Abstract
2α-Heteroarylethyl-1α,25-dihydroxyvitamin D3 analogues, which were designed to form a hydrogen bond between Arg274 of human vitamin D receptor (hVDR) and a nitrogen atom of the heteroaromatic ring at the 2α-position, were synthesized. Among them, 2α-[2-(tetrazol-2-yl)ethyl]-1α,25-dihydroxyvitamin D3 showed higher osteocalcin promoter transactivation activity in human osteosarcoma (HOS) cells and a greater therapeutic effect in ovariectomized (OVX) rats, osteoporosis model animals, on enhancing bone mineral density than those of active vitamin D3. X-ray cocrystallographic analysis of the hVDR-ligand complex confirms that the new hydrogen bond formation stabilized the complex.
Keywords: Vitamin D, vitamin D receptor, X-ray cocrystallographic analysis, osteocalcin, bone mineral density, in vivo antiosteoporotic effect
The pleiotropically hormonal active metabolite form of secosteroid vitamin D3, 1α,25-dihydroxyvitamin D3 [1α,25(OH)2D3], exerts its biological activity via binding and modulation of the vitamin D receptor (VDR), a member of the nuclear receptor superfamily of transcriptional regulators.1 1α,25(OH)2D3 plays pivotal roles in maintaining calcium and phosphorus homeostasis and bone remodeling. 1α,25(OH)2D3 also acts as a regulator in a wide range of fundamental physiological processes including cell growth and differentiation, immune function, embryonic development, and inflammatory reactions.2,3 One of the most important factors when 1α,25(OH)2D3 binds to the VDR is hydrogen bond formation between the 1α-OH group and the Arg274 residue of the hVDR.4
The ligand binding domain (LBD) of the hVDR contains water molecules from the A-ring anchoring moiety to the surface of the protein, called a water channel, to stabilize the VDR-[1α,25(OH)2D3] complex by forming hydrogen bond networks.5 X-ray crystallographic analyses of the VDR-[2α-(3-hydroxypropyl)-1α,25(OH)2D3 (O1C3)] and VDR-[2α-(3-hydroxypropoxy)-1α,25(OH)2D3 (O2C3)] complexes have clearly demonstrated that the terminal hydroxy group of both synthetic ligands forms a hydrogen bond with Arg274 and replaces one of the water molecules in the LBD of the hVDR to stabilize the complex;5 therefore, O1C3 and O2C3 showed 3- and 1.8-times greater binding affinity for the VDR than the natural hormone, respectively.6−8 Here we studied the effects of a heteroaromatic ring on binding to the hVDR instead of the OH group and on biological activities in vitro and in vivo.
We designed 2α-[2-(tetrazol-2-yl)ethyl]-1α,25(OH)2D3 (1a) and the related compounds 1b–f since the number of atoms from the 2α-position to the terminal oxygen of O1C3 is four, and compound 1a, for example, also consists of four atoms to the nitrogen that could coordinate to Arg274, but only 1f has five to the nitrogen atom (Figure 1).
Figure 1.
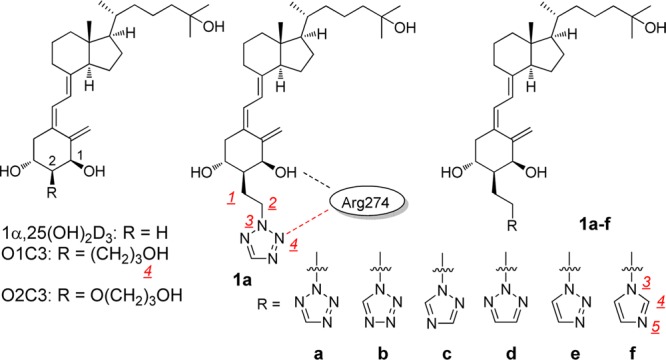
Structures of active vitamin D3 and 2α-substituted synthetic analogues, O1C3, O2C3, and 1a–f.
