Abstract
A series of noncytotoxic 4-aminoquinoline-3-hydroxypyridin-4-one hybrids were synthesized on the basis of a synergistic in vitro combination of a precursor N-alkyl-3-hydroxypyridin-4-one with chloroquine (CQ) and tested in vitro against CQ resistant (K1 and W2) and sensitive (3D7) strains of Plasmodium falciparum. In vitro antiplasmodial activity of the precursors was negated by blocking the chelator moiety via complexation with gallium(III) or benzyl protection. None of the precursors inhibited β-hematin formation. Most hybrids were more potent inhibitors of β-hematin formation than CQ, and a correlation between antiplasmodial activity and inhibition of β-hematin formation was observed. Potent hybrids against K1, 3D7, and W2, respectively, were 8c (0.13, 0.004, and 0.1 μM); 8d (0.08, 0.01, and 0.02 μM); and 7g (0.07, 0.03, and 0.08 μM).
Keywords: 4-Aminoquinoline, hydroxypyridinone, antiplasmodial, iron chelators
Malaria is estimated to affect 40% of the global population, with 330–660 million clinical cases reported annually.1 The disease is caused by mosquito-borne eukaryotic protists of the genus Plasmodium. Five species, namely, P. ovale, P. malariae, P. vivax, P. Knowlesi, and P. falciparum, have been associated with the disease in humans.2 Of these, P. falciparum is the most problematic, mainly due to its high prevalence, virulence and drug resistance.2 Currently, there are no vaccines available for malaria, so control relies on chemotherapy and insect control measures. However, resistance of malaria parasites to previously widely used drugs such as chloroquine (CQ) and of mosquitoes to pesticides remain big challenges.
Artemisinin based therapies (ACTs) are the most important drugs to treat falciparum malaria and have been recommended as first line treatment against falciparum malaria globally.3−6 However, early signs of resistance to artemisinins, with delayed parasite clearance in clinical trials in Southeast Asia are of great concern.7 There is therefore need for the discovery of new effective medicines, which are safe and efficacious against resistant P. falciparum. Many approaches including rescue of old drugs, synthesis of new pharmacophores, and use of the drugs in combination have been explored in the fight against malaria. Toward the rescue of old drugs, efforts have been made to synthesize analogues of proven pharmacophores such as endoperoxides and 4-aminoquinoline moieties. Some of the analogues have proven to be better against resistant parasites than the parent drugs.8 Knowledge of resistance mechanisms has also aided the discovery of new antimalarials. Groups that render drugs unable to act against resistant parasites have been modified. An example is CQ, where resistance is seen with the parent aminoalkyl side chain, but many CQ analogues are being synthesized with either shorter or longer alkyl chains to maintain full activity against resistant strains.9 Other approaches have included alterations of side chains via incorporation of various functional groups10−12 and metalation of the quinoline nitrogen.13−16 However, changes to the aminoquinoline nucleus alone have been shown to restore activity marginally.
Drugs with new mechanisms of action have also been explored. This is exemplified by the iron chelators; a group that works by arresting parasite growth by iron chelation. Examples of such drugs include deferiprone (DFP) and desferrioxamine (DFO), which have been evaluated in vitro, in animal models and in clinical trials.17,18
Another approach to antimalarial drug discovery is molecular hybridization. This involves the fusion of two drugs, usually via a covalent linker. The two drugs may be both active or have pharmacophoric units derived from known bioactive molecules.19,20 The approach can take advantage of good properties of one drug; for example, the transport mechanism of one compound can enhance access of the other drug to the active site. However, one drug may suffer from liabilities of the other drug. For instance, incorporation of 7-chloroquinoline could potentially result in cross-resistance of new hybrids with CQ. The 3-hydroxylpyridin-4-ones are known to chelate iron;21 thus, these compounds may exert optimal iron abrogation to limit parasite proliferation if it can access the parasitic digestive vacuole using the CQ transport.
