 0.78 | 0.04 | 0.01 | 1.54 |
| Taking Coumadin | 0.37 | 0.31 | −0.35 | 1.07 | ||||
| Deep vein thrombosis after UGS IPV | −0.66 | 0.84 | −1.16 | 0.94 | ||||
| Last UGS IPV injection a success | −0.0003 | 0.999 | −0.78 | 0.78 | ||||
| Initial follow up to time to first UGS IPV (days) | 0.0004 | 0.01 | 0.0001 | 0.001 | ||||
| Number of UGS IPV | 0.05 | 0.50 | −0.09 | 0.18 | ||||
| Initial follow up to last follow up (days) | 0.0004 | 0.005 | 0.0001 | 0.0006 | 0.0003 | 0.02 | 0.00005 | 0.0006 |
| % success UGS IPV | −0.58 | 0.19 | −1.47 | 0.30 | ||||
| Initial Ulcer Area (cm2) | −0.004 | 0.54 | −0.02 | 0.009 | ||||
Ulcer healing was predicted by IPV thrombosis. For each 10% increase in IPV thrombosis, we saw a 16% increase in the odds of healed venous ulcer status at the end of follow-up (OR 4.31, CI 1.04–17.95, p=.04). Patients with complete IPV closure (thrombosis of all perforating veins in a limb) on last UGS had a 3.5 times greater chance of ulcer healing compared to failure of complete closure (OR 4.50, CI 1.23–16.51, p=.02). Multivariable modeling demonstrated IPV complete closure positively predicted ultimate ulcer healing (OR 4.87, CI 1.28–18.5, p=.02), while each increase in cm2 initial ulcer area negatively predicted ultimate ulcer healing (OR 0.92, CI .83–1.0, p=.08). Increased age at initial visit predicted fewer recurrences of ulcers in the multivariable model, while increased follow-up time and hypertension were seen with increased ulcer recurrence.
Variables that negatively influenced ulcer healing on univariate analysis included: additional perforator injections, (OR .82, CI .65–1.03, p=.1) and increased initial ulcer size (OR .92, CI .84–1.01, p=.1). Time from initial visit to first perforator injection appeared to predict recurrence of ulcers. Each year of ulcer existence prior to injection predicted 0.14 more recurrences of ulceration (p=.01). In addition, each additional year of follow-up after injection predicted 0.14 ulcer recurrences (P=.01).
Follow-Up
Overall median follow-up (FU) + interquartile range (25th to 75th percentile) was 33.5 (8.9–79.9) months, and median FU + interquartile range for patients in various healing groups broke down as: healed ulcers = 12.2 (5.7–38.6) months, recurrent ulcers which were healed at last follow-up = 94.7 (60.3–103.0), ulcers which healed, recurred and were open at last follow-up = 71.0 (33.6–93.9), ulcers that never healed = 18.1 (6.1–44.7). We grouped the two recurrent ulcer groups together in comparison to ulcers that either healed without recurrence or never healed in a mixed effects model, which demonstrated that total follow-up time was strongly associated with recurrence. Each additional year of follow-up was associated with a 56% increase in chance of recurrence (p=.001). Other factors that were associated with ulcer recurrence were: younger patient age (p=.05) and hypertension (p= .04).
Because follow-up time strongly predicted recurrence, healing status is likely influenced by how long the patients’ ulcers were followed and reflects fluidity in the healing of this population. A multivariable logistic regression was run on 62 ulcers in 62 patients, comparing never healed ulcers with those ulcers that healed and/or recurred, in an effort to ascertain if there were factors that appeared to prohibit ulcer healing at any time point. Closure of IPVs and follow-up predicted increased ulcer healing (p=.04 and p=.09). If recurrent ulcers were analyzed separately from healed ulcers, multivariable logistic regression analysis revealed diuretic use (p=.07), more UGS IPV injections (p=.08), and longer follow-up time (p=.01) were associated with recurrence.
