Abstract
Objective
Although metastasis of hepatocellular carcinoma to the brain is uncommon, it is associated with a very high mortality rate and most patients usually expire within 1 year after brain metastasis. The aim of this study is to identify the effectiveness of the active interventions such as gamma knife radiosurgery or surgical intervention for these patients.
Methods
We retrospectively reviewed the medical records and imaging data of 59 patients with metastatic brain tumors from hepatocellular carcinoma from May 2004 to September 2012. The study included patients with available clinical and radiological data who had been diagnosed with metastatic hepatocellular carcinoma of the brain, confirmed by magnetic resonance imaging. The overall survival time was analyzed and compared according to each risk factor.
Results
The mean age at diagnosis of metastatic brain tumor was 52.2 years (14-77). The mean follow-up duration was 13.3 weeks (0.1-117.6). Overall median survival was 4.3 weeks (95% confidence interval, 2.2-6.4). The results from an analysis of clinical factors related to survival revealed that treatment modalities were significantly related to the patient's survival (log rank, p=0.006). Twenty patients (32.8%) experienced tumor bleeding, and the survival time of the patients with tumor bleeding tended to be shorter, although the result was not statistically significant (log rank, p=0.058). Hepatic reserve, by Child-Pugh classification, was grade A in 38 patients (64.4%), grade B in 16 patients (27.1%), and grade C in 5 patients (8.5%), and was significantly related to the patient's survival (log rank, p=0.000).
Conclusion
Although patients with metastatic brain tumors from hepatocellular carcinoma showed poor survival, active intervention including surgical resection or gamma knife radiosurgery may result in better survival, especially if patients have preserved liver function.
Keywords: Brain tumor, Hepatocellular carcinoma, Metastasis, Radiation therapy, Radiosurgery, Surgery
INTRODUCTION
Hepatocellular carcinoma (HCC) represents one of the most common causes of cancer related deaths world-wide [1]. The incidence of HCC demonstrates a striking geographic variability, with the highest rates in East and South-East Asia and Sub-Saharan Africa [1]. As Korea is an area of endemic Hepatitis B Virus, HCC is the third most common cancer and the third leading cause of cancer deaths in Korea.
Brain metastasis is the most common intracranial neoplasm in adults and is associated with significant morbidity and mortality. Considerable research has focused on improving both survival and quality of life for these patients. However, brain metastasis from HCC is extremely rare, with a reported frequency ranging from 0.2% to 2.2% [2-6]. Though uncommon, it is associated with a very high mortality rate and most patients die within 1 year [7-10].
The identification of prognostic factors and optimal treatment strategies are still being researched. We analyzed the treatment results of metastatic brain tumors from HCC, and compared the prognosis according to the treatment modality.
MATERIALS AND METHODS
From May 2004 to September 2012, a total of 182 patients were diagnosed with brain metastasis from HCC at our hospital. We retrospectively reviewed the clinical and imaging data. The medical records of these patients were reviewed and data collected regarding patient characteristics, clinical presentation, treatment, and clinical course. In all patients, brain metastases were diagnosed by computed tomography (CT) scans and magnetic resonance imaging (MRI). The study included patients with available clinical and radiological data who had been diagnosed with metastatic hepatocellular carcinoma of the brain, confirmed by MRI. A total of 59 patients met the inclusion critera and were enrolled. Overall survival time after diagnosis of brain metastasis was analyzed and compared according to risk factors such as gender, presenting symptoms, presence of tumor bleeding, and treatment modalities.
Treatment modalities for brain metastasis from HCC were surgical resection, gamma knife radiosurgery (GKS), whole brain radiation therapy (WBRT), and best supportive care (BSC). Based on T1 weighted images with gadolinium enhancement and T2 weighted images, GKS was performed using Leksell Gamma Knife type C or Perfexion (Elekta Instrument AB, Stockholm, Sweden). Mean GKS dosage was 15.3 Gy (range 14.5-20) at marginal dose. WBRT dosage range was 30 Gy to 45 Gy with 10 to 15 fractions. Supportive care included intravenous injections of steroid and hyperosmolar solutions.
Overall survival time and factors affecting survival time were analyzed. These were estimated according to the Kaplan-Meier method and measured from the date of diagnosis of brain metastasis until the date of last follow-up or until death. Survival curves were compared using the log-rank test, with p<0.05 considered statistically significant. Statistical analyses were performed using SPSS software version 13.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
Characteristics of patients
Patients' mean age at diagnosis of brain metastasis was 52.2 years (range 14-44). Mean follow-up duration was 13.4 weeks (0.1-117.6). Among 59 patients, 49 patients (83%) were male, 10 patients (17%) were female. Among 59 patients, hepatic reserve, by Child-Pugh classification, was grade A in 38 patients (64.4%), grade B in 16 patients (27.1%), and grade C in 5 patients (8.5%). Twenty patients (33.9%) experienced tumor bleeding. There were four types of initial treatment modality performed in our patients. Seventeen patients (28.8%) received supportive care. Twenty patients (33.9%) were treated with GKS, 14 patients (23.7%) were treated with surgical resection, and 8 patients (13.6%) were treated with WBRT. Fifty-one patients (86.4%) had symptoms such as motor weakness and mental status change. Fifty-five patients (93.2%) had other extracranial metastasis and 4 patients (6.7%) had no other extracranial metastasis. Among 59 patients, recursive partitioning analysis (RPA) classification was class A in 1 patient (1.6%), class B in 16 patients (59.3%), and class C in 5 patients (38.9%). Clinical characteristics of the patients are summarized in Table 1. There was no statistically significant difference among patients group by initial treatment modality (Table 2).
