Abstract
Objective
Malignant meningiomas are rare and have worse prognosis than benign meningiomas. We report our experience of a malignant meningioma and review relevant literature in an attempt to investigate the clinical features, treatment, and prognosis of these tumors.
Methods
Fifteen patients underwent surgical treatment for intracranial malignant meningiomas between year 1990 and 2012 in our institution. Anaplastic meningiomas were diagnosed in thirteen cases and papillary meningiomas in two. Fourteen patients (93.3%) received radiotherapy after surgical resection. All patients were followed regularly including clinical-neurological follow-up as well as magnetic resonance imaging. Progression was determined radiographically when there was more than 10% of mass volume increase or when there were onset or worsening of neurological symptoms not attributable to other causes.
Results
Six patients were male and nine were women, and their mean age was 56.9 years (range 36-78). The median follow-up was 54 months (range 3-246). According to our study result, the 5-year progression free survival rate of malignant meningiomas was 53.6%. There were 2 cases (13.3%) of postoperative complications. Recurrences were confirmed in 4 patients (26.7%) during follow-up, the median recurrence time was 35 months (range 12-61), and further procedures were performed. Two of the recurred patients were treated with radiosurgery after secondary tumor resection, and other two patients were treated with radiosurgery alone. There was no more recurred disease patients in the follow-up period after then.
Conclusion
We report the outcomes of the aggressive surgery with radiation of malignant meningiomas. Although the data is limited, we found that radiosurgery treatment had favorable tumor control on recurred patients from our experience.
Keywords: Malignant meningioma, Radiotherapy, Radiosurgery
INTRODUCTION
Meningioma has the second highest incidence rate among primary tumors occurring in the central nervous system, and it comprises 13-16% of intracranial tumors [1,2]. According to the fourth classification of World Health Organization (WHO) in 2007, 92% of meningiomas are divided into benign tumors (WHO Grade I) which comparatively grows in slowly [2-5]. However, 1-3% are known as malignant meningiomas [5-7] which has high recurrence rate, morbidity rate, and mortality rate even after surgical treatment. The treatment principle of meningioma is complete surgical removal (Simpson grade I-III), however, it was reported that the recurrence rate of malignant meningiomas after complete extirpation is 20-40% by the decade and it increases to 40-60% in partial extirpation patients (Simpson grade IV) [8]. Also, the 5-year overall survival rate of malignant meningiomas was 32-64%, so adjuvant radiation therapy or radiosurgery is attempted regardless of the extent of surgery [9-11]. Therefore, we analyzed the clinical aspects and treatment results of our series rather than the patients who received adjuvant radiation therapy or radiosurgery after surgical treatment of malignant meningiomas with long-term follow-up.
MATERIALS AND METHODS
Patients
A retrospective study was performed on 726 patients who received surgery and histopathologically confirmed as a meningioma in our institution from January 1990 to December 2012. All patients who had other intracranial tumors or existing nerve disorders, or patients who had received radiation therapy for other reasons were excluded to prevent any variables in the results. The histopathological diagnosis was based on the brain tumor classification by WHO. Out of the 726 meningioma patients, 15 patients (2.1%) were diagnosed as anaplastic, papillary meningiomas which are classified as WHO Grade III, according to the presence of each of the following features: increased cellularity, small cells with a high nuclear-cytoplasmic ratio, prominent nucleoli, uninterrupted patternless or sheet-like growth features, foci of 'spontaneous' or 'geographic' necrosis, over 20 mitotic count [per 10 high power field (HPF)], and any brain invasion [3,7,12]. The clinical aspects and neurological examination before surgery were confirmed by the medical records, and those were evaluated before surgery with the Karnofsky performance status score (KPS score) for risk stratification.