DISCUSSION
Despite the proven efficacy of compression therapy 5, 6, 22–26, a subset of compression-treated patients cannot heal venous ulcers despite strict compliance. Correction of great saphenous vein reflux is associated with significant ulcer healing and decreases in ulcer recurrence. 25 For ulcers that persist, minimally invasive elimination of pathologic perforating veins near the ulcer increases healing rates and may decrease recurrence with few wound complications and high rates of technical success. 10, 14, 27 Patients with healed ulcers that were previously treated with ablation of IPVs and continue to maintain compression therapy have significantly reduced recurrence rates compared to compression alone. 28 However, perforator ablation requires instrumentation into or near an active ulcer, is a more difficult technique to master, and does not treat the associated varicosities fed by an IPV.
Thermal ablation of perforators has a high overall technical closure rate (approximately 80–90%).29–31 Early results of sclerotherapy by Guex et al showed a comparable 90% occlusion rate after three or fewer sessions. 32 Ultrasound-guided perforator injection is attractive in that this therapy can be delivered through a varicosity remote from the ulcer and thus decrease wound complications and procedural discomfort. Additionally, this therapy can be used to eliminate multiple pathologic perforators and their associated varicosities in one sitting. It is rapidly performed and is technically straightforward. Unlike ablative techniques, sclerotherapy is able to be performed virtually 100% of the time. Many series of ablative perforator techniques appear to report closure rates for perforators successfully cannulated, not all attempts at cannulation and ablation. Additionally, USG sclerotherapy is much less expensive and could potentially represent significant savings to the health system. Previous studies of UGS have demonstrated thrombosis rates after 3 months varying from 69% to 96%, while follow-up studies at 1 to 2 years demonstrated rates of 53% to 80% in great saphenous veins and varicose veins 33, but little work has been done to illustrate the effect of UGS on ulcer healing when performed on patients without other treatable venous pathologies.
Our study population consisted of patients who had failed compression therapy for more than 2 years prior to treatment, and had no superficial reflux. In this very difficult population, perforator thrombosis was achieved in 54% of injections, demonstrating the complexity and severity of venous disease as well as a major drawback of this technique. Physiologic reasons for a decrease in successful IPV thrombosis in comparison to axial veins includes that IPV are short, high-flow vessels, multiple perforators may feed a network of varicosities, and that many patients (>30%) are on chronic anticoagulation. Previous work has demonstrated decreased thrombosis after UGS in patients with ulcer. 15 We found warfarin use resulted in a 20% decrease in thrombosis.
Each ulcer averaged 2.67 injections. Repeated injections were performed for incomplete thrombosis of initial injection, recanalized perforators, and treatment of new/additional perforators. Each additional injection predicted 35% lower odds of the eventual total IPV thrombosis after UGS. This likely reflects two potentially overlapping populations; patients with many perforators in the ulcerated limb who required several sessions to safely treat these veins, and patients who had a lower rate of perforator thrombosis. Regardless, both groups represent more severe venous and perforator disease. We found that repeated treatments had a success rate of 45%. Although this was lower than the initial injection thrombosis rate of 66% (p=.12), ulcer healing increased with successful thrombosis of IPVs. Thus, we endorse continued perforator injection or other methods of perforator ablation in the face of initial failure until thrombosis is achieved, as this was the most significant predictor of ulcer healing.