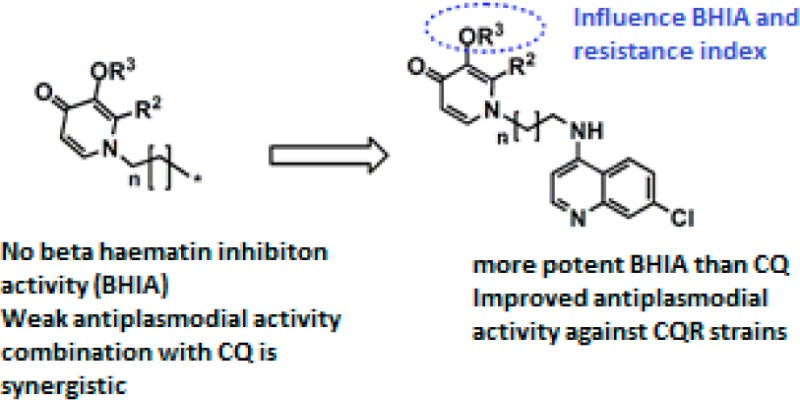
The aims of this study were to synthesize 4-aminoquinoline-3-hydroxypyridin-4-one hybrid analogues that have activity against CQ resistant P. falciparum, to study the mechanism of action of these compounds with respect to inhibition of β-hematin formation and to characterize these compounds with respect to cytotoxicity. The hybridization was justified by the synergistic antiplasmodial effect of combining N-alkyl-3-hydroxypyridin-4-ones with chloroquine, as observed in the preliminary studies (Supporting Information).
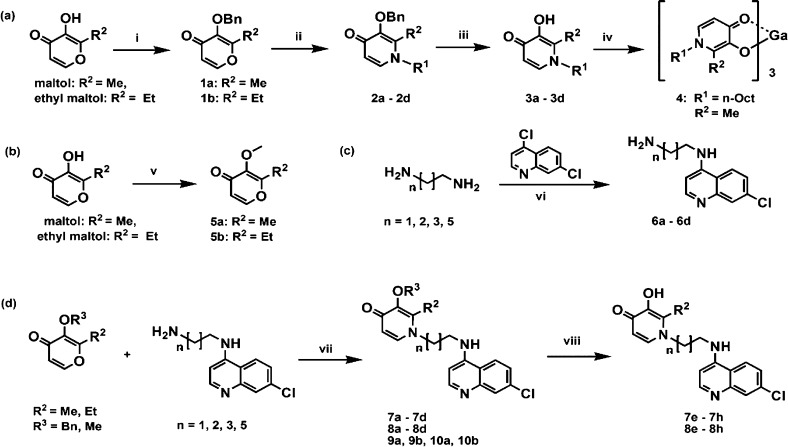
Documented procedures with some modifications were followed in the synthesis of 3-hydroxypyridin-4-ones [3,4-HPOs] (2a–2d; 3a–3d; 5a; and 5b),21N-(7-chloro-4-quinolinyl)-diaminoalkanes (6a–6d),22 and one of the gallium(III) complexes (4)23 (Scheme 1 and Table 1). The Michael addition of the N-(7-chloro-4-quinolinyl)-diaminoalkanes to the benzylated pyranones yielded the benzyl-protected hybrids as free bases (Scheme 1). The benzylated hybrid molecules were deprotected by catalytic hydrogenolysis at high pressure or by acid hydrolysis. N-(7-Chloro-4-quinolinyl)-1-(aminoalkyl)-3-(methoxy)-2-alkyl-4-(1H)-pyridinones [3-methoxylated hybrids] were prepared by reacting 3-methoxylated pyranones 5a or 5b with N-(7-chloro-4-quinolyl) alkanes (Scheme 1). Antiplasmodial activity, β-hematin inhibition, and cytotoxicity of the prepared compounds were evaluated using standard bioassays,24−26 and the results are shown in Table 1.
Scheme 1. Syntheses of N-Alkyl-3-hydroxypyridin-4-ones, Their Gallium(III) Complexes and the Hybrids.