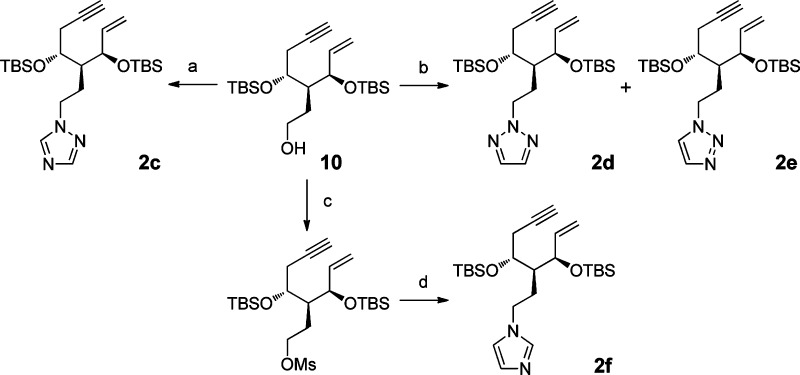
Synthesis of the A-ring precursors enyne 2a,b is shown in Scheme 1. Previously, we reported the preparation of methyl 3-deoxy-3-C-ethenylaltropyranoside 3 from methyl α-d-glucoside,9 and 3 was converted to alcohol 4 via a hydroboration–oxidation reaction after TBS protection at the 2-OH group of 3. The OH group of 4, protected by a pivaloyl group followed by our usual protocol, gave enyne 10 in good yield.10 Briefly, NBS treatment of benzylidene acetal gave bromide 5, which reacted with activated zinc powder in the presense of NaBH3CN to provide alcohol 6. The alcohol was converted to epoxide 7 through sulfonylation of the primary alcohol followed by TBAF treatment. Ethynylation of 7 using lithium TMS-acetylide in the presence of BF3·OEt2 in THF and subsequent solvolysis in K2CO3/MeOH supplied enyne 9.11 Bis-O-silylation with TBSOTf/2,6-lutidine followed by hydride reduction of the ester part afforded 10.10 Mitsunobu reaction between 10 and 1H-tetrazole gave the desired protected enynes 2a and 2b in 81% and 19% yields, respectively. When comparing 1H NMR12 and 13C NMR13 chemical shifts of appropriate methylene and methyne CHs of 2a and 2b, these two isomers were distinguishable, i.e., methylene protons of 2-N-alkyl substituted tetrazole 2a appeared in a lower field (4.68–4.82 ppm) than those of the corresponding 1-N-alkyl substituted tetrazole 2b (4.47–4.64 ppm). Instead, the methyne proton of 2a appeared in a higher field (8.46 ppm) than that of 2b (8.56 ppm) in 1H NMR; the methylene and methyne carbons of 2a (53.0 and 152.6 ppm) appeared in a lower field than those of 2b (48.2 and 142.1 ppm) in 13C NMR, respectively.12,13
Scheme 1. Synthesis of A-Ring Precursors 2a,b.
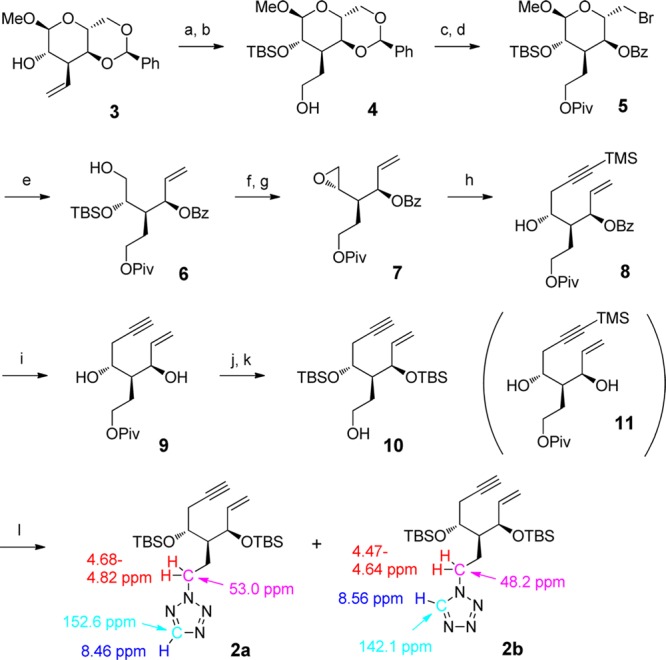
Reagents and conditions: (a) TBSOTf, 2,6-lutidine, CH2Cl2, 85%. (b) 9-BBN, THF, and then, H2O2, aq. NaOH, 92%. (c) PivCl, py, CH2Cl2, 95%. (d) NBS, BaCO3, CCl4, 87%. (e) Zn powder, NaBH3CN, 1-PrOH–H2O (5:1), 70%. (f) TsCl, py, 96%. (g) TBAF, THF, 80%. (h) BuLi, TMS-acetylene, BF3·OEt2, THF, 78%. (i) K2CO3, MeOH, 71%. (j) TBSOTf, 2,6-lutidine, CH2Cl2, 93%. (k) DIBAL-H, CH2Cl2, 92%. (l) DIAD, PPh3, 1H-tetrazole, THF, 2a 81% and 2b 19%.
