Abstract
Objective
Patients with symptomatic brain metastases secondary to mass effect are often candidates for surgery. However, many of these surgical candidates are also found to have multiple asymptomatic tumors. This study aimed to determine the outcome of surgical resection of symptomatic brain metastases followed by chemotherapy or radiotherapy (RT) for the remnant asymptomatic lesions in non-small cell lung cancer (NSCLC) patients with multiple brain metastases.
Methods
We conducted a retrospective review of the medical records of 51 NSCLC patients with symptomatic multiple brain metastases who underwent surgical resection, of whom 38 had one or more unresected asymptomatic lesions subsequently treated with chemotherapy and/or RT. Thirteen patients underwent resection of all metastatic lesions.
Results
Median survival for overall patient population after surgical resection was 10.8 months. Median survival for patients with surgical resection of all brain metastases was not significantly different with patients who underwent surgical resection of only symptomatic lesions (6.5 months vs. 10.8 months; p=0.97). There was no statistically significant difference in survival according to the number of tumors (p=0.86, 0.16), or post-surgical treatment modalities (p=0.69).
Conclusion
The survival time of NSCLC patients with multiple brain metastases after surgery for only symptomatic brain metastases is similar to that of patients who underwent surgery for all brain metastases. The remaining asymptomatic lesions may be treated with chemotherapy or radiotherapy. The optimal treatment modality, however, needs to be defined in prospective trials with larger patient cohort.
Keywords: Surgery, Multiple, Brain metastasis, Survival, Lung cancer
INTRODUCTION
Brain metastases develop in up to 50% of all patients with cancer [1]. Multiple brain metastases are a common occurrence in patients with cancer, with an incidence between 12 and 50% in this population [2-4]. Autopsy reports indicate that between 60% and 85% of patients with brain metastases have multiple lesions [5-7]. Recently, the detection sensitivity of brain metastases has increased secondary to improvement in imaging technology [8]. When only computed tomography (CT) was widely utilized, almost all detected brain metastases were symptomatic; however with the introduction of magnetic resonance imaging (MRI), detection of asymptomatic brain metastases has increased [9]. Brain metastases are symptomatic in approximately two-thirds of metastatic brain tumor patients [5,10]. Because of its poor prognosis, their mean survival is approximately 6 months, even after receiving whole brain radiation therapy (WBRT). Patients diagnosed with multiple (>3) metastatic lesions used to be treated with WBRT with or without stereotactic radiosurgery [11]. The current accepted practice to manage these patients is not to offer surgery except in the presence of a life-threatening lesion, the presence of two lesions that can be removed in a single craniotomy, and the need of pathologic diagnosis [12].
Recently, advances in microsurgical technology, such as navigation systems, intra-operative imaging, and cortical mapping, have elevated surgical intervention higher along the list of therapeutic options for these patients [13-15]. The primary benefit of surgical resection of symptomatic metastatic lesions is prompt eradication of mass effect and neurologic symptoms. Eventually, surgery may confer modest survival benefits in non-small cell lung cancer (NSCLC) patients with multiple brain metastases by providing them the opportunity to receive post-operative systemic chemotherapy. Therefore, cerebral metastatectomy should be considered in patients with controlled systemic disease, and concurrent, multiple symptomatic brain metastases that result in significantly deteriorated performance status, when reasonable systemic treatment options are available. Patients with one or more symptomatic brain metastases due to mass effect may be considered for metastatectomy. It must be considered that many of surgical candidates have also asymptomatic tumors and these patients may also be eligible for surgery.
This study aimed to investigate the outcome of surgical resection for the symptomatic cerebral lesions followed by chemotherapy or radiotherapy for asymptomatic lesions in NSCLC patients with multiple brain metastases.
MATERIALS AND METHODS
Clinical characteristics of the patients
Fifty one NSCLC patients with multiple brain metastases underwent surgical metastatectomy between 2001 and 2007. The mean age was 55.3 years (range 29-73). There were 36 men and 15 women. Thirty seven patients (72.5%) showed better Karnofsky performance scale score than 70. Of these 51 patients, seven patients were categorized to recursive partitioning analysis (RPA) class I, thirty two patients to RPA II, and twelve patients to RPA class III. Twenty five patients (49%) had stable systemic disease at the time of brain surgery. Twenty seven (52.9%) patients had synchronous brain metastases, and twenty four patients had metachronous metastases. The patients' clinical characteristics are summarized in Table 1. In terms of number of lesions, there were two lesions in 20 patients, three lesions in 12 patients, four lesions in 9 patients, and ten patients harbored more than five lesions. After surgical intervention, 38 patients had remnant asymptomatic lesions after resection of symptomatic lesions, and in 13 patients, all cerebral metastases were resected.
Table 1.