Table 1.
Clinical characteristics of patients
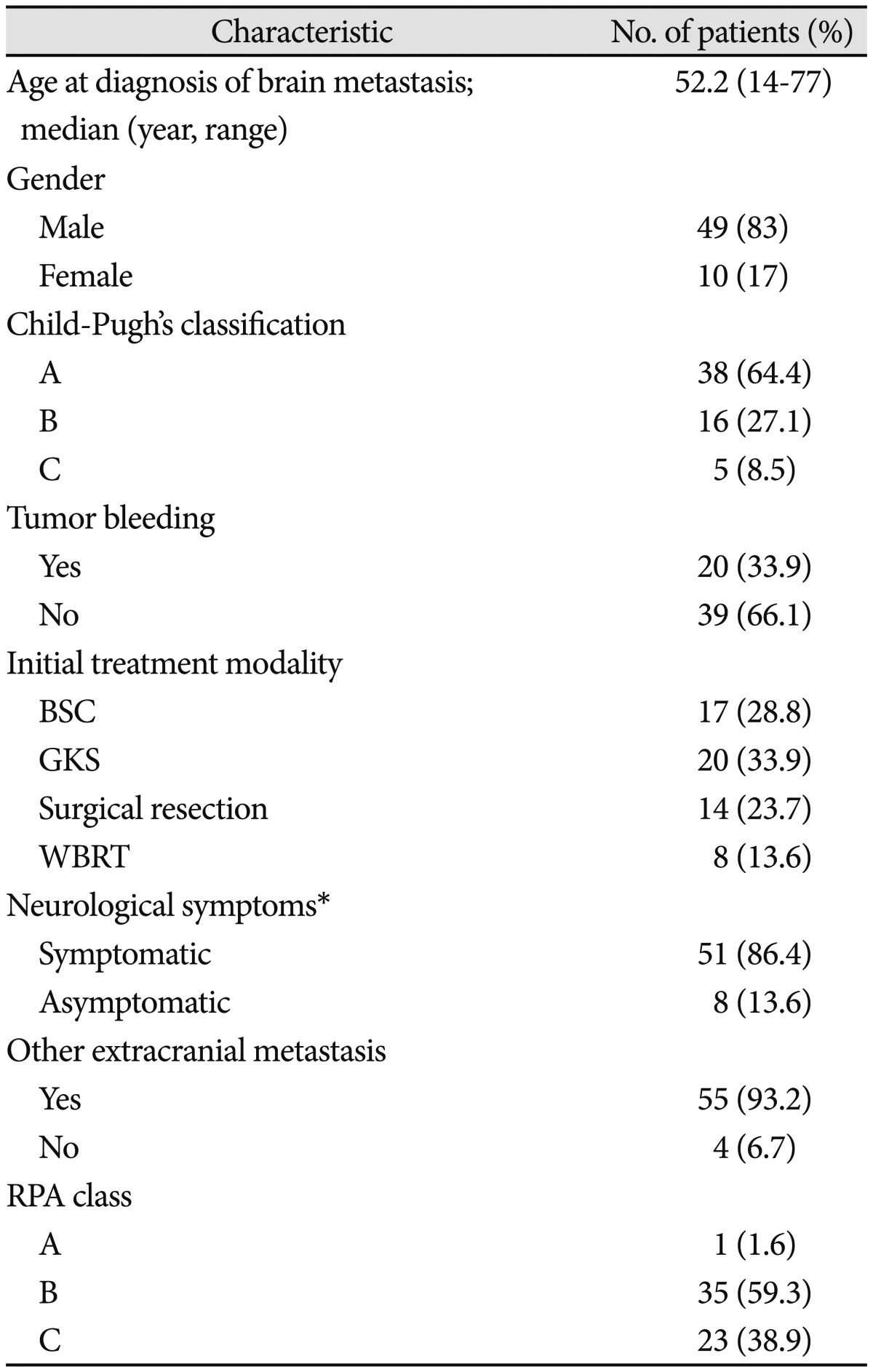
*Including headache, decreased mental status, weakness and so on. BSC: best supportive care, GKS: gamma knife radiosurgery, WBRT: whole brain radiation therapy, RPA: recursive partitioning analysis
Table 2.