Reagents and conditions: (i) BnCl, MeOH, 92 °C, 22 h; (ii) RNH2, pH 7 (10 M NaOH), 1:1 EtOH/H2O, 105 °C, 18 h; (iii) H2, Pd/C, 4 atm, 1:9 H2O/EtOH (aq), 4 h; (iv) Ga(NO3)3·9H2O, pH 8 (0.1 M NaOH), 80 °C, 4 h; (v) Me2SO4, (if pyranone R2 = Me) or MeI (if pyranone R2 = Et), 2.5 M KOH, acetone, rt, 12 h; (vi) neat, N2, 80 °C 1 h, then 130 °C, 3 h; (vii) 50% aq EtOH, pH 13 (2 M NaOH), 90–120 °C, 18–24 h; (viii) H2, Pd/C, 2 M ethanolic HCl, 4 atm or 1:2:3 H2O/EtOH/HCl, 74 °C, 24 h (Note: compd structural details can be found in Table 1).
Table 1. Antiplasmodial Activity, Resistance Indices, β-Hematin Inhibition, and Cytotoxicity Data for the Hybridsa.
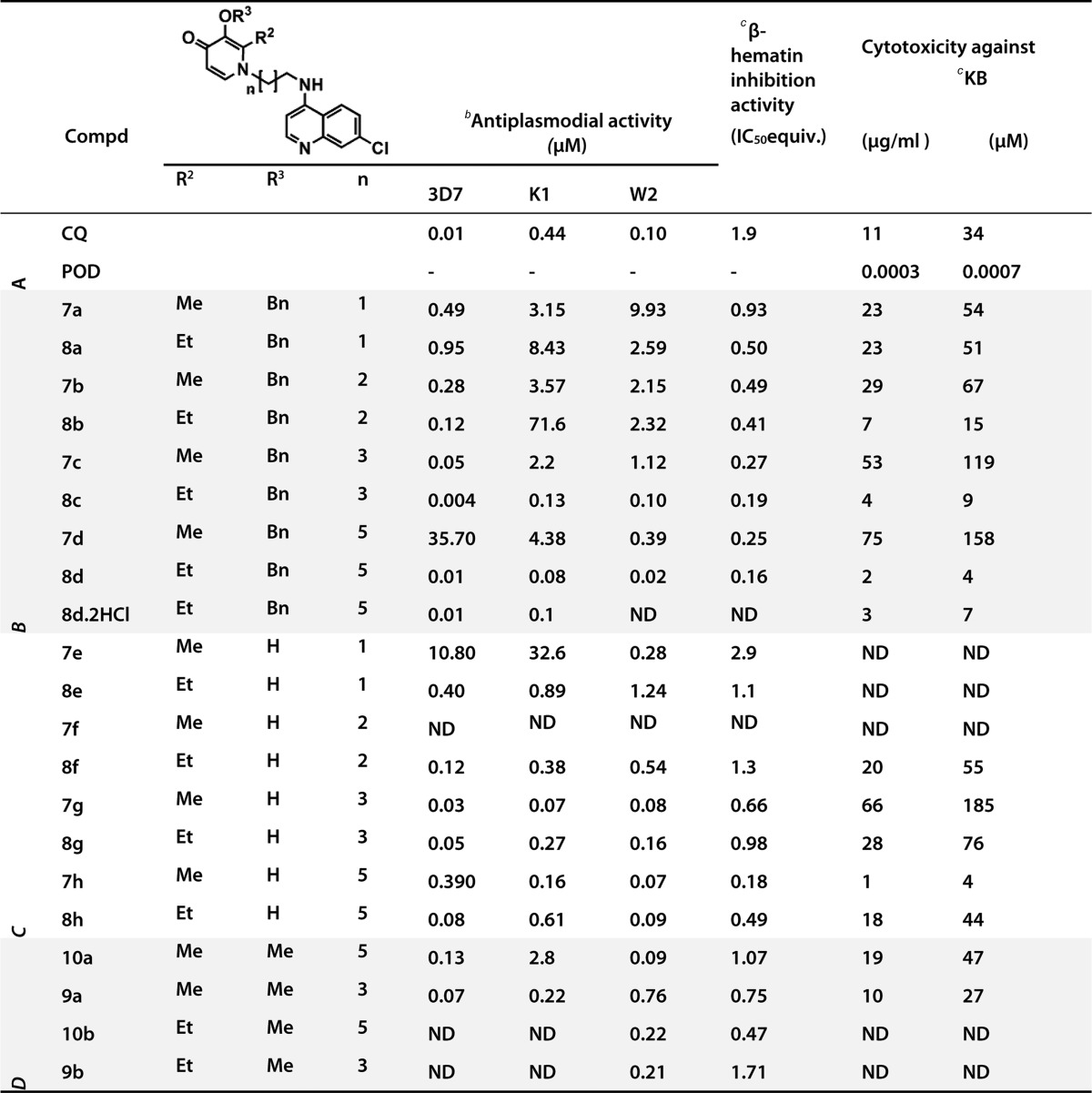
SE ≤ 7%, n = 3
IC50 (μM) antiplasmodial activity against P. falciparum.