Clinical characteristics of 51 patients with multiple brain metastases
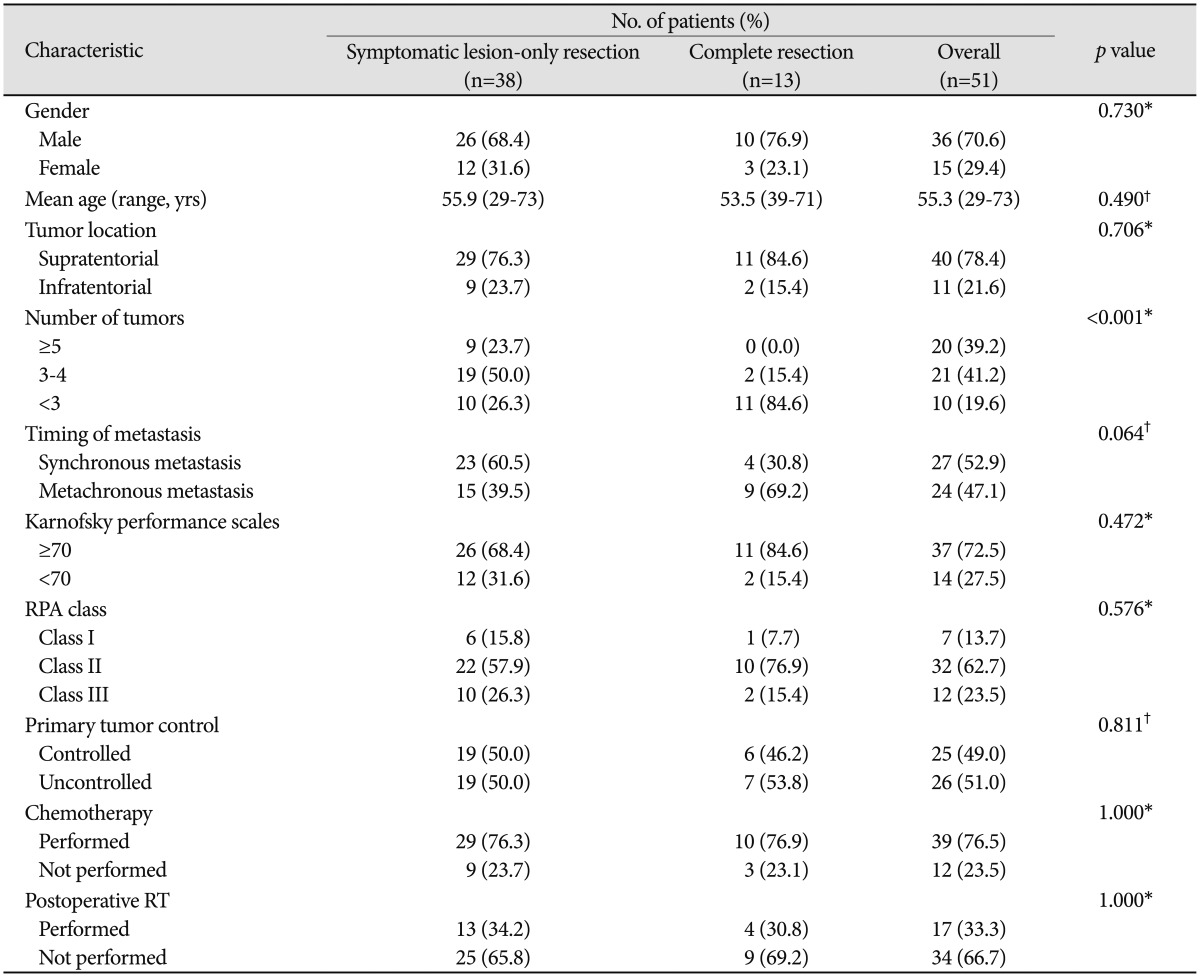
*Pearson's chi-square test, †t-test. RPA: recursive partitioning analysis, RT: radiotherapy
Surgical treatment and subsequent treatments after surgery
All patients were treated with surgery for brain metastases. We used a technique described in our previous report to promote completeness of resection [15]. We removed only symptomatic lesions in this study. Asymptomatic lesions were subsequently treated with chemotherapy and/or radiotherapy. Post-surgical treatment modalities for asymptomatic brain metastases are summarized in Table 2. Twenty two patients were treated with both chemotherapy and radiotherapy (radiotherapy after chemotherapy in 11, chemotherapy after radiotherapy in 11), seventeen patients were treated with chemotherapy alone, and eight patients were treated with radiotherapy alone.
Table 2.