Treatment and follow-up
The feeding vessels and tumor vascularity through were evaluated first by cerebral angiography before surgery. If preoperative embolization was available, embolization was conducted to reduce the hemorrhage during surgery and to ease removal of the tumor. The surgery was conducted 3-7 days after the embolization. The location and size of the craniotomy was changed for each tumor, and navigation system was introduced to correctly localize the location of tumors. We checked the residual tumor after the mass removal and conducted electric cauterization if there was any dura unavailable for removal. The result of the surgery were graded according to Simpson's classification [9,13,14]. For purposes of our analyses, gross total resection (GTR) included Simpson Grades 1-3; subtotal resection (STR) was equivalent to Simpson Grade 4. For adjuvant therapy after surgical removal, whole brain radiation therapy (WBRT) was performed before year 2006 in our hospital. After 2006, fractionated radiation therapy (RT) was given for postoperative adjuvant treatment. However, in patients with cerebrospinal fluid (CSF) seeding on preoperative MRI findings, WBRT was performed. Fractionated RT was delivered with a median daily fraction of 1.8 Gy (range 1.8-3 Gy) and a median total dose of 54 Gy (range, 25-65 Gy). Stereotactic radiosurgery was done on patients having remnant tumor or recurred tumor after surgery, or in patients who are intolerable to radiation therapy. Stereotactic radiosurgery using Gamma Knife was conducted with a median dose of 14 Gy (range 12-15 Gy). Tumor recurrence was followed up with brain MRI within 24 hours of surgery, after 3 months, then at 6- to 12-month intervals. However, when there were any signs or symptoms of neurological deficiency, MRI was performed at any point. In recurrence disease patients after the first surgery, stereotactic radiosurgery after a second surgical operation was performed when the size of the mass increased, or when neurologic changes occurred. If the patient showed no relevant symptoms, only stereotactic radiosurgery was performed.
Primary end point and statistical analysis
