Abstract
Cell-mediated immunity plays a major role in protecting the host from viral infections and tumor challenge. Here, we report the enzymatic stability and adjuvanticity of a peptiomimetic stereoisomer of the bovine neutrophil peptide indolicidin. The analogue, dubbed ld-indolicidin, contains the regular enantiomeric sequence of indolicidin and is synthesized by general stepwise solid-phase strategy. ld-Indolicidin possesses high resistance to enzymatic degradation and shows tolerance in mice. As vaccine adjuvant, ld-indolicidin is better able than the native form of indolicidin to enhance cell-mediated immune responses, using inactivated H5N1 virus as a model antigen. Taken together, these results open up a new approach to the development of vaccine adjuvants and immunotherapy technologies.
Keywords: Host defense peptides, immunoregulation, peptidomimetic stereoisomer, vaccine adjuvants
Vaccine adjuvants (from the Latin adjuvare, meaning ″to help″) are added to many vaccines to increase their immunogenicity and efficacy (see the Supporting Information for background and terminology).1−3 However, currently licensed adjuvants for human vaccines, such as aluminum-based mineral salts (Alum) and emulsion-based droplets, are either a poor adjuvant for the generation of cell-mediated immunity or a vigorous adjuvant for the induction of hypersensitive autoimmunity.3 Note that the former functions in removing virus-infected cells and in defending against cancers, and the latter plays a major role in trigging autoimmune disorders and hypersensitive reactions.3−5 Thus, a larger selection of vaccine adjuvants is needed for the design of new generation vaccines for specific applications. To this, synthetic peptides can be regarded as an interesting alternative because the precise chemical definition allows one to identify epitopes recognized by pathogen recognition receptors of the immune cells, including toll-like receptors.6,7
Indolicidin (ILPWKWPWWPWRR-NH2), isolated from the cytoplasmic granules of bovine neutrophils, is a tridecapeptide amide with boat-shaped structure and a proline- and tryptophan-rich host defense peptide (see the Supporting Information for background and terminology).8−11 Indolicidin is microbicidal in vitro against bacteria, fungi, protozoa, and human immunodeficiency virus (HIV-1).10,11 To improve the prospect for clinical application, one needs to develop adaptive immunity that has long-term protection against pathogen infections. In this case, indolicidin acts as a vaccine adjuvant to promote the antigen-specific immune responses when coadministrated with the antigen. Heretofore, the antigen-specific antibody- and cell-mediated immune responses were enhanced only in the case when indolicidin synergistically combined with CpG oligodeoxynucleotides and polyphosphazene.9 Current research generally focuses on the discovery and development of new sequence peptides with improved activity.9,10 One example is omiganan (ILRWPWWPWRRK-NH2), which has demonstrated efficacy in phase III clinical trials as a topical gel for prevention of catheter-associated infections.8 Another challenge for clinical use peptide drugs is their instability, which is probably due to the degradation in vivo by proteases. To this end, there are different strategies to protect biological active peptides from enzymatic degradation, such as cross-linkage,11 and cyclization of peptide backbone.12,13 Recently, total synthesis of peptides containing d-amino acids has been carried out, and the conformational characteristics have been analyzed.13 A particular attractive system is a peptide with regular enantiomeric sequence.13 However, little is known so far regarding the chain stereoregularity on vaccine immunogenicity.
Here, we report the enzymatic stability and immunoregulatory efficacy of an indolicidin analogue. The analogue, named ld-indolicidin, was designed to contain indolicidin peptide chain composed of alternating l and d amino acid enantiomers (Figure 1). Synthesis of native- and ld-indolicidin involves the solid-phase method using Fmoc strategy. The variable chain stereoregularity was found to be more resistant to enzymatic degradation, distinguishing it from parent peptide. As vaccine adjuvant, the peptidomimetic stereoisomer was better able than the native form of indolicidin to regulate cell-mediated immune responses.