Additional treatments after brain surgery
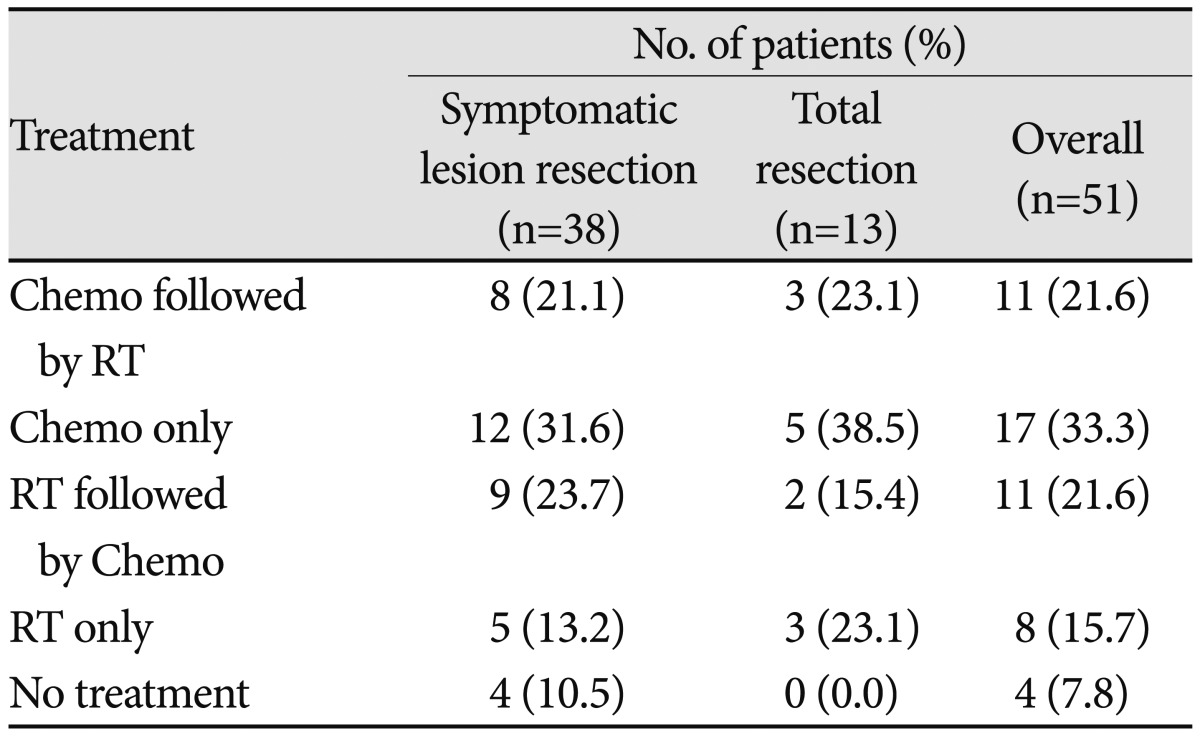
Chemo: chemotherapy, RT: radiationtherapy
Follow-up evaluation
Patients were examined clinically monthly, and brain MRIs were obtained every 3 months following surgery.
Statistical analysis
Overall survival was calculated from the date of brain surgery using the Kaplan-Meier method. Univariate comparisons of survival between different groups were performed using the log-rank test. Variables compared include, the number of metastases, types of metastatectomy (resection of all lesions, one or more lesions left unresected), and post-surgical treatments (radiotherapy, chemotherapy). Multivariate analysis was performed to determine if the variables significantly correlated with survival difference under the Cox regression model. The following variables were examined: type of metastatectomy, controlled or uncontrolled systemic disease, RPA class and timing of metastases.
RESULTS
Survival analysis
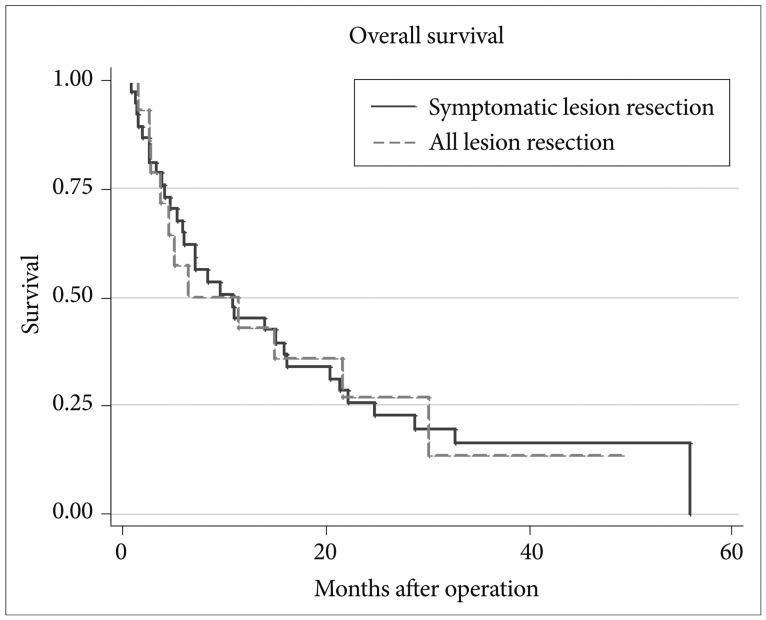
Median survival after brain surgery in patients with multiple brain metastases was 10.8 months in the study population. Median survival of patients with unresected asymptomatic lesions was 10.8 months, and that of patients with no evaluable lesion was 6.5 months. Survival duration did not differ significantly between the two groups (p=0.97) (Fig. 1). The overall survival was 44.4% at year 1 and 25.8% at year 2. The survival in the patients with unresected, asymptomatic lesions were 46.5% at year 1 and 24.6% at year 2, and that in patients with no evaluable lesions were 38.5% at year 1 and 28.9% at year 2.
Fig. 1.
Survival curve for multiple brain metastases according to types of lesion resection.