We employed radiographic reports, pathology reports, and all available inpatient and outpatient records to follow-up patients after primary surgery. In measuring the time-to-progression interval, progression was determined radiographically when there was more than 10% of mass volume increase, or when there was onset or worsening of neurological symptoms not attributable to other causes [2,14-16]. Progression was analyzed as the primary end point. Progression free survival rates were generated using the Kaplan-Meier method. The analyzed characteristics included age, KPS, gender, degree of resection (GTR vs. STR), use of embolization before primary surgery. Statistical significance was determined by a p value of 0.05. The Statistical Package for the Social Sciences (SPSS V 20.0, SPSS Inc., Chicago, IL, USA) was used for statistical analysis.
RESULTS
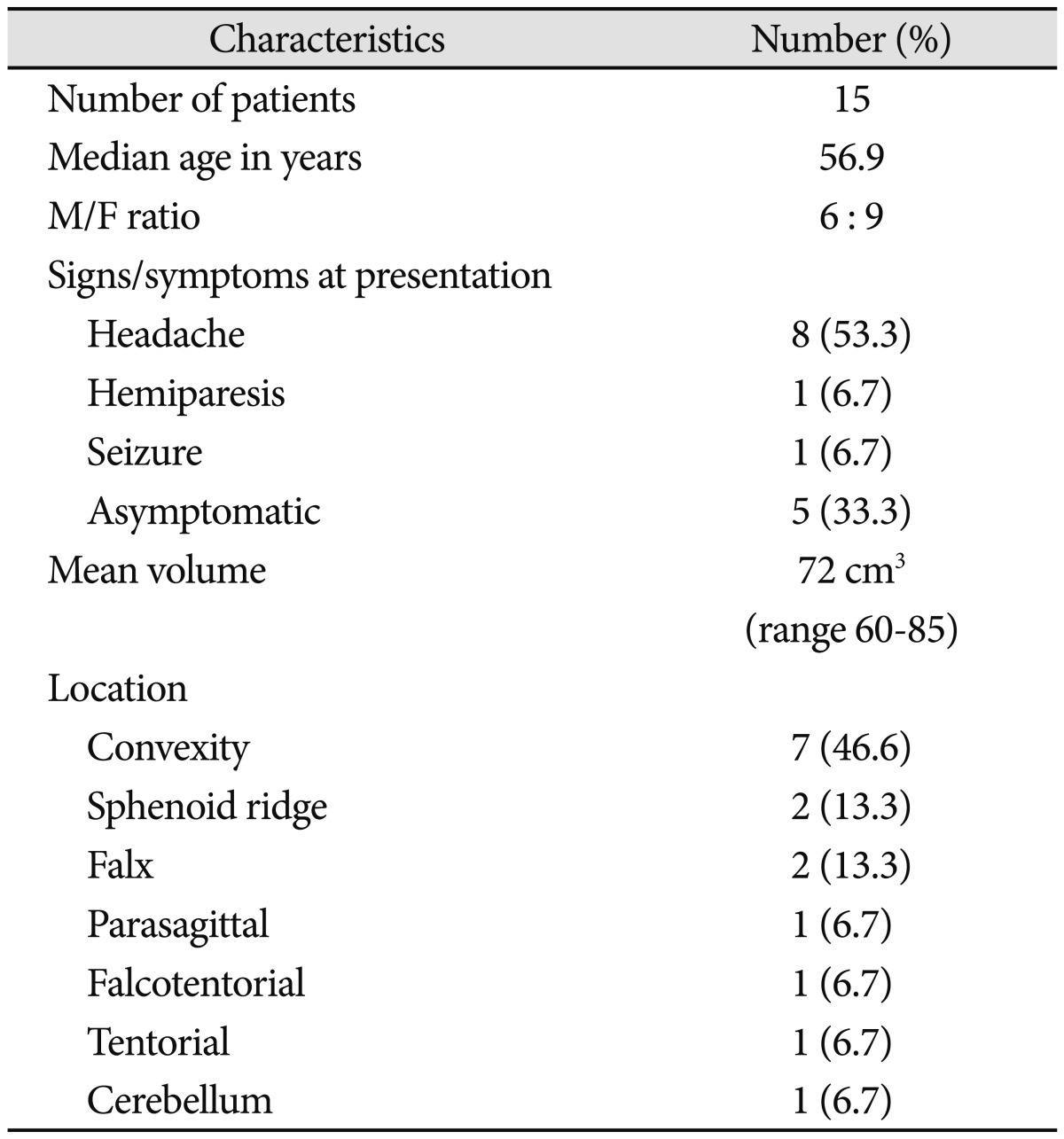
There were 15 malignant meningioma patients (2.4%) among 726 patients, who received meningioma surgery at our institution over a 23-year period. Pathologic features showed that there were 13 cases (86.7%) of anaplastic meningiomas and 2 cases (13.3%) of papillary meningiomas. The average age was 56.9 (range 36-75) years, and there were more woman as there were 6 men and 9 women. There were 7 cases (46.6%) located in the convexity, 2 cases (13.3%) located in the sphenoid ridge, and rest 6 cases located respectively in the falx, parasagittal, tentorium, falcotentorial, and cerebellum. Most patients showed mass effect of the tumor. Eight (53.3%) had headaches, one (6.7%) had hemiparesis, and one (6.7%) had seizure, but the remaining 5 patients (33.3%) had no specific symptoms. The mean KPS score before the initial surgery was 95±5.2 (Table 1).
Table 1.
Data in 15 patients with malignant meningioma
Treatment and complication
Eight patients (53.3%) underwent preoperative embolization. The surgical results were Simpson grade 1 in 2 patients (13.2%), Simpson Grade 2 in 5 patients (33.4%), Simpson Grade 3 in 3 patients (20%), Simpson Grade 4 in 5 patients (33.4%). Out of 15 patients, 12 patients received radiation therapy and 2 patients underwent radiosurgery as adjuvant treatment, while the other 1 patient had no additional therapy because of old age. Transient mild side effects of radiation treatment including skin irritation, fatigue, dizziness, headache were observed in most patients. Two patients (13.3%) had serious complications after the surgery, and they were, respectively, 1 case of post-operative intracranial hematoma and 1 case of status epilepticus elicited from leptomeningeal seeding. The patient who showed an intracranial hematoma presented as hemiparesis after surgical treatment, and another patient who showed status epilepticus died after 3 months.