UGS of IPVs is a safe treatment, with few complications and an easy ability to retreat in the setting of initial failure of thrombosis. After polidocanol (POL) was approved by the FDA as a sclerosant, we changed from STS due to evidence indicating POL may have a better safety protocol, be as or more effective than STS, and be better tolerated. 34 Our incidence of DVT was low and comparable to other known studies. 15, 35–37 Side effects of perforator ablation include ecchymosis, induration, and pain in the majority of cases, while paresthesias, hyperpigmentation, and phlebitis occur in the minority of cases. 27, 38, 39 Multiple needle punctures during sclerotherapy can lead to vasospasm or hematoma, but in our population, the common side effects seen during ablation were minimal. Patients tolerated sclerotherapy much more comfortably than ablation therapy and often recover faster. 40, 41 Thrombosis of incompetent perforator veins was the most powerful predictor of venous ulcer healing, and ulcer healing was achieved in more than 50% of patients. This was a population of patients who had suffered with venous stasis wound(s) for years and had few remaining therapeutic options. Large initial ulcer area, however, predictably demonstrated a lower chance of ultimate healed ulcer, even with successful perforator thrombosis.
Healing status of ulcers was determined at an arbitrarily defined study end period, and thus, recurrence of venous ulcers incorporates the time-dependent nature of the disease. Our data demonstrate recurrence of ulcers was significantly predicted by length of follow-up, a finding that is consistent with the natural history of venous ulceration and represents a selection bias in that patients who heal are less likely to return to the clinic even when prompted. 42 Increased ulceration with long-term follow-up also speaks to the nature of new perforating veins appearing in the at-risk areas, or the occurrence of late recanalizations. Unfortunately, our largely retrospective review did not provide adequate data on the exact perforator locations to enable accurate reporting of whether new injections a significant time after demonstrated thrombosis represented de novo perforators versus recanalization. We did find that continued therapy and repeat injections with IPV thrombosis led to healing. Thus, improving comorbidities such as hypertension, continued use of compressive therapy and aggressive surveillance and perforator ablation may all contribute to maintenance of ulcer healing.
This study has a number of limitations. The most important is the largely retrospective nature of the review. Variances in long-term follow-up have prohibited standardized healing curves. Our small sample size, combined with high variance in initial ulcer sizes and heterogeneity in recurrence rates precluded cumulative analysis of healing rates of all ulcers.
Due to the inconsistency of quality of life data being collected on patients, it was not valuable in our current analysis. Similarly, it was not possible to control for wound care methods. Ointments and exact methods of compression (Unna’s boot vs. short-stretch bandages, e.g.) often changed based on patient preferences, and ability to comply with the prescribed regimen and perceived success of the current therapy. In addition, although detailed documentation of perforator location was recorded during initial ultrasound evaluation and foam injection, variability in ultrasound sonographers may make follow-up comparison difficult in determining whether a suspected perforator recurrence was newly formed or indeed recanalized.
CONCLUSION