Clinical characteristics of patients by initial treatment modality
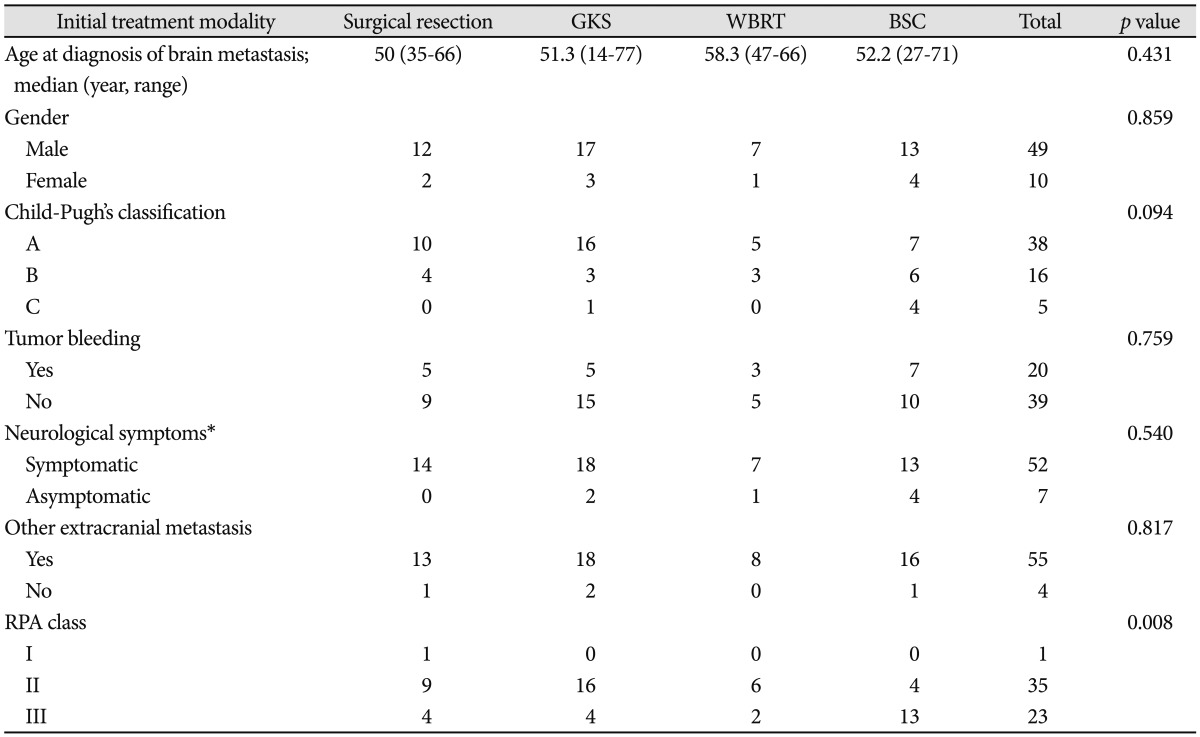
*Including headache, decreased mental status, weakness and so on. BSC: best supportive care, GKS: gamma knife radiosurgery, WBRT: whole brain radiation therapy, RPA: recursive partitioning analysis
Overall Survival time and prognostic factors
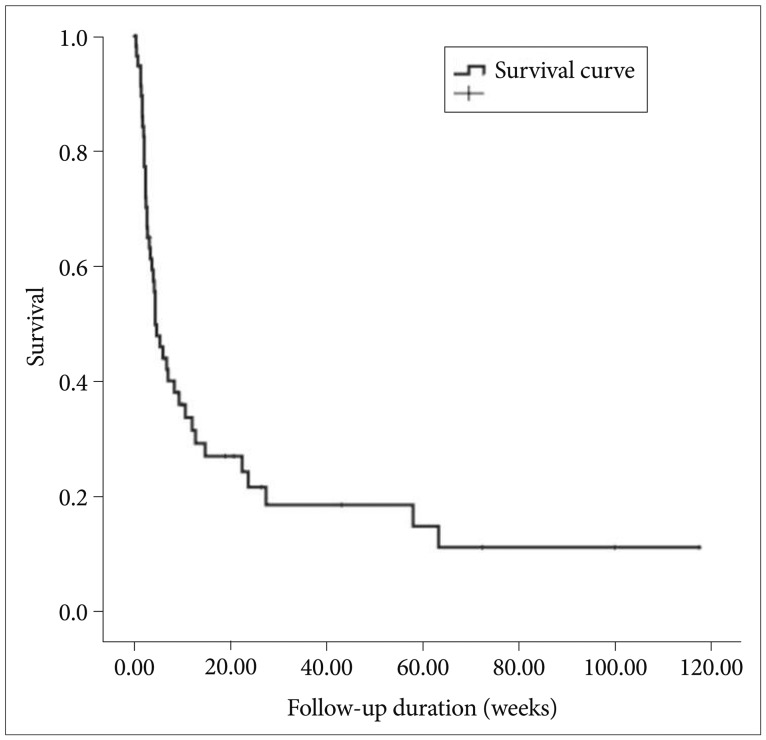
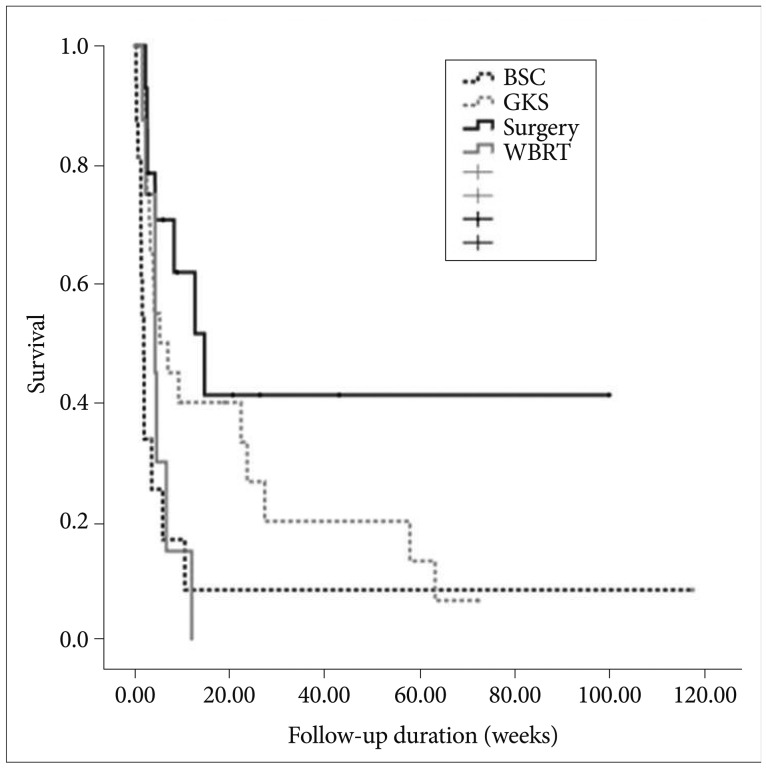
Median overall survival after diagnosis of brain metastases was 4.3 weeks (95% confidence interval 2.2-6.3 weeks) (Fig. 1). Overall survival was analyzed according to patient characteristics (Table 3). The median survival varied by initial treatment modality. Median survival was for patients treated with WBRT was 4.3 weeks, and the median survival for patients treated with GKS was 5.3 weeks. Patients receiving surgical resection had a median survival of 14.7 weeks, and those receiving supportive care had a median survival of 1.9 weeks. These