Outcome and prognostic factors for recurrence
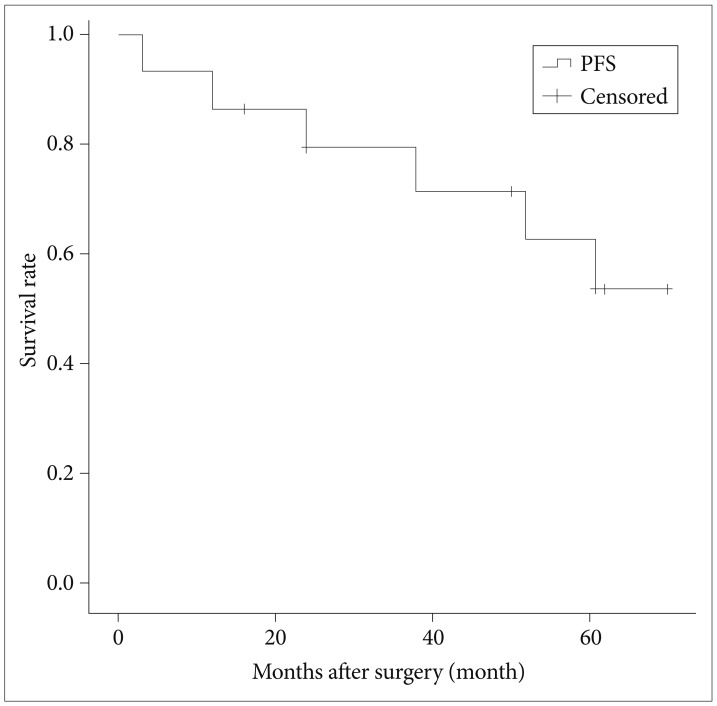
The median follow-up was 54 months (range 3-246). One patient died of brain swelling and status epilepticus due to leptomeningeal seeding, and 4 patients (31%) showed progression of tumor in the follow-up period. Two patients were treated with radiation therapy after gross total removal, one patient was treated with radiation therapy after subtotal removal, and one patient was treated with stereotactic radio surgery after subtotal removal. The median recurrence time was at 35 months (range 12-61). The 5-year progression free survival rate was 53.6% (Fig. 1). There was one case of tumor progression out of six patients who had radiosurgery treatment. But no variables, including mass volume, degree of resection, KPS, gender, or preoperative embolization, affected the survival and interval to recurrence (Table 2).
Fig. 1.
Kaplan-Meier plot of 5-year progression free survival. PFS: progression free survival.
Table 2.
Analysis of factors affecting survival and recurrence rate
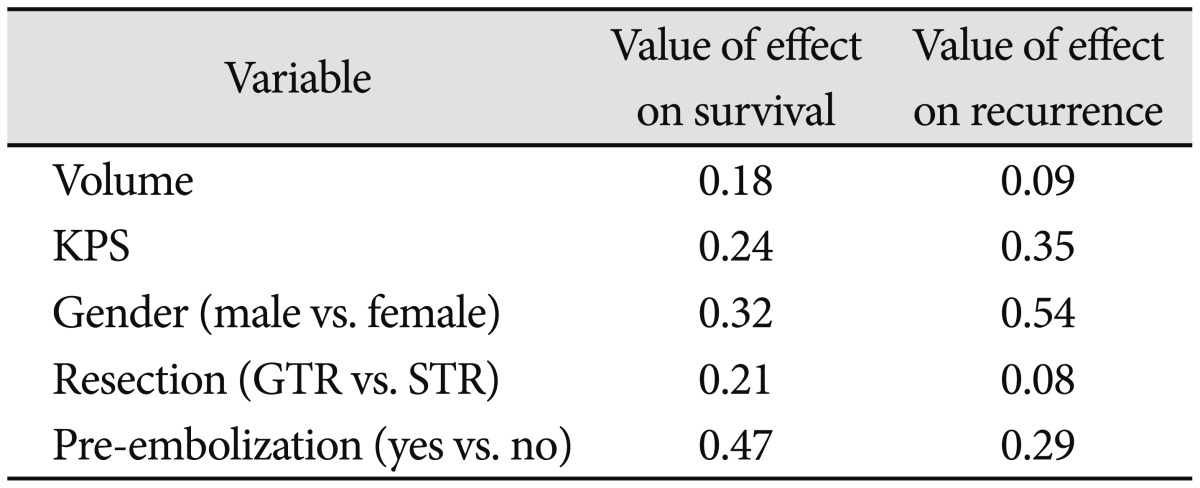
KPS: Karnofsky performance status, GTR: gross total resection, STR: subtotal resection
Treatment after recurrence
Four patients had local tumor recurrence and stereotactic radiosurgery was done on all recurred cases. Two of recurred patients were treated with radiosurgery after secondary tumor resection, and other two patients were treated with radiosurgery alone. Stereotactic radiosurgery treatment on recurred patients applied a median dose of 14 Gy (range 12-16 Gy). There was no more recurred patients in the follow-up period after then.