IC50 represent the molar equivalents of test compound relative to β-hematin required to inhibit β-hematin formation by 50%; A = controls; B = benzylated analogues, C = deprotected analogues, D = methoxy analogues, and ND = not determined.
The activities of the most potent N-alkyl-3-hydroxypyridin-4-one 3d were IC50 = 2.38 and 1.8 μM against sensitive strain 3D7 and resistant strain K1 of P. falciparum, respectively (Supporting Information). In vitro antiplasmodial activity of these precursors was negated by blocking the chelator moiety via complexation with gallium(III) or protection by a benzyl group (Supporting Information). None of these compounds inhibited β-hematin formation up to a concentration of 10 IC50 equivalents of the test compounds. Antiplasmodial activity of the combinations of 2d or 3d with CQ or dihydroartemisinin was studied using the isobologram method.263d-CQ combinations were predominantly synergistic in both K1 and 3D7, and this justified the synthesis of the hybrids. In contrast, the 2d-CQ combinations were predominantly antagonistic.26 In these tests, up to a maximum concentration of 0.05 μM of 3d was used.
The hybrids proved to be potent β-hematin inhibitors unlike the N-alkyl-3-hydroxypyridin-4-ones (Table 1). Overall the β-hematin inhibition activity of all the hybrids was superior to that of CQ except for 7e and was in the range of 0.01–1.71 IC50 equiv. For both the benzylated and deprotected analogues, β-hematin inhibition activity improved with increased lipophilicity or longer alkyl linker. However, no significant difference was observed between compounds with alkyl linkers of 4 carbons or 6 carbons, e.g., 8c versus 8d.
For the most part, deprotection was observed to cause a decrease in β-hematin inhibition activity as was the replacement of the 3-benzyl group with a methoxy. This implied the involvement of the 3-benzyl group or the group at the C-3 position in the inhibition of β-hematin formation.
Strong correlations were observed between β-hematin inhibition and antiplasmodial activity in 3D7 (R2 = 0.96) and W2 (R2 = 0.93) for the benzylated analogues.26 At first sight, the correlation also appears to be strong in 3D7 (R2 = 0.92) and W2 (R2 = 0.95) for the deprotected analogues. However, if one data point (2.9, 10.8) is omitted in the determination of the correlation for deprotected analogues in 3D7, the R2 value drops to 0.045, strongly suggesting that the correlation is fortuitous. Despite this, the other significant correlations observed suggest that β-hematin inhibition is a major mode of action of these conjugates against both resistant and sensitive strains, at least for the protected analogues. Positive association between β-hematin inhibition and antiplasmodial activity was also observed for the W2 strain. Deprotection caused a decline in β-hematin inhibition but did not translate into decline in antiplasmodial activity in all compounds. This could be an indication that other mechanisms may be playing a role in maintaining antiplasmodial potency.
Generally, deprotection was found to lower the resistance indices of most of the compounds.26 Thus, the chelation or the free chelator moiety in these hybrid molecules could be responsible for enhancing the potency of the hybrids against resistant strains. However, data from the N-alkyl-3-hydroxypyridin-4-ones indicate that iron chelation by itself is not enough to cause significant antiplasmodial activity, as demonstrated by the poor activity of the precursor compounds.26 It may be hypothesized that the antiplasmodial activity of the precursor was enhanced in hybrid molecules due to accumulation in the digestive vacuole mediated by the CQ moiety.