results reveal that initial treatment modalities impacted the patient's survival (log rank, p=0.006) (Fig. 2). After surgical resection, all patients were planned to undergo tumor bed GKS or WBRT as adjuvant treatment. However, only 11 patients were completed adjuvant treatment and the remaining 3 patients did not complete the treatment course because of poor neurological or systemic condition for receiving adjuvant treatment. Median survival of patients without tumor bleeding was 4.6 weeks and that of patients with tumor bleeding was 3.9 weeks. Tumor bleeding was correlated with poor outcomes, although the presence of tumor bleeding had no statistically significant difference (log rank, p=0.08).
Fig. 1.
Overall survival.
Table 3.
Overall survival time according to patient's characteristics
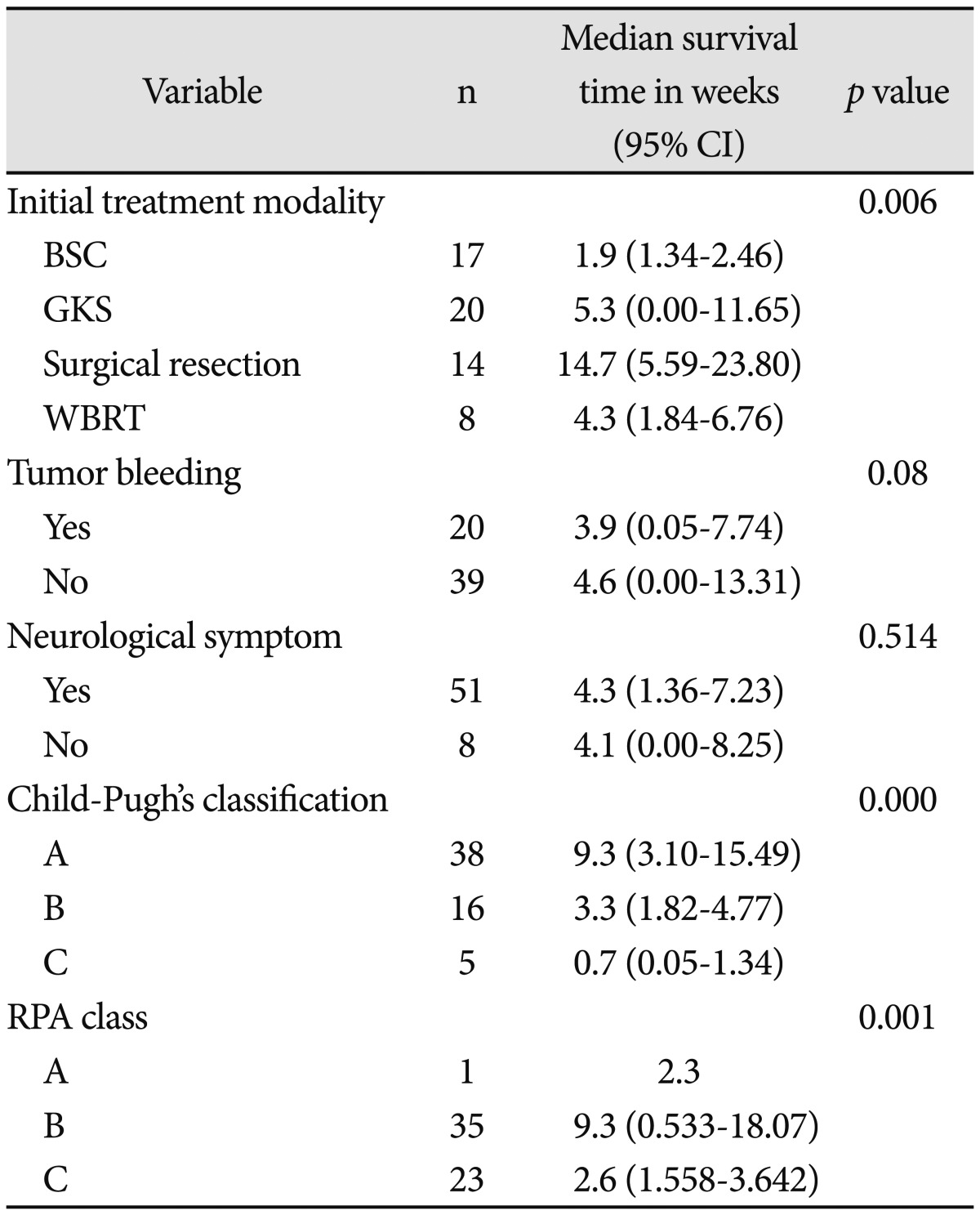
CI: confidence interval, BSC: best supportive care, GKS: gamma knife radiosurgery, WBRT: whole brain radiation therapy
Fig. 2.