Case presentation 1
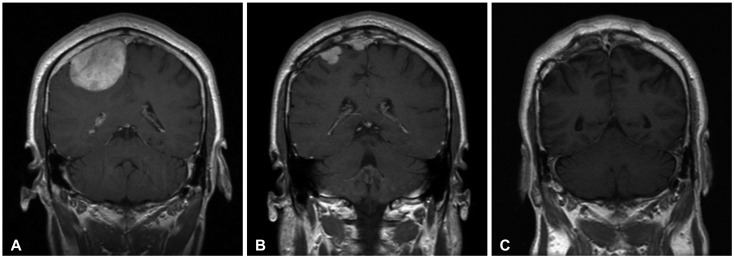
A 45-year-old man presented to our Neurology clinic with a 3-week history of worsening headaches, giddiness and vomiting. Magnetic resonance imaging (MRI) confirmed a solid mass (5×6 cm) in the right frontal convexity adjacent to the superior sagittal sinus. The pathological examination confirmed the lesion as an anaplastic meningioma. After surgery, adjuvant radiotherapy was provided. But the patient was lost in the follow-up 6 months after the operation. He then returned to our hospital with severe headaches and vomiting 24 months post-operation. Two separated enhancing masses were observed at the previous operation site on the MRI, and the patient received secondary operation combined with radiosurgery. The patient's symptoms improved and the former mass was seen to be totally removed on the follow-up MRI at 37 months after operation with radiosurgery. He is still being followed up after the second operation without recurrence (Fig. 2).
Fig. 2.
Enhanced coronal magnetic resonance imaging (MRI) series in a 45-year-old man (Case 11). A: Preoperative MRI showing a homogenously enhanced mass around right frontal convexity. B: MR imaging obtained 24 months after surgery and conventional radiotherapy revealing the recurrence of meningioma at previously surgical site with two separated enhancing mass. C: Thirty-seven months after operation with radiosurgery, a coronal MRI with gadolinium enhancement demonstrating disappearance of the meningioma.
Case presentation 2
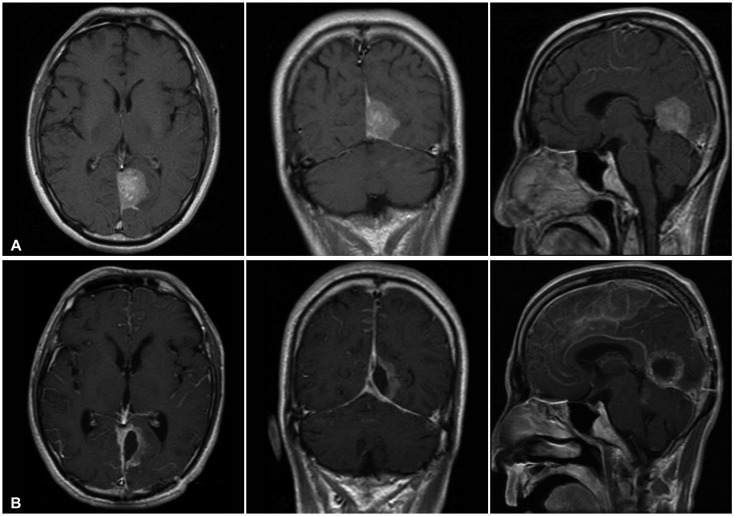
A 46-year-old man presented to the hospital for a persistent headache. Neurological examination demonstrated fluent speech when prompted and a flat affect. No focal motor or sensory deficits could be elicited. The initial T1-weighted MR image demonstrated an isolated, homogeneous, Gd-enhancing mass measuring approximately 4×3 cm with a prominent dural-based tail which bases upon the falcotentorium of the left occipital lobe. The mass was resected via a left occipital craniotomy. The diagnosis was confirmed as a papillary meningioma. After surgery, status epilepticus was elicited from the leptomeningeal seeding. A dysfunction in CSF pathway occurred by the leptomeningeal seeding and the patient died because of brain swelling after 3 months (Fig. 3).
Fig. 3.
A: Preoperative magnetic resonance imaging with gadolinium enhancement showing homogenously enhancing mass which bases upon falcotentorium (Case 8). B: MR imaging obtained 3 month after surgery and conventional radiation therapy revealing diffuse enhancement on leptomeninges which are consistent with leptomeningeal seeding. The patient exhibited status epilepticus and expired.