Other azols, 1,2,4-triazole, 1,2,3-triazole, and imidazole, were also attached to enyne unit 10 through SN2 reactions (Scheme 2). Alkylation of 1,2,3-triazole gave two products, and 2-N-alkyl triazole 2d was the main product. For imidazole, it was necessary to produce imidazole anion by NaH since the N-alkylation reaction failed under the Mitsunobu conditions.
Scheme 2. Preparation of A-Ring Precursors 2c–f from Enyne Alcohol 10.
Reagents and conditions: (a) DIAD, PPh3, 1,2,4-triazole, THF, 94%. (b) DIAD, PPh3, 1H-1,2,3-triazole, THF, 2d 85% and 2e 15%. (c) MsCl, Et3N, CH2Cl2, 91%. (d) NaH, imidazole, THF, 71%.
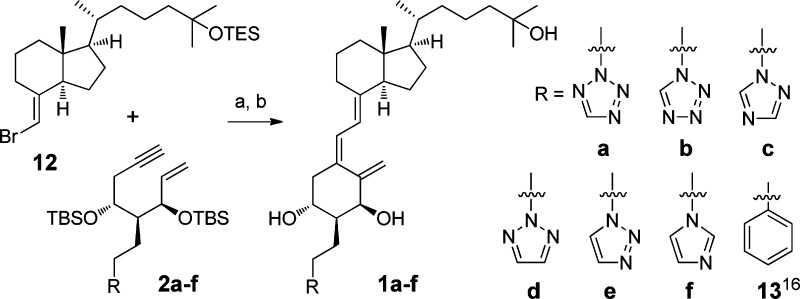
As shown in Scheme 3, the CD-ring bromoolefin 12(14) and enynes 2a–f were coupled under Trost coupling conditions15 followed by deprotection of O-silyl groups using TBAF to give the new 2α-heteroarylalkyl vitamin D3 analogues 1a–f, respectively.
Scheme 3. Trost Coupling between 12 and 2a–f.
Reagents and conditions: (a) Pd(PPh3)4, Et3N–toluene (1:1), reflux. (b) TBAF, THF, for two steps: 1a 40%, 1b 37%, 1c 29%, 1d 27%, 1e 49%, and 1f 48%.
The preliminary biological activities, hVDR binding affinity and osteocalcin promoter transactivation activity in human osteosarcoma (HOS) cells, of the new compounds 1a–f were evaluated (Table 1). Among them, 2α-[2-(tetrazol-2-yl)ethyl]-1α,25(OH)2D3 (1a) showed potent binding affinity for hVDR and greater transactivation activity (EC50 9.8 pM) than that of the natural hormone, 1α,25(OH)2D3. Analogue 1d showed moderate hVDR binding affinity and almost the same level of transactivation activity as 1α,25(OH)2D3. Compounds 1b, 1e, and 1f showed no effective binding affinity for hVDR, just like 2α-phenethyl-1α,25(OH)2D3 (13, R = Ph in Scheme 3).16
Table 1. hVDR Binding Affinity and Osteocalcin Promoter Transactivation Activity in HOS Cells.