Median survival by initial treatment modality (log-rank, p=0.006). BSC: best supportive care, GKS: gamma knife radiosurgery, WBRT: whole brain radiation therapy.
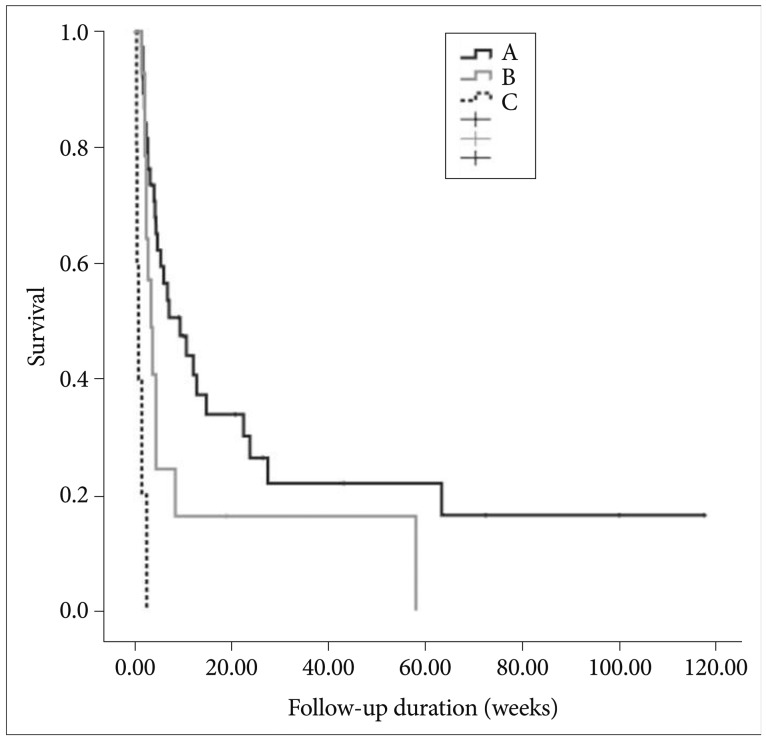
Median survival of patients without neurological symptom was 4.1 weeks, while that of patients with neurological symptoms was 4.3 weeks. The presence of neurological symptoms was not statistically significant (log rank, p=0.514). With respect to hepatic reserve, by Child Pugh's classification, median survival was 9.3 weeks for Grade A patients, 3.3 weeks for Grade B patients and 0.7 weeks for Grade C patients (Fig. 3). There was a significant survival difference between Grade A or B patient groups compared to the Grade C patient groups (log rank, p=0.00).
Fig. 3.
Median survival by Child-Pugh's classification (log-rank, p=0.000).
With respect to RPA classification, the median survival was 2.3 weeks for Grade A patients, 9.3 weeks for Grade B patients and 2.6 weeks for Grade C patients. There was a significant survival difference according to RPA classification (log rank, p=0.001).
Results using Cox's proportional hazards model were described in Table 4. Child-Pugh's classification was statistically significant (p=0.000) and the odds ratio was 3.325. Initial treatment modality was statistically significant (p=0.009) and the odds ratio was 1.526. The odds ratio of RPA classification was 1.826, although there was no statistical significance (p=0.108). The other factors were not statistically significant.
Table 4.
p value and hazard ratio using Cox's proportional hazards model
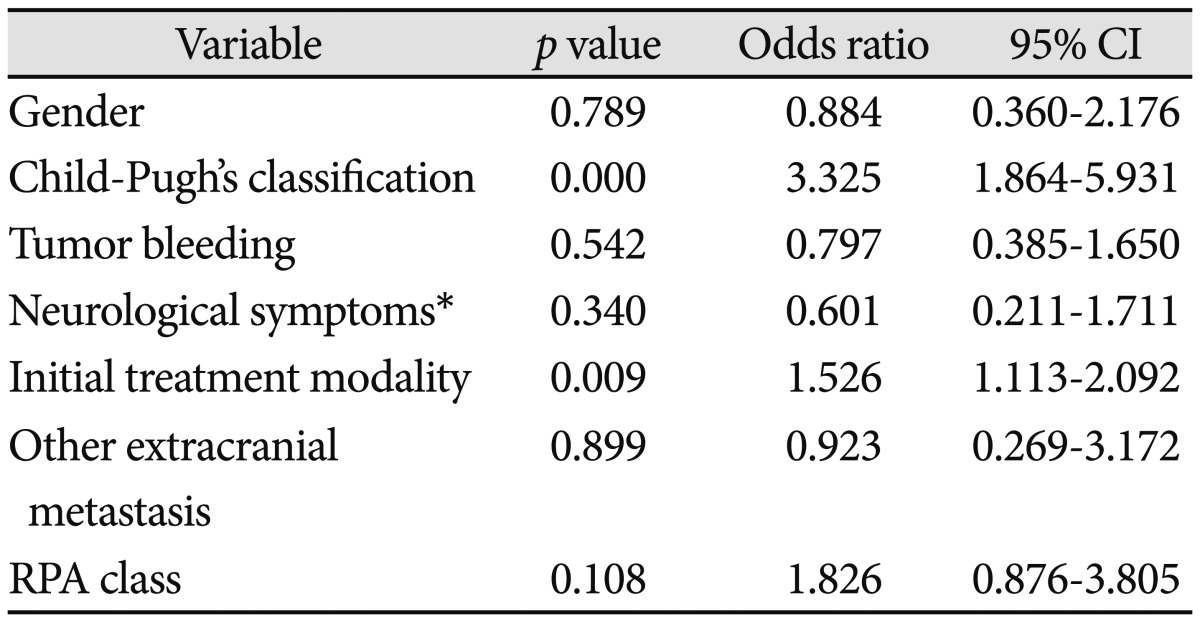
*Including headache, decreased mental status, weakness and so on. CI: confidence interval, RPA: recursive partitioning analysis
DISCUSSION
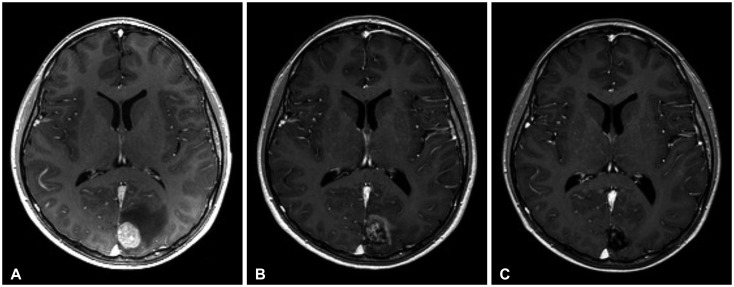