Multivariate analysis
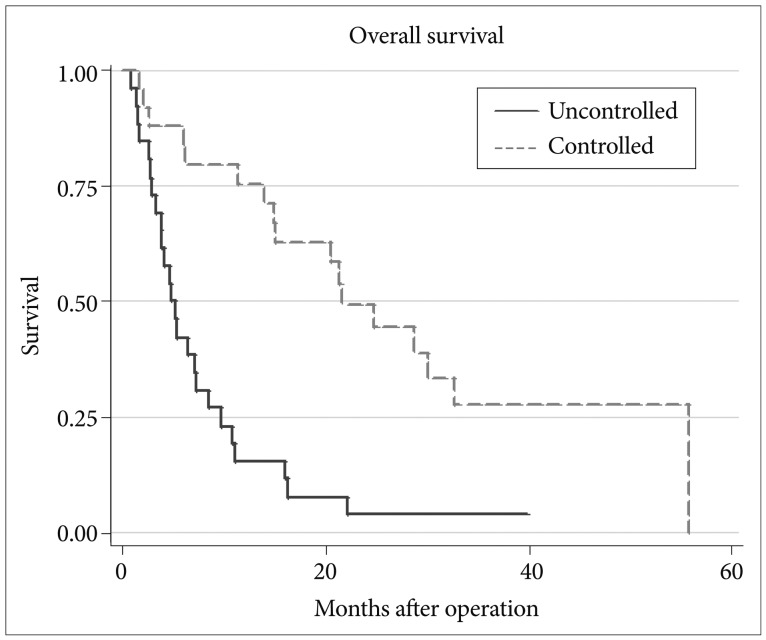
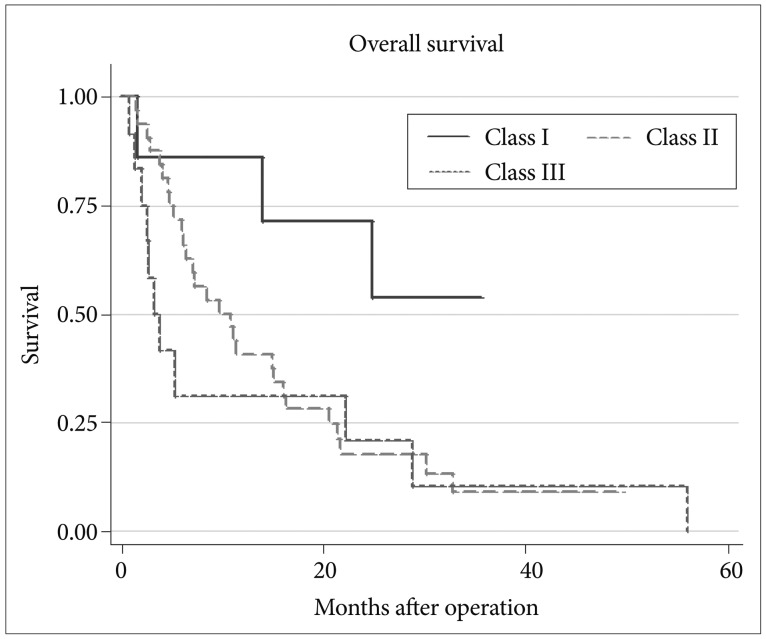
Multivariate analysis using the Cox regression model indicated that variables significantly affecting survival were primary tumor control (p<0.001) and RPA classes (p=0.044, 0.018) (Table 3). Patients with uncontrolled systemic disease survived 4.7 months and those with controlled systemic disease survived 21.7 months (p=0.001) (Fig. 2). Median survival has not been reached in RPA class I, and were 9.6 and 3.3 months in RPA class II and III, respectively (p=0.001) (Fig. 3).
Table 3.
Factors affecting overall survival in univariate and multivariate analyses (Cox regression model)
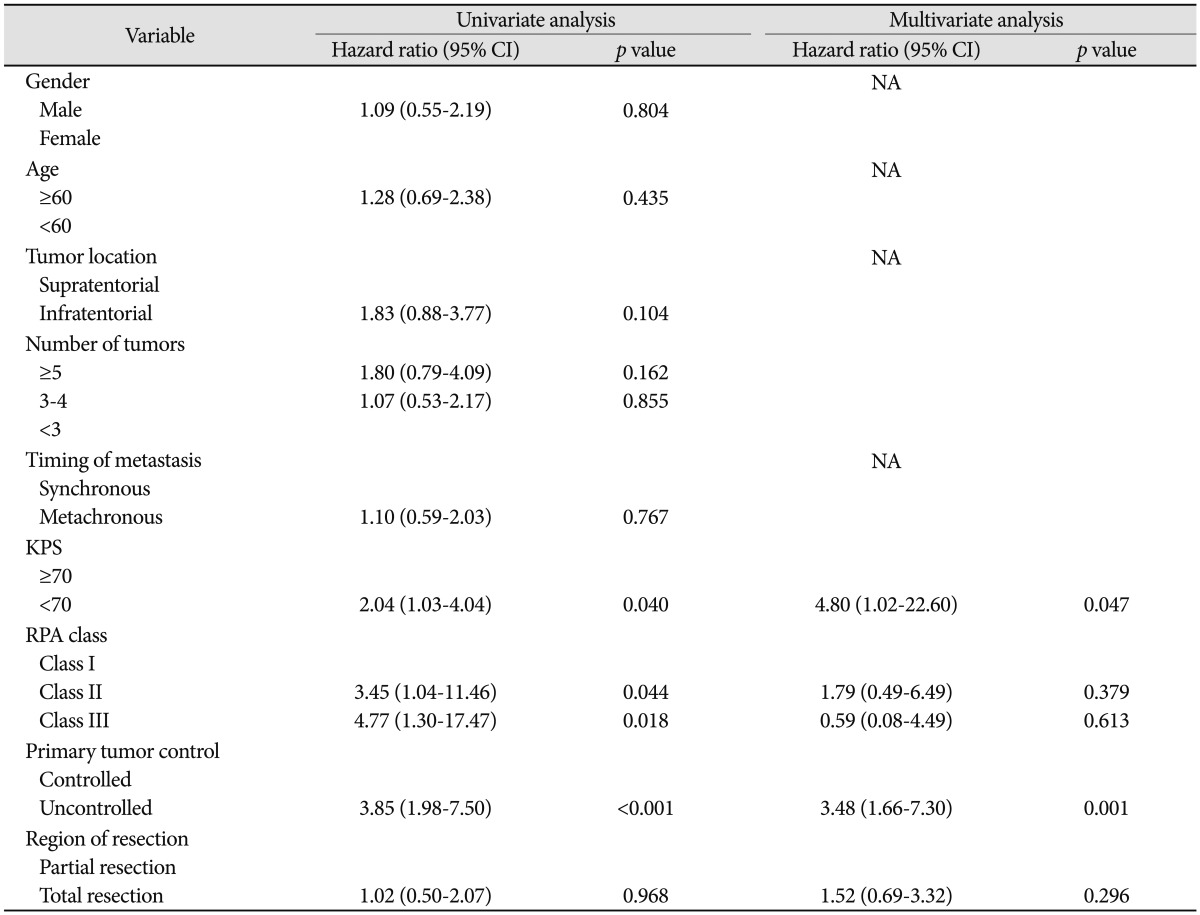
CI: confidence interval, NA: not assessed, KPS: Karnofsky performance scale, RPA: recursive partitioning analysis
Fig. 2.