Cytotoxicity studies in the mammalian cell line KB revealed that most of the hybrids (11 out of 16) tested were not cytotoxic as evidenced by IC50 > 10 μg/mL (Table 1). These hybrid compounds had a toxicity profile comparable to or better than that of CQ, and they were far less toxic than the standard podophyllotoxin (POD). However, there is a possibility that solubility may have precluded detecting cytotoxicity of a few of the compounds whose solubility was <10 μg/mL. For the majority compounds (12 out of 16), the cytotoxicity IC50 values were ≥10 μg/mL implying that they were soluble throughout the assay conditions. Greater selective indices (Supporting Information) were observed in the 3D7 strain compared to the KB line, with the exception of 8c. None of the compounds had better selective indices than CQ, although compounds 7c, 7g, 8c, 8g, and 8d·2HCl were comparable to CQ.
This study confirms that the antiplasmodial activity of the synthesized hybrid molecules depends largely on β-hematin inhibition. However, the iron chelator group has potential to enhance activity against resistant strains of P. falciparum for these types of compounds. Conjugation with 3,4-HPOs seems to lower the cytotoxicity of CQ at least in vitro. Currently, we are investigating the in vitro and in vivo ADME/PK properties of these hybrids alongside in vivo efficacy.
Acknowledgments
Susan Little of the London School of Hygiene and Tropical Medicine is acknowledged for performing the antiplasmodial tests against the K1 and 3D7 strains of P. falciparum as well as cytotoxicity assays.
Supporting Information Available
Experimental procedures and analytical data for some compounds including 2d, 3d, 8c, 8d, and 7g and a table showing antiplasmodial activity of N-alkyl-3-hydroxypyridin-4-ones and a gallium(III) complex. This material is available free of charge via the Internet at http://pubs.acs.org.
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript. All the authors contributed equally to this manuscript.
T.J.E. is grateful for the funding from National Research Foundation (NRF) and the Medical Research Council (MRC) of South Africa. K.C. gratefully acknowledges funding from the MRC of South Africa, University of Cape Town, and South African Research Chairs Initiative of the Department of Science and Technology administered through the NRF.
The authors declare no competing financial interest.
This letter was published ASAP on May 29, 2013, with several small errors in the text and in Table S1 of the Supporting information. The corrected version published ASAP on June 21, 2013.
Supplementary Material
References
- Snow R. W.; Guerra C. A.; Noor A. M.; Myint H. Y.; Hay S. I. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature 2005, 434, 214–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sermwittayawong N.; Singh B.; Nishibuchi M.; Sawangjaroen N.; Vuddhakul V. Human Plasmodium knowlesi infection in Ranong province, southwestern border of Thailand. Malaria J. 2012, 11361–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. Guidelines for the treatment of malaria; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- Fidock D. A. Drug discovery: Priming the antimalarial pipeline. Nature 2010, 465, 297–298. [DOI] [PubMed] [Google Scholar]
- Wiwanitkit V. New emerging drug-resistant malaria. Int. J. Gen. Med. 2010, 3, 327–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cibulskis R. E.; Bell D.; Christophel E. M.; Hii J.; Delacollette C.; Bakyaita N.; Aregawi M. W. Estimating trends in the burden of malaria at country Level. Am. J. Trop. Med. Hyg. 2007, 77, 133–137. [PubMed] [Google Scholar]
- Dondorp A. M.; Nosten F.; Yi P.; Das D.; Phyo A. P.; Tarning J.; Lwin K. M.; Ariey F.; Hanpithakpong W.; Lee S. J.; Ringwald P.; Silamut K.; Imwong M.; Chotivanich K.; Lim P.; Herdman T.; An S. S.; Yeung S.; Singhasivanon P.; Day N. P. J.; Lindegardh N.; Socheat D.; White N. J. Artemisinin resistance in Plasmodium falciparum malaria. N. Engl. J. Med. 2009, 361, 455–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan T. Quinoline antimalarials. Expert Opin. Ther. Pat. 2001, 11, 185–209. [Google Scholar]