| compd | relative hVDR binding affinity (%) | osteocalcin transactivation activity in HOS/SF cells (EC50, nM) |
|---|---|---|
| 1α,25(OH)2D3 | 100 | 0.026 |
| 1a | 64 | 0.010 |
| 1b | <1 | 0.29 |
| 1c | 4 | 0.15 |
| 1d | 12 | 0.044 |
| 1e | <1 | 0.21 |
| 1f | 2 | 0.68 |
| 13 | 1 | – |
Effects of the 2-N-alkylated tetrazole ring of 1a on the in vitro biological results were strong, and next we tested the in vivo therapeutic effect of 1a using an ovariectomized (OVX) rat as an osteoporosis model animal. Twelve-week-old Sprague–Dawley female rats were ovariectmized and fed a normal diet containing 1.0% Ca ad libitum for 4 weeks. The rats were then administrated 1a at doses of 0.007 μg/kg/day and 0.02 μg/kg/day, 5 times a week for 4 weeks. Twenty-four hours after the final administration, blood was collected under anesthesia, the rats were euthanized, and bone mineral density (BMD) of the spine (L4–L5) bone mass was measured by dual X-ray absorption meter. The results of BMD and serum Ca density are shown in Tables 2 and 3. The newly synthesized analogue 1a (Table 2) showed an increase of BMD at low doses of 0.007 and 0.02 μg/kg/day without the significant side effect of increased serum calcium, i.e., hypercalcemia compared to 1α,25(OH)2D3 (Table 3).
Table 2. Therapeutic Effects of 1a Using Ovariectomized (OVX) Rat Model.
| dose (μg/kg/day) |
BMD (g/cm2) |
serum Ca (mg/dL) |
|
|---|---|---|---|
| control (sham) | 0.231 ± 0.015 | 9.71 ± 0.27 | |
| control (OVX) | 0.209 ± 0.011 | 9.25 ± 0.11 | |
| 1a (OVX) | 0.007 | 0.214 ± 0.012 | 9.41 ± 0.20 |
| 1a (OVX) | 0.02 | 0.223 ± 0.017 | 9.66 ± 0.25 |
Table 3. Therapeutic Effects of 1α,25(OH)2D3 Using Ovariectomized (OVX) Rat Model.
| dose (μg/kg/day) | BMD (g/cm2) |
serum Ca (mg/dL) |
|
|---|---|---|---|
| control (sham) | 0.245 ± 0.010 | 9.72 ± 0.40 | |
| control (OVX) | 0.209 ± 0.019 | 9.14 ± 0.24 | |
| 1α,25(OH)2D3 (OVX) | 0.025 | 0.218 ± 0.010 | 10.14 ± 0.37 |
| 1α,25(OH)2D3 (OVX) | 0.1 | 0.228 ± 0.013 | 10.35 ± 0.27 |
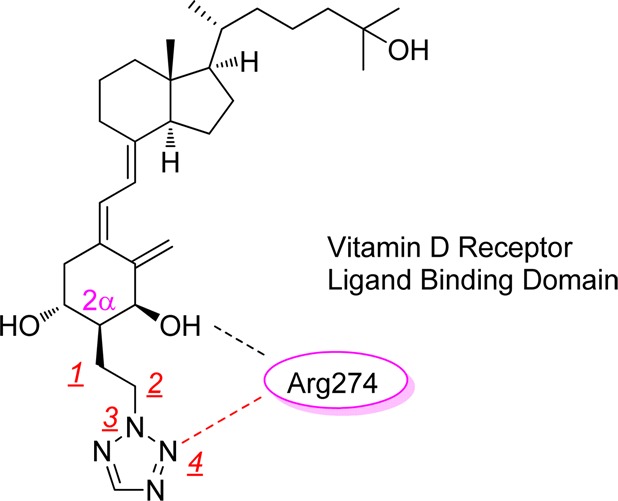
Next, we studied the crystal structure of the complex between truncated hVDR LBD and 1a to see new interactions of the tetrazole ring and amino acid residues of the LBD (Figure 2).17 The hVDR LBD–1a complex was superimposed on the hVDR LBD–1α,25(OH)2D3 complex with an root-mean-square deviation (rmsd) of 0.45 Å on all Cα atoms. Clearly, one of the nitrogen atoms of the 2-N-alkylated tetrazole ring formed a direct hydrogen bond with Arg274 (2.99 Å distance), which was also shown in the hVDR LBD–O1C3 complex between the 2α-terminal OH group of O1C3 and Arg274 to stabilize the complex after missing two water molecules from the original hVDR LBD–1α,25(OH)2D3 complex.5 Although we were also able to analyze the crystal structure of the hVDR LBD–1b complex and found that ligands 1a and 1b were superimposable in the hVDR (data not shown), it was difficult to explain why 1a showed much higher binding affinity than 1b from the crystal structures. 1-N-Alkylated tetrazole ring might have an equilibrium relationship between nitrogen and carbon atoms against Arg274 by free rotation of the 1-N-alkylated single bond in the solution. The dynamic difference in solution might reflect the lower binding affinity of the 1-N-alkylated tetrazole ring.