Differences in survival according to the status of primary disease in multiple brain metastases.
Fig. 3.
Differences in survival difference according to recursive partitioning analysis class in multiple brain metastases.
Number of tumors
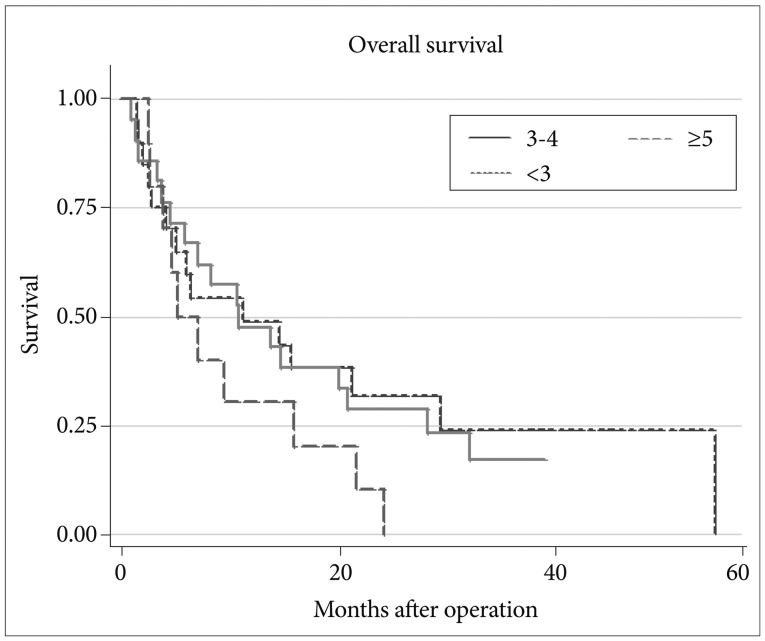
There was no statistically significant difference in survival according to the number of tumors (p=0.86, 0.16) (Fig. 4). However, there appeared to be a trend for shorter survival when the number of metastatic brain tumors was more than 5.
Fig. 4.
Comparison of survival according to the number of tumors.
Post-surgical treatment
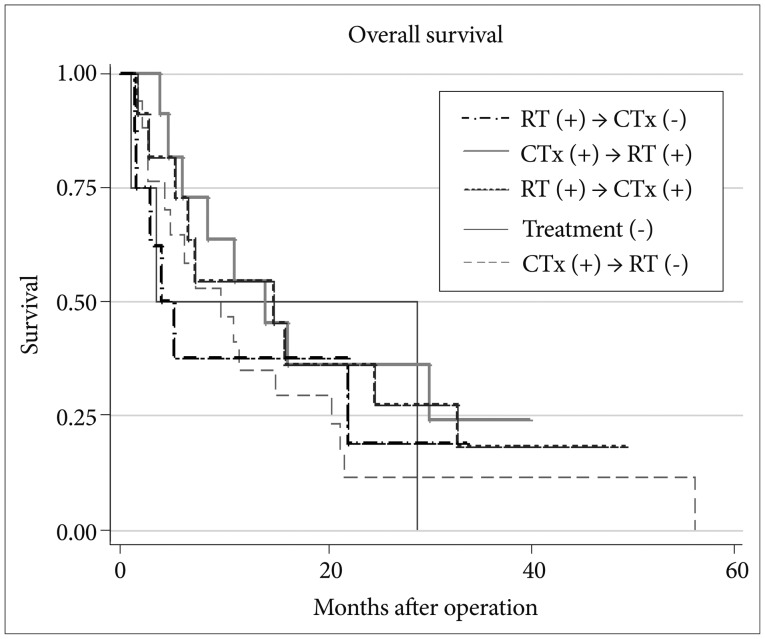
Survival data according to additional treatment modalities in patients with multiple brain metastases were shown in Fig. 5. There was no significant difference in survival with different post-surgical treatment modalities (p=0.69). However, patients who did not receive any additional treatment, and who only received radiotherapy to the brain without chemotherapy appeared to have shorter survival.