Ultrasound-guided injection of refluxing perforator veins in CEAP 6 patients was found to be safe and to predict ulcer healing. Thrombosis of pathologic perforators was the most powerful predictor of ulcer healing in our analysis. Perforator closure may require multiple injections but is associated with low complication rates. Foam sclerotherapy, or other methods of perforator closure, are therefore recommended for treatment of non-healing venous ulcers.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Ibegbuna V, Delis KT, Nicolaides AN. Haemodynamic and clinical impact of superficial, deep and perforator vein incompetence. Eur J Vasc Endovasc Surg. 2006;31(5):535–41. doi: 10.1016/j.ejvs.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 2.Erickson CA, Lanza DJ, Karp DL, Edwards JW, Seabrook GR, Cambria RA, et al. Healing of venous ulcers in an ambulatory care program: the roles of chronic venous insufficiency and patient compliance. J Vasc Surg. 1995;22(5):629–36. doi: 10.1016/s0741-5214(95)70051-x. [DOI] [PubMed] [Google Scholar]
- 3.Gloviczki P, Gloviczki ML. Evidence on efficacy of treatments of venous ulcers and on prevention of ulcer recurrence. Perspect Vasc Surg Endovasc Ther. 2009;21(4):259–68. doi: 10.1177/1531003510373660. [DOI] [PubMed] [Google Scholar]
- 4.Milic DJ, Zivic SS, Bogdanovic DC, Perisic ZD, Milosevic ZD, Jankovic RJ, et al. A randomized trial of the Tubulcus multilayer bandaging system in the treatment of extensive venous ulcers. J Vasc Surg. 2007;46(4):750–5. doi: 10.1016/j.jvs.2007.04.062. [DOI] [PubMed] [Google Scholar]
- 5.Mayberry JC, Moneta GL, Taylor LM, Jr, Porter JM. Fifteen-year results of ambulatory compression therapy for chronic venous ulcers. Surgery. 1991;109(5):575–81. [PubMed] [Google Scholar]
- 6.Partsch H, Flour M, Smith PC. Indications for compression therapy in venous and lymphatic disease consensus based on experimental data and scientific evidence. Under the auspices of the IUP. Int Angiol. 2008;27(3):193–219. [PubMed] [Google Scholar]
- 7.Guyatt G, Gutterman D, Baumann MH, Addrizzo-Harris D, Hylek EM, Phillips B, et al. Grading strength of recommendations and quality of evidence in clinical guidelines: report from an american college of chest physicians task force. Chest. 2006;129(1):174–81. doi: 10.1378/chest.129.1.174. [DOI] [PubMed] [Google Scholar]
- 8.Korn P, Patel ST, Heller JA, Deitch JS, Krishnasastry KV, Bush HL, et al. Why insurers should reimburse for compression stockings in patients with chronic venous stasis. J Vasc Surg. 2002;35(5):950–7. doi: 10.1067/mva.2002.121984. [DOI] [PubMed] [Google Scholar]
- 9.Linton RR. The Communicating Veins of the Lower Leg and the Operative Technic for Their Ligation. Ann Surg. 1938;107(4):582–93. doi: 10.1097/00000658-193804000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gloviczki P, Cambria RA, Rhee RY, Canton LG, McKusick MA. Surgical technique and preliminary results of endoscopic subfascial division of perforating veins. J Vasc Surg. 1996;23(3):517–23. doi: 10.1016/s0741-5214(96)80020-6. [DOI] [PubMed] [Google Scholar]
- 11.Tenbrook JA, Jr, Iafrati MD, O’Donnell TF, Jr, Wolf MP, Hoffman SN, Pauker SG, et al. Systematic review of outcomes after surgical management of venous disease incorporating subfascial endoscopic perforator surgery. J Vasc Surg. 2004;39(3):583–9. doi: 10.1016/j.jvs.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 12.Lang W. Development of perforator vein surgery from the Linton and Cockett procedure to endoscopic dissection. Zentralbl Chir. 2001;126(7):495–500. doi: 10.1055/s-2001-16274. [DOI] [PubMed] [Google Scholar]