In the past, HCC was often diagnosed at an advanced stage, and therefore had a very poor prognosis [11]. However, recently, close surveillance with advanced diagnostic modalities on high-risk patients has facilitated the detection of HCC at a much earlier stage. Together with considerable advances in treatment for HCC, such as surgical resection, percutaneous ablation, transcatheter arterial chemoembolization, and liver transplantation, the survival of HCC patients has improved considerably in recent years [12-16] (Fig. 4).
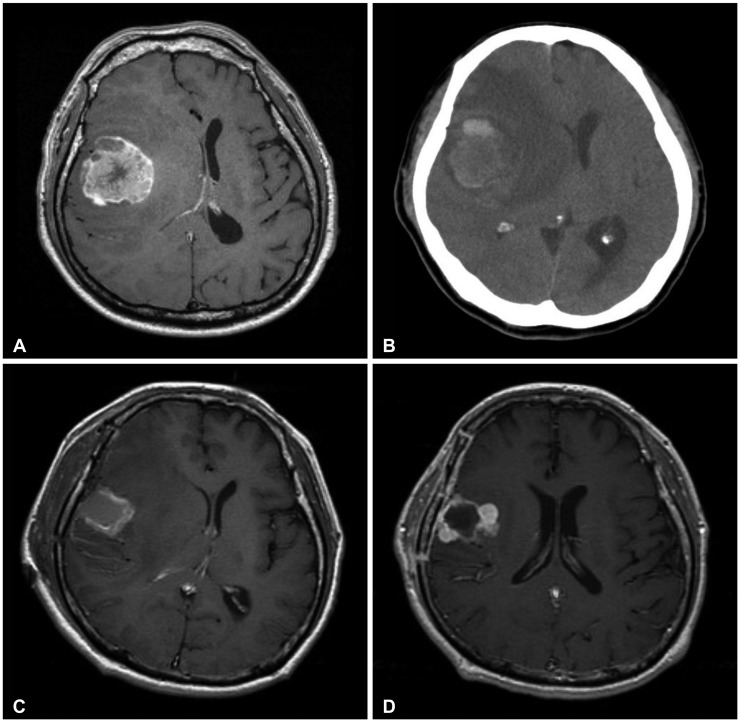
Fig. 4.
A: Preoperative MRI revealed a metastatic brain tumor at right frontal lobe with tumor bleeding. B: Preoperative CT revealed a metastatic brain tumor at right frontal lobe with tumor bleeding. C: Postoperative MRI revealed that the tumor was removed. D: Follow-up MRI (two months after operation) revealed that the tumor had recurred along the resection margin. After that, further gamma knife radiosurgery was done.
With survival time increasing due to controlled intrahepatic recurrence, the reported incidence of extra-hepatic metastasis in patients with HCC patients is gradually increasing [6,17]. Choi et al. [2] reported when HCC does produce extra-hepatic metastasis, the most common site for spread is the lung (34-70%), with additional metastatic sites including regional lymph nodes (16-45%), bone (6%), adrenal glands, and occasionally the peritoneum, pancreas, or kidney.
There are some reports on the incidence of brain metastasis from HCC. Brain parenchymal metastasis is relatively uncommon, even in Asian patients. However, with survival time increasing, several reports pertaining to brain metastasis from HCC were published, although the reported incidence varies from 0.2% to 2.2% in the literature [1,4,5]. Some studies attribute the infrequency of brain metastasis from HCC to low affinity between HCC cells and the brain [5,9,10,18,19].