Figure 2.
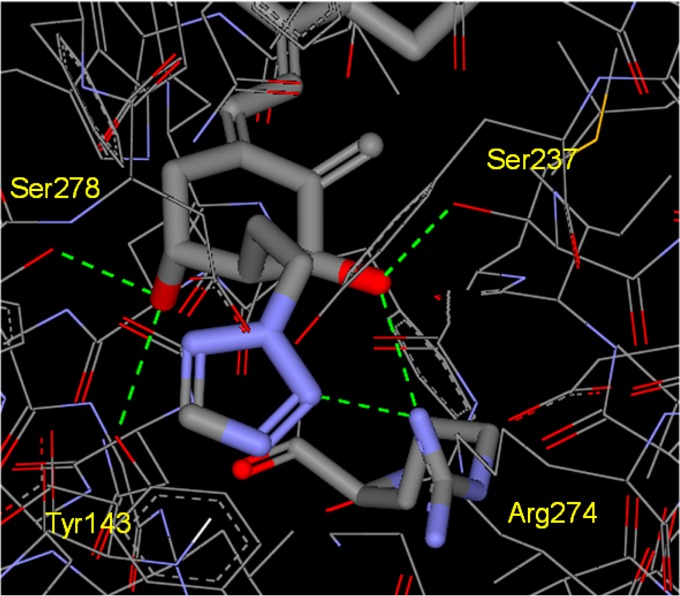
X-ray studies on hVDR LBD–1a complex. The A-ring part is magnified.
In summary, we synthesized 2α-heteroarylalkyl-1α,25-dihydroxyvitamin D3 analogues 1a–f for the first time and tested their biological activity. Among them, we found that 1a showed higher osteocalcin promoter transactivation activity in HOS cells and a greater therapeutic effect in vivo in OVX rats on enhancing bone mineral density without hypercalcemic side effects than those of the natural hormone. X-ray cocrystallographic analysis of the hVDR LBD–1a complex confirmed the new hydrogen bond formation between the nitrogen atom of the tetrazole ring and Arg274. We believe that 2α-heteroarylalkyl active vitamin D analogues would be potent candidates for osteoporosis chemotherapy.
Acknowledgments
We thank Ms. Junko Shimode and Ms. Miki Takahashi (Teikyo University) for the spectroscopic measurements.
Glossary
Abbreviations
- VDR
vitamin D receptor
- LBD
ligand binding domain
- OVX
ovariectomized
- BMD
bone mineral density
- TBS
tert-butyldimethylsilyl
- 9-BBN
9-borabicyclo[3.3.1]nonane
- PivCl
pivaloyl chloride
- NBS
N-bromosuccinimide
- TBAF
tetrabutylammonium fluoride
- TMS
trimethylsilyl
- DIBAL-H
diisobutylaluminium hydride
- DIAD
diisopropyl azodicarboxylate
Supporting Information Available
Detailed experimental procedures and spectral data for all new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
This work was supported in part by a Grant-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology (No. 23790021 to M.T.) as well as Grants-in-Aid from the Japan Society for the Promotion of Science (No. 23590015 to D.S. and No. 24590021 to A.K.).
The authors declare no competing financial interest.