- Dibyendu D.; Krogstad F. M.; Byers L. D.; Krogstad D. J. 4-Aminoquinolines active against chloroquine-resistant Plasmodium falciparum: basis of antiparasite activity and quantitative structure-activity relationship analyses. J. Med. Chem. 1998, 41254918–4926.9836608 [Google Scholar]
- Biot C.; Pradines B.; Sergeant M. H.; Gut J.; Rosenthal P. J.; Chibale K. Design, synthesis, and antimalarial activity of structural chimeras of thiosemicarbazone and ferroquine analogues. Bioorg. Med. Chem. Lett. 2007, 17, 6434–6438. [DOI] [PubMed] [Google Scholar]
- Burrows J. N.; Chibale K.; Wells T. N. C. The state of the art in anti-malarial drug discovery and development. Curr. Top. Med. Chem. 2011, 11, 1226–1254. [DOI] [PubMed] [Google Scholar]
- Wells T. N. C.; Alonso P. L.; Gutteridge W. E. New medicines to improve control and contribute to the eradication of malaria. Nat. Rev. Drug. Discovery 2009, 8, 879–891. [DOI] [PubMed] [Google Scholar]
- Navarro M. Gold complexes as potential anti-parasitic agents. Coord. Chem. Rev. 2009, 253, 1619–1626. [Google Scholar]
- Raynes K. Bisquinoline antimalarials: their role in malaria chemotherapy. Int. J. Parasitol. 1999, 29, 367–379. [DOI] [PubMed] [Google Scholar]
- Ajibade P.; Kolawole G. Synthesis, characterization and antiprotozoal studies of some metal complexes of antimalarial drugs. Transition Met. Chem. 2008, 33, 493–497. [Google Scholar]
- Navarro M.; Pérez H.; Sánchez-Delgado R. A. Toward a novel metal-based chemotherapy against tropical diseases. 3. Synthesis and antimalarial activity in vitro and in vivo of the new gold–chloroquine complex [Au(PPh3)(CQ)]PF6. J. Med. Chem. 1997, 40, 1937–1939. [DOI] [PubMed] [Google Scholar]
- Hershko C.; Theanacho E.; Spira D.; Peter H.; Dobbin P.; Hider R. The effect of N-alkyl modification on the antimalarial activity of 3-hydroxypyridin-4-one oral iron chelators. Blood 1991, 77, 637–643. [PubMed] [Google Scholar]
- Heppner D.; Hallaway P.; Kontoghiorghes G.; Eaton J. Antimalarial properties of orally active iron chelators. Blood 1988, 72, 358–361. [PubMed] [Google Scholar]
- Viegas-Junior C.; Danuello A.; da Silva Bolzani V.; Barreiro E. J.; Fraga C. A. M. Molecular hybridization: A useful tool in the design of new drug prototypes. Curr. Med. Chem. 2007, 14, 1829–1852. [DOI] [PubMed] [Google Scholar]
- Walsh J. J.; Bell A. Hybrid drugs for malaria. Curr. Pharm. Des. 2009, 15, 2970–2985. [DOI] [PubMed] [Google Scholar]
- Dobbin P.; Hider R.; Hall A.; Taylor P. D.; Sarpong P.; Porter J.; Xiao G.; Helm D. Synthesis, physicochemical properties, and biological evaluation of N-substituted 2-alkyl-3-hydroxypyridinones: Orally active iron chelators with clinical potential. J. Med. Chem. 1993, 36, 2448–2458. [DOI] [PubMed] [Google Scholar]
- Solomon V. R.; Haq W.; Srivastava K.; Puri S. K.; Katti S. B. Synthesis and antimalarial activity of side chain modified 4-aminoquinoline derivatives. J. Med. Chem. 2007, 50, 394–398. [DOI] [PubMed] [Google Scholar]
- Xiao G.; Van der Helm D.; Hider R. C.; Dobbin P. S. Structure–stability relationships of 3-hydroxypyridin-4-one complexes. Dalton Trans. 1992, 22, 3265–3271. [Google Scholar]
- Guantai E. M.; Ncokazi K.; Egan T. J.; Gut J.; Rosenthal P. J.; Smith P. J.; Chibale K. Design, synthesis, and in vitro antimalarial evaluation of triazole linked chalcone and dienone hybrid compounds. Bioorg. Med. Chem. 2010, 18, 8243–8256. [DOI] [PubMed] [Google Scholar]
- Ncokazi K. K.; Egan T. J. A colorimetric high-throughput beta-hematin inhibition screening assay for use in the search for antimalarial compounds. Anal. Biochem. 2005, 338, 306. [DOI] [PubMed] [Google Scholar]
- See the Supporting Information for full details.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.