Fig. 5.
Comparison of survival according to additional treatments modalities in multiple metastases. RT: radiotherapy, CTx: chemotherapy.
Comparison of multiple metastases with single metastasis
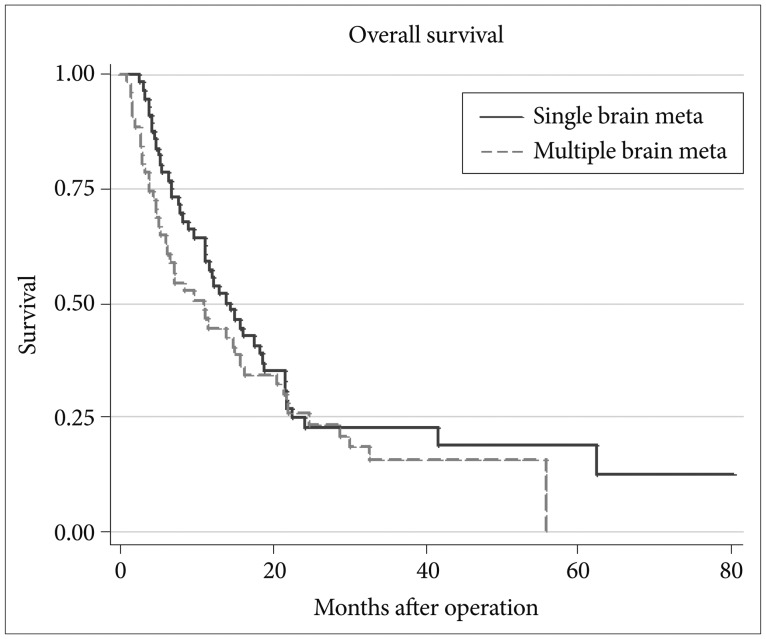
We compared survival in patients with multiple brain metastases from data we published previously in patients with single brain metastasis after complete resection of the lesion [15]. Comparison of survival data for patients with single and multiple brain metastases are shown in Fig. 6. Median survival was 14 months in patients with single brain metastasis, 10.8 months in patients with multiple brain metastases. Survival for single and multiple brain metastases groups were 57.1% and 44.4%, respectively, at year 1, and 24.6% and 25.8%, respectively, at year 2. Survival duration did not differ significantly between the two groups (p=0.22).
Fig. 6.
Comparison of survival between single and multiple brain metastases.
DISCUSSION
Treatment outcomes of multiple brain metastases after surgery
Management of patients with multiple brain metastases is considered to be difficult due to the historically poor prognosis of these patients when compared to patients with solitary brain metastasis. Treatment generally consisted of WBRT once the diagnosis of multiple brain metastases was made.
The authors of several reports have shown that tumor resection in a subset of patients with multiple brain metastases is associated with improved outcomes [16-20]. Bindal et al. [16] reported a median survival of 10 months in 82 patients who underwent resection combined with WBRT. In that retrospective study, the authors reported a 14-month average survival after complete removal of multiple metastases in 26 cases, similar to the 14-month survival observed in a control group with matched single tumors [16]. However, in 30 patients with incomplete resection, survival was not longer than that conferred by radiation treatment alone (average approximately 6 months). Hazuka et al. [21], demonstrated a 5-month average survival in 18 patients who underwent resection of multiple metastases. In that study, one patient achieved complete resection of all metastases and survived for 48 months.
Our study suggests that survival of patients with multiple symptomatic brain metastases who underwent metastatectomy while leaving asymptomatic lesions unresected is equivalent to that in patients who underwent complete cerebral metastatectomy.
Patients with multiple metastases who underwent surgery lived substantially longer than the 3-6 month survival time reported by numerous investigators in patients who received radiation treatment alone.
The median survival was 11 months in patients with multiple brain metastases, and this result was comparable to that of 14 months we previously reported in patients with single brain metastasis after resection [15]. This is comparable to the results reported in other series, suggesting that in patients with brain metastases, surgical resection of only symptomatic brain metastases does not result in poorer survival when compared to patients with only single brain metastasis or multiple brain metastases, in which all lesions were resected. Bindal et al. [16] and Hazuka et al. [21], reported poor survival when one or more metastases remained unresected. This finding is different from ours in that there were no significant survival differences between the different types of resection: total resection of single metastasis, complete resection of all metastases, and resection of symptomatic lesions only with one or more remaining asymptomatic metastases. This may suggest that post-operative chemotherapy may confer a survival advantage because most patients with brain metastases die of primary disease progression. We believe that surgical resection remains important for the removal of large symptomatic metastases and if successful, would possibly allow patients to undergo other treatments for the remaining asymptomatic metastatic tumors, due to improved performance status.