Supplementary Material
References
- Mangelsdorf D. J.; Thummel C.; Beato M.; Herrlich P.; Schütz G.; Umesono K.; Blumberg B.; Kastner P.; Mark M.; Chambon P.; Evans R. M. The nuclear receptor superfamily: The second decade. Cell 1995, 83, 835–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouillon R.; Carmeliet G.; Verlinden L.; van Etten E.; Verstuyf A.; Luderer H. F.; Lieben L.; Mathieu C.; Demay M. Vitamin D and human health: lessons from vitamin D receptor null mice. Endocr. Rev. 2008, 29, 726–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman D., Pike J. W., Adams J. S., Eds. Vitamin D, 3rd ed.; Academic Press: London, U.K., 2011. [Google Scholar]
- Rochel N.; Wurtz J. M.; Mitschler A.; Klaholz B.; Moras D. The crystal structure of the nuclear receptor for vitamin D bound to its natural ligand. Mol. Cell 2000, 5, 173–179. [DOI] [PubMed] [Google Scholar]
- Hourai S.; Fujishima T.; Kittaka A.; Suhara Y.; Takayama H.; Rochel N.; Moras D. Probing a water channel near the A-ring of receptor-bound 1α,25-dihydroxyvitamin D3 with selected 2α-substituted analogues. J. Med. Chem. 2006, 49, 5199–5205. [DOI] [PubMed] [Google Scholar]
- Suhara Y.; Nihei K.; Kurihara M.; Kittaka A.; Yamaguchi K.; Fujishima T.; Konno K.; Miyata N.; Takayama H. Efficient and versatile synthesis of novel 2α-substituted 1α,25-dihydroxyvitamin D3 analogues and their docking to vitamin D receptors. J. Org. Chem. 2001, 66, 8760–8771. [DOI] [PubMed] [Google Scholar]
- Saito N.; Suhara Y.; Kurihara M.; Fujishima T.; Honzawa S.; Takayanagi H.; Kozono T.; Matsumoto M.; Ohmori M.; Miyata N.; Takayama H.; Kittaka A. Design and efficient synthesis of 2α-(ω-hydroxyalkoxy)-1α,25-dihydroxyvitamin D3 including 2-epi-ED-71 and its 20-epimers with HL-60 cell differentiation activity. J. Org. Chem. 2004, 69, 7463–7471. [DOI] [PubMed] [Google Scholar]
- 2α-Substituted active vitamin D analogues, O1C3, O2C3, and MART-10, for example, were CYP24A1 resistant and showed long half-life activity in the target cells, see:Yasuda K.; Ikushiro S.; Kamakura M.; Takano M.; Saito N.; Kittaka A.; Chen T. C.; Ohta M.; Sakaki T. Human cytochrome P450-dependent differential metabolism among three 2α-substituted-1α,25-dihydroxyvitamin D3 analogs. J. Steroid Biochem. Mol. Biol. 2013, 133, 84–92. [DOI] [PubMed] [Google Scholar]
- Honzawa S.; Yamamoto Y.; Hirasaka K.; Takayama H.; Kittaka A. Synthesis of A-ring synthon of 2α-substituted vitamin D3 analogues utilizing Grignard reaction towards methyl 2,3-anhydro-4,6-O-benzylidene-α-d-mannopyranoside. Heterocycles 2003, 61, 327–338. [Google Scholar]
- Previously, we synthesized 10 from d-xylose; see ref (6) (spectral data included).
- Under high dilution conditions (0.02 M), 8 was converted to 9 and 11 in 52% and 36% yields, respectively.
- Butler R. N.Tetrazoles. In Comprehensive Heterocyclic Chemistry; Katritzky A. R., Charles W. R., Eds.; Pergamon Press Ltd.: Oxford, U.K., 1984; Vol. 5, pp 791–838. [Google Scholar]
- Elguero J.; Marzin C.; Roberts J. D. Carbon-13 magnetic resonance studies of azoles. Tautomerism, shift reagents, and solvent effects. J. Org. Chem. 1974, 39, 357–363. [Google Scholar]
- Maeyama J.; Hiyamizu H.; Takahashi K.; Ishihara J.; Hatakeyama S.; Kubodera N. Two convergent approaches to the synthesis of 1α,25-dihydroxy-2β-(3-hydroxypropoxy)vitamin D3 (ED-71) by the Lythgoe and the Trost coupling reactions. Heterocycles 2006, 70, 295–307for spectroscopic data of 12, see Supporting Information. [Google Scholar]
- Trost B. M.; Dumas J.; Villa M. New strategies for the synthesis of vitamin D metabolites via palladium-catalyzed reactions. J. Am. Chem. Soc. 1992, 114, 9836–9845. [Google Scholar]
- Honzawa S.; Hirasaka K.; Yamamoto Y.; Peleg S.; Fujishima T.; Kurihara M.; Saito N.; Kishimoto S.; Sugiura T.; Waku K.; Takayama H.; Kittaka A. Design, synthesis and biological evaluation of novel 1α,25-dihydroxyvitamin D3 analogues possessing aromatic ring on 2α-position. Tetrahedron 2005, 61, 11253–11263. [Google Scholar]
- The Protein Data Bank accession numbers for the coordinates of the structures of the VDR complex with 1a and 1b reported in this letter are 4ITE and 4ITF, respectively.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.