- 13.Lee DW, Chan AC, Lam YH, Wong SK, Fung TM, Mui LM, et al. Early clinical outcomes after subfascial endoscopic perforator surgery (SEPS) and saphenous vein surgery in chronic venous insufficiency. Surg Endosc. 2001;15(7):737–40. doi: 10.1007/s004640090050. [DOI] [PubMed] [Google Scholar]
- 14.Harlander-Locke M, Lawrence PF, Alktaifi A, Jimenez JC, Rigberg D, DeRubertis B. The impact of ablation of incompetent superficial and perforator veins on ulcer healing rates. J Vasc Surg. 55(2):458–64. doi: 10.1016/j.jvs.2011.08.054. [DOI] [PubMed] [Google Scholar]
- 15.Masuda EM, Kessler DM, Lurie F, Puggioni A, Kistner RL, Eklof B. The effect of ultrasound-guided sclerotherapy of incompetent perforator veins on venous clinical severity and disability scores. J Vasc Surg. 2006;43(3):551–6. doi: 10.1016/j.jvs.2005.11.038. discussion 556–7. [DOI] [PubMed] [Google Scholar]
- 16.Burnand K, Thomas ML, O’Donnell T, Browse NL. Relation between postphlebitic changes in the deep veins and results of surgical treatment of venous ulcers. Lancet. 1976;1(7966):936–8. doi: 10.1016/s0140-6736(76)92714-8. [DOI] [PubMed] [Google Scholar]
- 17.Gohel MS, Taylor M, Earnshaw JJ, Heather BP, Poskitt KR, Whyman MR. Risk factors for delayed healing and recurrence of chronic venous leg ulcers--an analysis of 1324 legs. Eur J Vasc Endovasc Surg. 2005;29(1):74–7. doi: 10.1016/j.ejvs.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 18.Kulkarni SR, Barwell JR, Gohel MS, Bulbulia RA, Whyman MR, Poskitt KR. Residual venous reflux after superficial venous surgery does not predict ulcer recurrence. Eur J Vasc Endovasc Surg. 2007;34(1):107–11. doi: 10.1016/j.ejvs.2006.12.033. [DOI] [PubMed] [Google Scholar]
- 19.Magnusson MB, Nelzen O, Volkmann R. Leg ulcer recurrence and its risk factors: a duplex ultrasound study before and after vein surgery. Eur J Vasc Endovasc Surg. 2006;32(4):453–61. doi: 10.1016/j.ejvs.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 20.Padberg FT., Jr CEAP classification for chronic venous disease. Dis Mon. 2005;51(2–3):176–82. doi: 10.1016/j.disamonth.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 21.Tessari L, Cavezzi A, Frullini A. Preliminary experience with a new sclerosing foam in the treatment of varicose veins. Dermatol Surg. 2001;27(1):58–60. [PubMed] [Google Scholar]
- 22.van Gent WB, Hop WC, van Praag MC, Mackaay AJ, de Boer EM, Wittens CH. Conservative versus surgical treatment of venous leg ulcers: a prospective, randomized, multicenter trial. J Vasc Surg. 2006;44(3):563–71. doi: 10.1016/j.jvs.2006.04.053. [DOI] [PubMed] [Google Scholar]
- 23.O’Meara S, Cullum NA, Nelson EA. Compression for venous leg ulcers. Cochrane Database Syst Rev. 2009;(1):CD000265. doi: 10.1002/14651858.CD000265.pub2. [DOI] [PubMed] [Google Scholar]
- 24.Morrell CJ, Walters SJ, Dixon S, Collins KA, Brereton LM, Peters J, et al. Cost effectiveness of community leg ulcer clinics: randomised controlled trial. BMJ. 1998;316(7143):1487–91. doi: 10.1136/bmj.316.7143.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barwell JR, Davies CE, Deacon J, Harvey K, Minor J, Sassano A, et al. Comparison of surgery and compression with compression alone in chronic venous ulceration (ESCHAR study): randomised controlled trial. Lancet. 2004;363(9424):1854–9. doi: 10.1016/S0140-6736(04)16353-8. [DOI] [PubMed] [Google Scholar]
- 26.Howard DP, Howard A, Kothari A, Wales L, Guest M, Davies AH. The role of superficial venous surgery in the management of venous ulcers: a systematic review. Eur J Vasc Endovasc Surg. 2008;36(4):458–65. doi: 10.1016/j.ejvs.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 27.Lawrence PF, Alktaifi A, Rigberg D, DeRubertis B, Gelabert H, Jimenez JC. Endovenous ablation of incompetent perforating veins is effective treatment for recalcitrant venous ulcers. J Vasc Surg. 54(3):737–42. doi: 10.1016/j.jvs.2011.02.068. [DOI] [PubMed] [Google Scholar]