Treatment outcomes of patients with metastatic brain tumors from non-small cell lung cancer
In most clinical studies concerning brain metastases, multiple different cancers were involved in each study which made the ratio of each pathological entity different between series. We believe that it is important to analyze survival in a single pathological entity. There have been several retrospective studies analyzing survival of NSCLC patients with brain metastases. Bonnette et al. [22] reported a median survival of 12.6 months in 103 NSCLC patients with synchronous brain metastases, who underwent brain and pulmonary lesions resection. In that series, the overall survival was 56% at 1 year, 28% at 2 years, and 11% at 5 years. However, only 4 patients from the study population had multiple brain metastases. Iwasaki et al. [23] analyzed 70 NSCLC patients with brain metastases. The authors reported that in patients who underwent lung and brain metastases resection, the survival was 66.4% and 21.9% at 1 year and 3 years, respectively. In the lung lesion-only resection group, the 1 and 3 years survival were 33.2% and 6.6%, respectively. Abrahams et al. [24] reported a median survival of 12.9 months in their series of 70 NSCLC patients with brain metastases. In their series, 1-, 2-, 3-years survivals were 52.2, 30.7, 18.1%, respectively. Our results were similar to these previous reports. Survival of the single and multiple brain metastases groups were 57.1% and 44.4%, respectively, at 1 year and 24.6% and 25.8%, respectively, at 2 years. Five-year survival for the single brain metastases group was 18.8%.
Treatment for the asymptomatic brain metastases
In our previous study, we demonstrated that patients with asymptomatic brain metastases may be treated with systemic chemotherapy alone as an initial treatment without jeopardizing their clinical outcomes [25]. WBRT may be reserved for the future as long as the brain metastases are controlled with systemic chemotherapy. This appears to be a reasonable approach when recent advances in systemic chemotherapy and molecular-targeted therapy are taken into consideration. Most NSCLC patients with brain metastases died of progressive systemic disease rather than the progression of metastatic brain lesions, even in patients who did not receive WBRT [25]. This may suggest that WBRT may not always be necessary in patients with asymptomatic brain metastases. Recently, in one retrospective study, Kim et al. [26] suggested a potential role of systemic chemotherapy instead of WBRT as an initial treatment of NSCLC patients with synchronous, asymptomatic brain metastases. After surgical resection of symptomatic lesions in patients with multiple brain metastases, the clinical status of patients is similar to those with asymptomatic brain metastases. Therefore, post cerebral metastatectomy, systemic chemotherapy may have a role in the treatment of the remaining cerebral and systemic disease. In our series, when systemic chemotherapy was administered following resection of symptomatic brain lesions, although median survival appeared to be longer than in patients who did not receive chemotherapy (12 months vs. 4 months), the difference was not statistical significant (p=0.50).
Recommendations for the treatment of multiple brain metastases
We advocate the role of systemic chemotherapy in the initial treatment of NSCLC patients with advanced systemic disease and concurrent asymptomatic or minimally symptomatic brain metastases. Patients with one or more symptomatic brain metastases due to mass effect may be considered for surgery. If one or two lesions are life threatening or highly symptomatic, surgical removal may improve the quality of life and survival of the patient. Chemotherapy and/or radiotherapy including radiosurgery may be used to treat the remaining lesions. Based on the results of our current and previous studies, primary chemotherapy for asymptomatic brain metastases may be more efficacious than WBRT and radiosurgery in case of advanced and progressive systemic disease. WBRT and radiosurgery may be helpful when the systemic disease is controlled and/or when brain lesions progress even after receiving effective systemic chemotherapy.
Survival in NSCLC patients with multiple brain metastases who underwent resection for symptomatic lesions alone is similar to that in patients who underwent complete cerebral metastatectomy. The remaining asymptomatic lesions may be treated with chemotherapy or radiotherapy. The optimal treatment modality, however, needs to be defined in prospective trials encompassing larger patient cohort.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Tsukada Y, Fouad A, Pickren JW, Lane WW. Central nervous system metastasis from breast carcinoma. Autopsy study. Cancer. 1983;52:2349–2354. doi: 10.1002/1097-0142(19831215)52:12<2349::aid-cncr2820521231>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 2.Arbit E, Wronski M. Clinical decision making in brain metastases. Neurosurg Clin N Am. 1996;7:447–457. [PubMed] [Google Scholar]
- 3.Delattre JY, Krol G, Thaler HT, Posner JB. Distribution of brain metastases. Arch Neurol. 1988;45:741–744. doi: 10.1001/archneur.1988.00520310047016. [DOI] [PubMed] [Google Scholar]
- 4.Posner JB, Chernik NL. Intracranial metastases from systemic cancer. Adv Neurol. 1978;19:579–592. [PubMed] [Google Scholar]