DISCUSSION
In the classification of meningiomas based on the WHO system (2007), it is reported that benign meningiomas occupies 92%, atypical meningioma occupies 5-7%, and malignant meningioma occupies 1-3% of the total [6,13,14,17,18]. Papillary, anaplastic and rhabdoid meningiomas belong to the malignant meningiomas, and cytologically their feature shows over 20 mitoses in HPF similar to sarcomas or melanomas [19,20]. The treatment principle of a meningioma is surgical removal, and for that reason, surgical status becomes an important factor for the prediction of recurrence [12,16,21,22]. Prognosis of incomplete mass removal is not good, including recurrence and others [9,10,15,17]. Benign meningiomas have comparatively has low invasiveness, however, malignant meningiomas often recur even after complete surgical removal and radiation therapy [5,7,11,21]. Goldsmith et al. [20] reported a 48% 5-year progression free survival rate and Hug et al. [10] reported 52% 5-year progression free survival for malignant meningiomas. This study showed a 5-year progression free survival rate of 53.6%. Although direct comparisons with previous studies are complicated for its small sample sizes and different combined management strategies, the rates of local tumor control in our study appear to be similar with other studies. Many cases reported the difficulty in complete removal of tumor by surgery and consequent high recurrence rate, so adjuvant treatment after surgery is required [8,23,24]. Radiation therapy and radiosurgery are commonly used as adjuvant treatment [6,21]. In particular, it is reported that fractionated radiation therapy may effectively control the recurrence and progress of tumors [16,20,25]. For malignant tumors, additional radiation therapy is recommended regardless of the Simpson grade because of its high recurrence rate [15,25]. Many literatures report that adjuvant radiation therapy after surgery has to be vitally conducted because malignant meningiomas have high mortality and morbidity rates [7,11,19,26,27]. Milosevic et al. [12] reported the results of mean 50 Gy radiation for total 59 patients (17 cases of atypical meningiomas and 42 cases of malignant meningiomas), and the tumor recurred in 39 patients (66%) and did not recur in 20 patients (34%). Hug et al. [10] reported that high dose radiation therapy (≥60 Gy) increased the survival rate, improving control rate for 16 patients with malignant meningiomas. Goldsmith et al. [20] presented data concerning 23 patients with aggressive meningiomas according to a variety of pathologic factors, including brain invasion. They found that the radiation dose was positively associated with increased survival rate when analyzed as a continuous variable. However, Al-Mefty et al. [6] reported that the fractionated radiation therapy on meningiomas may cause complications in the long term follow-up period, such as decreased visual acuity, hypopituitarism, delayed radiation induction cerebral injury, and secondary tumor recurrence by radiation. Likewise, Hug et al. [10] reported about 9% patients had side effects from the radiation.
Recently, stereotactic radiosurgery is conducted as an important auxiliary simultaneous treatment which has comparatively lower rate of complications than fractionated radiation therapy [3,13,15,28]. Also stereotactic radiosurgery is effective for controlling tumor recurrence and improving survival rate of patients [15,20]. It has been reported stereotactic radiosurgery can be a safe treatment for small sized meningiomas [21,22]. Harris et al. [14] reported 72% of control rate after 5 years from stereotactic radiosurgery after surgical mass removal for 12 patients of malignant meningiomas, and suggested the additional radiosurgery for such patients. Ojemann et al. [4] retrospectively reviewed 19 patients who received SRS for lesions recurring after craniotomy and fractionate radiation therapy. The 5-year progression free survival rate was 34%. They suggested that age less than 50 years and a target volume of less than 8 cm3 were found to be significant factors with respect to tumor control progression by multivariate analysis. In this study, we conducted additional radiosurgery in 2 patients after the first surgical treatment and additional radiosurgery in 4 recurred patients. No recurrence was found in the 4 recurred patients who received radiosurgery. If a focal recurrence develops, early radiosurgery is advocated. However, the statistical value of this study is not effective as the number of cases in our series is small, and therefore further long-term follow-up is required later. Mattozo et al. [22] suggested that if a patient is found later to have a nodular tumor recurrence away from critical structures, then stereotactic radiosurgery is recommended. Conversely, if a patient has a diffuse tumor recurrence or the tumor is in direct contact with the anterior visual pathways, then radiation therapy is recommended.