Although brain metastasis from HCC is rare, the incidence of brain metastasis from HCC may be underestimated. One reason may be hepatic encephalopathy, an important finding in late-stage HCC patients that can mask neurological presentations of patients with brain metastasis from HCC [9,10,20]. Moreover, brain imaging studies are not routinely examined in most institutes. Accordingly, brain metastasis in asymptomatic patients would go undetected until they cause neurological symptoms [21]. For estimating the exact incidence, and proper diagnosis with treatment, active consideration of neuroimaging studies such as CT or MRI in HCC patients is recommended. This may improve patient's prognosis by early detection and intervention for brain metastasis from HCC.
Brain metastasis from HCC is frequently associated with tumor bleeding, because HCC is hypervascular and most patients have coagulopathy due to decreased liver function [22]. Jiang et al. [21] reported 46.3% of patients (19/41) with brain metastasis from HCC experienced tumor bleeding. In our study, 33.9% of patients (20/59) presented with hemorrhagic brain metastases. Our results showed that patients who experienced tumor bleeding had poorer outcomes (3.9 vs. 4.6 week survival time, log rank p=0.08), though the result was not statistically significant. Although these results may be caused by more severe neurological dysfunction due to increased intracranial pressure, with adequate surgical intervention, patient survival improved (Fig. 5).
Fig. 5.
A: Pre-GKS MRI revealed a metastatic brain tumor at the left occipital lobe. B: Follow-up MRI (three months after GKS) shows a decrease in the extent of the enhancing portion of the mass. C: Follow-up MRI (six month after GKS) shows no evidence of tumor recurrence. GKS: gamma knife radiosurgery.
Current study results are consistent with previous reports that the 1-year survival rate is approximately 40% for patients with brain metastasis of HCC. In our study, the median overall survival was 4.3 weeks (95% confident interval, 2.2-6.4) and the prognosis of patients with brain metastasis from HCC was poor.
Our study results revealed statistically significant differences according to initial treatment modality. Median survival time of patients who underwent surgical resection and GKS was statistically longer than for those receiving supportive care and WBRT (surgical resection group, 14.7; GKS group, 5.3 weeks; WBRT group, 4.3 weeks; BSC group, 1.9 weeks). Choi et al. [2] also reported patient groups treated with surgical resection or GKS showed better survival compared to the patient groups treated with BSC or WBRT. Chang et al. [9] found that patients with brain metastasis from HCC who underwent surgery and/or radiation therapy had a survival time of more than 4 months, while those who received only supportive care died within 1 month. The prognosis with supportive care is poor due to spread of HCC to multiple organs and repeated intracranial hemorrhage [9,23].
Also, there was a statistically significant difference based on hepatic reserve by Child-Pugh's classification. While the median survival of Child-Pugh's classification of Grade A patients was 9.3 weeks, that of Grade C patients was only 0.7 weeks. A number of authors have reported the importance of the Child-Pugh's classification score as a prognostic indicator. Considering that almost all patients with HCC have decreased liver function and that hepatic failure is a major cause of death, liver function may be a factor in the overall survival after treatment in patients with HCC [2]. Another factor affecting the survival of patients was RPA classification. RPA classification is well known prognostic factor for metastatic brain tumors [24]. In our study, RPA classification was one of the significant prognostic factors, which was similar to previous studies.
Based on these results, for patients with Child-Pugh's classification Grade A or B, RPA classification, and active treatments such as surgical resection or GKS seem more appropriate than conservative treatments such as WBRT or supportive care.
Contrary to our expectations, there was no statistically significant difference based on the presence of neurological symptoms (log rank, p=0.514). In general, the prognosis for patients with brain metastasis depended on the number of metastases and the Karnofsky performance status scale (KPS) score [25]. In our study, the presence of neurological symptoms, generally a a factor on KPS score, was not prognostic (log rank, p=0.514). This may be due to the very short survival time of patients with brain metastasis from HCC. Further study is needed to investigate this.
Using Cox's proportional hazards model, factors affecting survival time of metastatic brain tumor from HCC was analysed and p value and odds ratio was calculated. Child-Pugh's classification and initial treatment modality was significant factors affecting survival time and such odds ratio were described in Table 4.