- 5.Amer MH, Al-Sarraf M, Baker LH, Vaitkevicius VK. Malignant melanoma and central nervous system metastases: incidence, diagnosis, treatment and survival. Cancer. 1978;42:660–668. doi: 10.1002/1097-0142(197808)42:2<660::aid-cncr2820420237>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 6.Chason JL, Walker FB, Landers JW. Metastatic carcinoma in the central nervous system and dorsal root ganglia. A prospective autopsy study. Cancer. 1963;16:781–787. doi: 10.1002/1097-0142(196306)16:6<781::aid-cncr2820160614>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 7.Galluzzi S, Payne PM. Brain metastases from primary bronchial carcinoma: a statistical study of 741 necropsies. Br J Cancer. 1956;10:408–414. doi: 10.1038/bjc.1956.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rubin P, Brasacchio R, Katz A. Solitary metastases: illusion versus reality. Semin Radiat Oncol. 2006;16:120–130. doi: 10.1016/j.semradonc.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 9.Seute T, Leffers P, ten Velde GP, Twijnstra A. Detection of brain metastases from small cell lung cancer: consequences of changing imaging techniques (CT versus MRI) Cancer. 2008;112:1827–1834. doi: 10.1002/cncr.23361. [DOI] [PubMed] [Google Scholar]
- 10.Cairncross JG, Posner JB. The management of brain metastases. In: Walker MD, editor. Oncology of the Nervous System. Boston: Martinus Nijhoff Publishers; 1983. pp. 341–377. [Google Scholar]
- 11.Pollock BE. Management of patients with multiple brain metastases. Contemp Neurosurg. 1999;21:1–6. [Google Scholar]
- 12.Kalkanis SN, Kondziolka D, Gaspar LE, et al. The role of surgical resection in the management of newly diagnosed brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol. 2010;96:33–43. doi: 10.1007/s11060-009-0061-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paek SH, Audu PB, Sperling MR, Cho J, Andrews DW. Reevaluation of surgery for the treatment of brain metastases: review of 208 patients with single or multiple brain metastases treated at one institution with modern neurosurgical techniques. Neurosurgery. 2005;56:1021–1034. discussion 1021-34. [PubMed] [Google Scholar]
- 14.Stark AM, Tscheslog H, Buhl R, Held-Feindt J, Mehdorn HM. Surgical treatment for brain metastases: prognostic factors and survival in 177 patients. Neurosurg Rev. 2005;28:115–119. doi: 10.1007/s10143-004-0364-3. [DOI] [PubMed] [Google Scholar]
- 15.Yoo H, Kim YZ, Nam BH, et al. Reduced local recurrence of a single brain metastasis through microscopic total resection. J Neurosurg. 2009;110:730–736. doi: 10.3171/2008.8.JNS08448. [DOI] [PubMed] [Google Scholar]
- 16.Bindal RK, Sawaya R, Leavens ME, Lee JJ. Surgical treatment of multiple brain metastases. J Neurosurg. 1993;79:210–216. doi: 10.3171/jns.1993.79.2.0210. [DOI] [PubMed] [Google Scholar]
- 17.Joseph J, Adler JR, Cox RS, Hancock SL. Linear accelerator-based stereotaxic radiosurgery for brain metastases: the influence of number of lesions on survival. J Clin Oncol. 1996;14:1085–1092. doi: 10.1200/JCO.1996.14.4.1085. [DOI] [PubMed] [Google Scholar]
- 18.Kondziolka D, Patel A, Lunsford LD, Kassam A, Flickinger JC. Stereotactic radiosurgery plus whole brain radiotherapy versus radiotherapy alone for patients with multiple brain metastases. Int J Radiat Oncol Biol Phys. 1999;45:427–434. doi: 10.1016/s0360-3016(99)00198-4. [DOI] [PubMed] [Google Scholar]
- 19.Loeffler JS, Alexander E., III . Radiosurgery for the treatment of intracranial metastasis. In: Alexander E III, Loeffler JS, Lunsford LD, editors. Stereotactic Radiosurgery. New York: Mc-Graw-Hill; 1993. pp. 197–206. [Google Scholar]
- 20.Shu HK, Sneed PK, Shiau CY, et al. Factors influencing survival after gamma knife radiosurgery for patients with single and multiple brain metastases. Cancer J Sci Am. 1996;2:335–342. [PubMed] [Google Scholar]
- 21.Hazuka MB, Burleson WD, Stroud DN, Leonard CE, Lillehei KO, Kinzie JJ. Multiple brain metastases are associated with poor survival in patients treated with surgery and radiotherapy. J Clin Oncol. 1993;11:369–373. doi: 10.1200/JCO.1993.11.2.369. [DOI] [PubMed] [Google Scholar]
- 22.Bonnette P, Puyo P, Gabriel C, et al. Surgical management of non-small cell lung cancer with synchronous brain metastases. Chest. 2001;119:1469–1475. doi: 10.1378/chest.119.5.1469. [DOI] [PubMed] [Google Scholar]
- 23.Iwasaki A, Shirakusa T, Yoshinaga Y, Enatsu S, Yamamoto M. Evaluation of the treatment of non-small cell lung cancer with brain metastasis and the role of risk score as a survival predictor. Eur J Cardiothorac Surg. 2004;26:488–493. doi: 10.1016/j.ejcts.2004.05.049. [DOI] [PubMed] [Google Scholar]
- 24.Abrahams JM, Torchia M, Putt M, Kaiser LR, Judy KD. Risk factors affecting survival after brain metastases from non-small cell lung carcinoma: a follow-up study of 70 patients. J Neurosurg. 2001;95:595–600. doi: 10.3171/jns.2001.95.4.0595. [DOI] [PubMed] [Google Scholar]
- 25.Lee DH, Han JY, Kim HT, et al. Primary chemotherapy for newly diagnosed nonsmall cell lung cancer patients with synchronous brain metastases compared with whole-brain radiotherapy administered first: result of a randomized pilot study. Cancer. 2008;113:143–149. doi: 10.1002/cncr.23526. [DOI] [PubMed] [Google Scholar]
- 26.Kim KH, Lee J, Lee JI, et al. Can upfront systemic chemotherapy replace stereotactic radiosurgery or whole brain radiotherapy in the treatment of non-small cell lung cancer patients with asymptomatic brain metastases? Lung Cancer. 2010;68:258–263. doi: 10.1016/j.lungcan.2009.06.008. [DOI] [PubMed] [Google Scholar]