This study has several weaknesses that constrain its findings and clinical application. First, the series is a retrospective review performed over 2 decades, and thus patient selection bias and lack of a standardized treatment regimen was unavoidable. Second, the small sample size limits the power of our findings. Third, our series did not permit a comparison of the relative effectiveness of stereotactic radiosurgery and radiation therapy for patients with recurrent or progressing malignant meningioma. A randomized trial comparing tumor control and patient outcomes for the different radiation techniques after surgical resection, or for patients with recurrent tumors would be needed to determine whether one approach is more efficacious.
We demonstrated outcomes of the aggressive surgery with radiation treatment of malignant meningiomas. Although the data is limited, we found that radiosurgery treatment had favorable tumor control on recurred patients from our experience. Thus, additional prospective study regarding the role of radiosurgery is needed.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Hoessly GF, Olivecrona H. Report on 280 cases of verified parasagittal meningioma. J Neurosurg. 1955;12:614–626. doi: 10.3171/jns.1955.12.6.0614. [DOI] [PubMed] [Google Scholar]
- 2.Mirimanoff RO, Dosoretz DE, Linggood RM, Ojemann RG, Martuza RL. Meningioma: analysis of recurrence and progression following neurosurgical resection. J Neurosurg. 1985;62:18–24. doi: 10.3171/jns.1985.62.1.0018. [DOI] [PubMed] [Google Scholar]
- 3.Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ojemann SG, Sneed PK, Larson DA, et al. Radiosurgery for malignant meningioma: results in 22 patients. J Neurosurg. 2000;93(Suppl 3):62–67. doi: 10.3171/jns.2000.93.supplement. [DOI] [PubMed] [Google Scholar]
- 5.Willis J, Smith C, Ironside JW, Erridge S, Whittle IR, Everington D. The accuracy of meningioma grading: a 10-year retrospective audit. Neuropathol Appl Neurobiol. 2005;31:141–149. doi: 10.1111/j.1365-2990.2004.00621.x. [DOI] [PubMed] [Google Scholar]
- 6.Al-Mefty O, Kadri PA, Pravdenkova S, Sawyer JR, Stangeby C, Husain M. Malignant progression in meningioma: documentation of a series and analysis of cytogenetic findings. J Neurosurg. 2004;101:210–218. doi: 10.3171/jns.2004.101.2.0210. [DOI] [PubMed] [Google Scholar]
- 7.Maier H, Ofner D, Hittmair A, Kitz K, Budka H. Classic, atypical, and anaplastic meningioma: three histopathological subtypes of clinical relevance. J Neurosurg. 1992;77:616–623. doi: 10.3171/jns.1992.77.4.0616. [DOI] [PubMed] [Google Scholar]
- 8.Palma L, Celli P, Franco C, Cervoni L, Cantore G. Long-term prognosis for atypical and malignant meningiomas: a study of 71 surgical cases. J Neurosurg. 1997;86:793–800. doi: 10.3171/jns.1997.86.5.0793. [DOI] [PubMed] [Google Scholar]
- 9.Adegbite AB, Khan MI, Paine KW, Tan LK. The recurrence of intracranial meningiomas after surgical treatment. J Neurosurg. 1983;58:51–56. doi: 10.3171/jns.1983.58.1.0051. [DOI] [PubMed] [Google Scholar]
- 10.Hug EB, Devries A, Thornton AF, et al. Management of atypical and malignant meningiomas: role of high-dose, 3D-conformal radiation therapy. J Neurooncol. 2000;48:151–160. doi: 10.1023/a:1006434124794. [DOI] [PubMed] [Google Scholar]
- 11.Jääskeläinen J, Haltia M, Servo A. Atypical and anaplastic meningiomas: radiology, surgery, radiotherapy, and outcome. Surg Neurol. 1986;25:233–242. doi: 10.1016/0090-3019(86)90233-8. [DOI] [PubMed] [Google Scholar]
- 12.Milosevic MF, Frost PJ, Laperriere NJ, Wong CS, Simpson WJ. Radiotherapy for atypical or malignant intracranial meningioma. Int J Radiat Oncol Biol Phys. 1996;34:817–822. doi: 10.1016/0360-3016(95)02166-3. [DOI] [PubMed] [Google Scholar]