This study found that active treatments such as surgical resection or GKS may increase the survival time of selected patients with brain metastasis from HCC. Favorable prognostic factors were Child-Pugh's classification Grade A or B. Further study regarding detail indications for active treatment seems to give longer survival time for the patients with brain metastasis from HCC.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Choi HJ, Cho BC, Sohn JH, et al. Brain metastases from hepatocellular carcinoma: prognostic factors and outcome: brain metastasis from HCC. J Neurooncol. 2009;91:307–313. doi: 10.1007/s11060-008-9713-3. [DOI] [PubMed] [Google Scholar]
- 3.Katyal S, Oliver JH, 3rd, Peterson MS, Ferris JV, Carr BS, Baron RL. Extrahepatic metastases of hepatocellular carcinoma. Radiology. 2000;216:698–703. doi: 10.1148/radiology.216.3.r00se24698. [DOI] [PubMed] [Google Scholar]
- 4.Kim M, Na DL, Park SH, Jeon BS, Roh JK. Nervous system involvement by metastatic hepatocellular carcinoma. J Neurooncol. 1998;36:85–90. doi: 10.1023/a:1005716408970. [DOI] [PubMed] [Google Scholar]
- 5.Murakami K, Nawano S, Moriyama N, et al. Intracranial metastases of hepatocellular carcinoma: CT and MRI. Neuroradiology. 1996;38(Suppl 1):S31–S35. doi: 10.1007/BF02278115. [DOI] [PubMed] [Google Scholar]
- 6.Seinfeld J, Wagner AS, Kleinschmidt-DeMasters BK. Brain metastases from hepatocellular carcinoma in US patients. J Neurooncol. 2006;76:93–98. doi: 10.1007/s11060-005-4175-3. [DOI] [PubMed] [Google Scholar]
- 7.Carr BI. Hepatocellular carcinoma: current management and future trends. Gastroenterology. 2004;127(5 Suppl 1):S218–S224. doi: 10.1053/j.gastro.2004.09.036. [DOI] [PubMed] [Google Scholar]
- 8.Lai CR, Liu HC. Hepatocellular carcinoma in Taiwan: clinicopathological study of 440 cases from a consecutive 6000 autopsies. Zhonghua Yi Xue Za Zhi (Taipei) 1993;51:249–256. [PubMed] [Google Scholar]
- 9.Chang L, Chen YL, Kao MC. Intracranial metastasis of hepatocellular carcinoma: review of 45 cases. Surg Neurol. 2004;62:172–177. doi: 10.1016/j.surneu.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 10.Chen SF, Tsai NW, Lui CC, et al. Hepatocellular carcinoma presenting as nervous system involvement. Eur J Neurol. 2007;14:408–412. doi: 10.1111/j.1468-1331.2007.01681.x. [DOI] [PubMed] [Google Scholar]
- 11.Okuda K, Ohtsuki T, Obata H, et al. Natural history of hepatocellular carcinoma and prognosis in relation to treatment. Study of 850 patients. Cancer. 1985;56:918–928. doi: 10.1002/1097-0142(19850815)56:4<918::aid-cncr2820560437>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 12.Arii S, Yamaoka Y, Futagawa S, et al. The Liver Cancer Study Group of Japan. Results of surgical and nonsurgical treatment for small-sized hepatocellular carcinomas: a retrospective and nationwide survey in Japan. Hepatology. 2000;32:1224–1229. doi: 10.1053/jhep.2000.20456. [DOI] [PubMed] [Google Scholar]
- 13.Shiina S, Teratani T, Obi S, et al. A randomized controlled trial of radiofrequency ablation with ethanol injection for small hepatocellular carcinoma. Gastroenterology. 2005;129:122–130. doi: 10.1053/j.gastro.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 14.Shiina S, Tagawa K, Niwa Y, et al. Percutaneous ethanol injection therapy for hepatocellular carcinoma: results in 146 patients. AJR Am J Roentgenol. 1993;160:1023–1028. doi: 10.2214/ajr.160.5.7682378. [DOI] [PubMed] [Google Scholar]
- 15.Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 16.Llovet JM, Real MI, Montaña X, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734–1739. doi: 10.1016/S0140-6736(02)08649-X. [DOI] [PubMed] [Google Scholar]
- 17.Kanda M, Tateishi R, Yoshida H, et al. Extrahepatic metastasis of hepatocellular carcinoma: incidence and risk factors. Liver Int. 2008;28:1256–1263. doi: 10.1111/j.1478-3231.2008.01864.x. [DOI] [PubMed] [Google Scholar]
- 18.El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999;340:745–750. doi: 10.1056/NEJM199903113401001. [DOI] [PubMed] [Google Scholar]
- 19.Yang WT, Yeo W, Leung SF, Chan YL, Johnson PJ, Metreweli C. MRI and CT of metastatic hepatocellular carcinoma causing spinal cord compression. Clin Radiol. 1997;52:755–760. doi: 10.1016/s0009-9260(97)80154-7. [DOI] [PubMed] [Google Scholar]
- 20.Hsieh MJ, Lu CH, Tsai NW, et al. Prediction, clinical characteristics and prognosis of intracerebral hemorrhage in hepatocellular carcinoma patients with intracerebral metastasis. J Clin Neurosci. 2009;16:394–398. doi: 10.1016/j.jocn.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 21.Jiang XB, Ke C, Zhang GH, et al. Brain metastases from hepatocellular carcinoma: clinical features and prognostic factors. BMC Cancer. 2012;12:49. doi: 10.1186/1471-2407-12-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uchino K, Tateishi R, Shiina S, et al. Hepatocellular carcinoma with extrahepatic metastasis: clinical features and prognostic factors. Cancer. 2011;117:4475–4483. doi: 10.1002/cncr.25960. [DOI] [PubMed] [Google Scholar]
- 23.Parkin DM. Global cancer statistics in the year 2000. Lancet Oncol. 2001;2:533–543. doi: 10.1016/S1470-2045(01)00486-7. [DOI] [PubMed] [Google Scholar]
- 24.Chen PG, Lee SY, Barnett GH, et al. Use of the Radiation Therapy Oncology Group recursive partitioning analysis classification system and predictors of survival in 19 women with brain metastases from ovarian carcinoma. Cancer. 2005;104:2174–2180. doi: 10.1002/cncr.21472. [DOI] [PubMed] [Google Scholar]
- 25.Chang WS, Kim HY, Chang JW, Park YG, Chang JH. Analysis of radiosurgical results in patients with brain metastases according to the number of brain lesions: is stereotactic radiosurgery effective for multiple brain metastases. J Neurosurg. 2010;113(Suppl):73–78. doi: 10.3171/2010.8.GKS10994. [DOI] [PubMed] [Google Scholar]