- 28.Harlander-Locke M, Lawrence P, Jimenez JC, Rigberg D, DeRubertis B, Gelabert H. Combined treatment with compression therapy and ablation of incompetent superficial and perforating veins reduces ulcer recurrence in patients with CEAP 5 venous disease. J Vasc Surg. 55(2):446–50. doi: 10.1016/j.jvs.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 29.Proebstle TM, Herdemann S. Early results and feasibility of incompetent perforator vein ablation by endovenous laser treatment. Dermatol Surg. 2007;33(2):162–8. doi: 10.1111/j.1524-4725.2006.33034.x. [DOI] [PubMed] [Google Scholar]
- 30.Ozkan U. Endovenous laser ablation of incompetent perforator veins: a new technique in treatment of chronic venous disease. Cardiovasc Intervent Radiol. 2009;32(5):1067–70. doi: 10.1007/s00270-009-9646-z. [DOI] [PubMed] [Google Scholar]
- 31.Peden E, Lumsden A. Radiofrequency ablation of incompetent perforator veins. Perspect Vasc Surg Endovasc Ther. 2007;19(1):73–7. doi: 10.1177/1531003507299478. [DOI] [PubMed] [Google Scholar]
- 32.Guex JJ. Ultrasound guided sclerotherapy (USGS) for perforating veins (PV) Hawaii Med J. 2000;59(6):261–2. [PubMed] [Google Scholar]
- 33.Stucker M, Kobus S, Altmeyer P, Reich-Schupke S. Review of published information on foam sclerotherapy. Dermatol Surg. 36(Suppl 2):983–92. doi: 10.1111/j.1524-4725.2009.01406.x. [DOI] [PubMed] [Google Scholar]
- 34.Rabe E, Schliephake D, Otto J, Breu FX, Pannier F. Sclerotherapy of telangiectases and reticular veins: a double-blind, randomized, comparative clinical trial of polidocanol, sodium tetradecyl sulphate and isotonic saline (EASI study) Phlebology. 25(3):124–31. doi: 10.1258/phleb.2009.009043. [DOI] [PubMed] [Google Scholar]
- 35.Cabrera J, Redondo P, Becerra A, Garrido C, Cabrera J, Jr, Garcia-Olmedo MA, et al. Ultrasound-guided injection of polidocanol microfoam in the management of venous leg ulcers. Arch Dermatol. 2004;140(6):667–73. doi: 10.1001/archderm.140.6.667. [DOI] [PubMed] [Google Scholar]
- 36.Darvall KA, Bate GR, Adam DJ, Silverman SH, Bradbury AW. Ultrasound-guided foam sclerotherapy for the treatment of chronic venous ulceration: a preliminary study. Eur J Vasc Endovasc Surg. 2009;38(6):764–9. doi: 10.1016/j.ejvs.2009.05.027. [DOI] [PubMed] [Google Scholar]
- 37.Bergan J, Pascarella L, Mekenas L. Venous disorders: treatment with sclerosant foam. J Cardiovasc Surg (Torino) 2006;47(1):9–18. [PubMed] [Google Scholar]
- 38.Roth SM. Endovenous radiofrequency ablation of superficial and perforator veins. Surg Clin North Am. 2007;87(5):1267–84. xii. doi: 10.1016/j.suc.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 39.Rueda CA, Bittenbinder EN, Buckley CJ, Bohannon WT, Atkins MD, Bush RL. The management of chronic venous insufficiency with ulceration: the role of minimally invasive perforator interruption. Ann Vasc Surg. 27(1):89–95. doi: 10.1016/j.avsg.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 40.Guex JJ. Complications and side-effects of foam sclerotherapy. Phlebology. 2009;24(6):270–4. doi: 10.1258/phleb.2009.009049. [DOI] [PubMed] [Google Scholar]
- 41.Darvall KA, Bate GR, Adam DJ, Bradbury AW. Recovery after ultrasound-guided foam sclerotherapy compared with conventional surgery for varicose veins. Br J Surg. 2009;96(11):1262–7. doi: 10.1002/bjs.6754. [DOI] [PubMed] [Google Scholar]
- 42.Vasquez MA, Munschauer CE. Venous Clinical Severity Score and quality-of-life assessment tools: application to vein practice. Phlebology. 2008;23(6):259–75. doi: 10.1258/phleb.2008.008018. [DOI] [PubMed] [Google Scholar]