- 13.Gold JR, Ellison JR. Silver staining for nucleolar organizing regions of vertebrate chromosomes. Stain Technol. 1983;58:51–55. doi: 10.3109/10520298309066749. [DOI] [PubMed] [Google Scholar]
- 14.Harris AE, Lee JY, Omalu B, Flickinger JC, Kondziolka D, Lunsford LD. The effect of radiosurgery during management of aggressive meningiomas. Surg Neurol. 2003;60:298–305. doi: 10.1016/s0090-3019(03)00320-3. discussion 305. [DOI] [PubMed] [Google Scholar]
- 15.Borovich B, Doron Y. Recurrence of intracranial meningiomas: the role played by regional multicentricity. J Neurosurg. 1986;64:58–63. doi: 10.3171/jns.1986.64.1.0058. [DOI] [PubMed] [Google Scholar]
- 16.Jung HW, Yoo H, Paek SH, Choi KS. Long-term outcome and growth rate of subtotally resected petroclival meningiomas: experience with 38 cases. Neurosurgery. 2000;46:567–574. doi: 10.1097/00006123-200003000-00008. discussion 574-5. [DOI] [PubMed] [Google Scholar]
- 17.Kallio M, Sankila R, Hakulinen T, Jääskeläinen J. Factors affecting operative and excess long-term mortality in 935 patients with intracranial meningioma. Neurosurgery. 1992;31:2–12. doi: 10.1227/00006123-199207000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Sughrue ME, Sanai N, Shangari G, Parsa AT, Berger MS, McDermott MW. Outcome and survival following primary and repeat surgery for World Health Organization Grade III meningiomas. J Neurosurg. 2010;113:202–209. doi: 10.3171/2010.1.JNS091114. [DOI] [PubMed] [Google Scholar]
- 19.Commins DL, Atkinson RD, Burnett ME. Review of meningioma histopathology. Neurosurg Focus. 2007;23:E3. doi: 10.3171/FOC-07/10/E3. [DOI] [PubMed] [Google Scholar]
- 20.Goldsmith BJ, Wara WM, Wilson CB, Larson DA. Postoperative irradiation for subtotally resected meningiomas. A retrospective analysis of 140 patients treated from 1967 to 1990. J Neurosurg. 1994;80:195–201. doi: 10.3171/jns.1994.80.2.0195. [DOI] [PubMed] [Google Scholar]
- 21.Huffmann BC, Reinacher PC, Gilsbach JM. Gamma knife surgery for atypical meningiomas. J Neurosurg. 2005;102(Suppl):283–286. doi: 10.3171/jns.2005.102.s_supplement.0283. [DOI] [PubMed] [Google Scholar]
- 22.Mattozo CA, De Salles AA, Klement IA, et al. Stereotactic radiation treatment for recurrent nonbenign meningiomas. J Neurosurg. 2007;106:846–854. doi: 10.3171/jns.2007.106.5.846. [DOI] [PubMed] [Google Scholar]
- 23.Hsu CC, Pai CY, Kao HW, Hsueh CJ, Hsu WL, Lo CP. Do aggressive imaging features correlate with advanced histopathological grade in meningiomas? J Clin Neurosci. 2010;17:584–587. doi: 10.1016/j.jocn.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 24.al-Mefty O, Kersh JE, Routh A, Smith RR. The long-term side effects of radiation therapy for benign brain tumors in adults. J Neurosurg. 1990;73:502–512. doi: 10.3171/jns.1990.73.4.0502. [DOI] [PubMed] [Google Scholar]
- 25.Nagar VA, Ye JR, Ng WH, et al. Diffusion-weighted MR imaging: diagnosing atypical or malignant meningiomas and detecting tumor dedifferentiation. AJNR Am J Neuroradiol. 2008;29:1147–1152. doi: 10.3174/ajnr.A0996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ohba S, Kobayashi M, Horiguchi T, et al. Long-term surgical outcome and biological prognostic factors in patients with skull base meningiomas. J Neurosurg. 2011;114:1278–1287. doi: 10.3171/2010.11.JNS10701. [DOI] [PubMed] [Google Scholar]
- 27.Toh CH, Castillo M, Wong AM, et al. Differentiation between classic and atypical meningiomas with use of diffusion tensor imaging. AJNR Am J Neuroradiol. 2008;29:1630–1635. doi: 10.3174/ajnr.A1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yue Q, Isobe T, Shibata Y, et al. New observations concerning the interpretation of magnetic resonance spectroscopy of meningioma. Eur Radiol. 2008;18:2901–2911. doi: 10.1007/s00330-008-1079-6. [DOI] [PubMed] [Google Scholar]