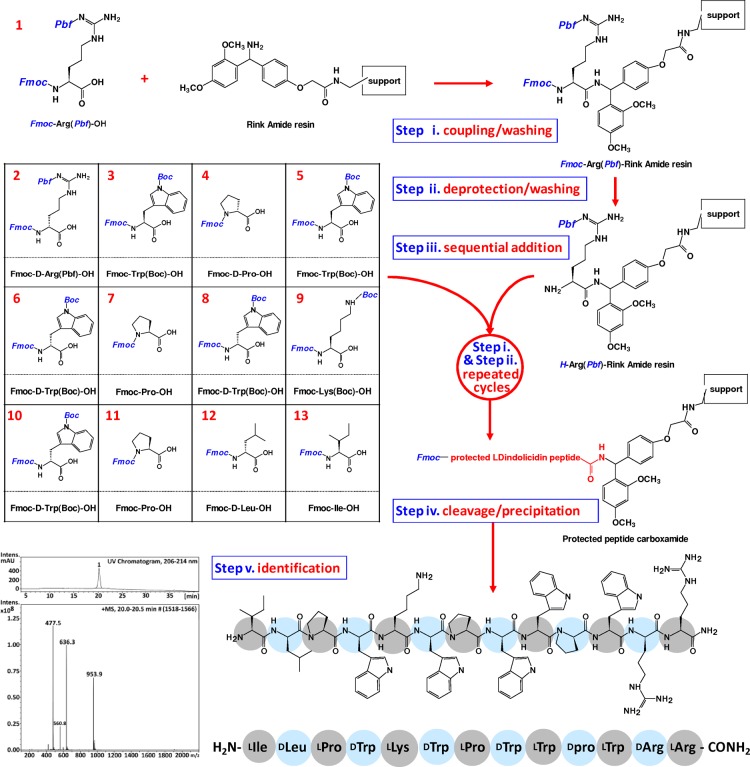
Figure 1.
Schematic representation of SPPS of ld-indolicidin in Fmoc synthesis.
The general procedure of solid-phase peptide synthesis (SPPS) consists of repeated coupling/washing/deprotecting/washing cycles of a side-chain protected amino acid stepwise attached onto a peptide-resin. This procedure allows the incorporation of d-amino acids in the design of peptides assuming well-defined configuration. Figure 1 shows the schematic representative of SPPS of ld-indolicidin in Fmoc chemistry and LC/MSD trace of the crude ld-indolicidin peptide from the recovery of precipitation. The synthesis of the ld-indolicidin peptide amide began with the loading of Fmoc-Arg(Pdf)–OH onto the resin support derivatized with the Rink Amide linker, followed by standard deprotecting of Fmoc-Arg(Pdf)-Ring Amide resin to give H-Arg(Pdf)-Rink Amide resin. The next 12 residues were then coupled sequentially on peptidyl resin, with alternating l and d amino acid enantiomers, to give Fmoc-protected ld-indolicidin peptide carboxamide. After cleavage and precipitation, ld-indolicidin peptide with a purity of at least 99% was obtained. It is noteworthy that the crude native-indolicidin from the recovery of precipitation (purity about 80%) needs to be further purified by a semipreparative HPLC.
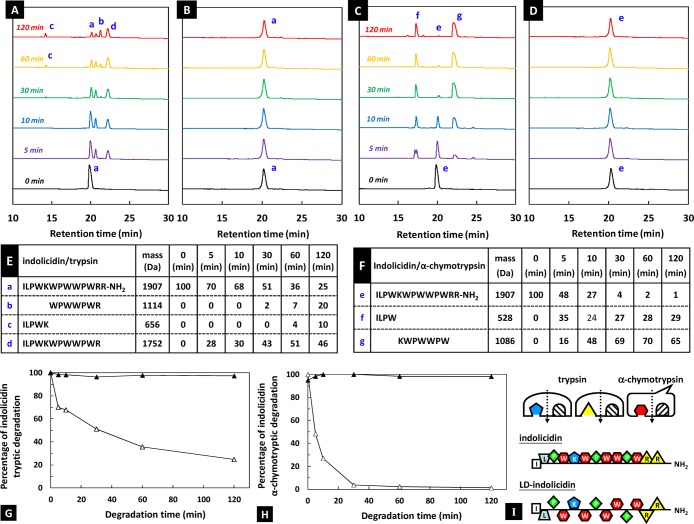
The enzymatic stability of all-l-amino acid and alternating l- and d-amino acid substitution peptide derived from indolicidin sequence was investigated in PBS at 37 °C in the presence of either trypsin (which cleaves peptide chains mainly at the carboxyl side of the amino acids lysine or arginine) or α-chymotrypsin (which preferentially cleaves peptide amide bonds where the carboxyl side of the amide bond is a tyrosine, tryptophan, or phenylalanine).11 The HPLC chromatograms of the enzymatic degradation of indolicidin and ld-indolicidin are given in Figure 2A–D, respectively.
Figure 2.
Enzymatic degradation of indolicidin and ld-indolicidin. (A–D) HPLC chromatograms of indolicidin andld-indolicidin after 0, 5, 10, 30, 60, and 120 min of tryptic or α-chymotryptic degradation. (E,F) Covalent structure of peptide fragments and (G,H) percentage of indolicidin (△) and ld-indolicidin (▲) in the PBS buffer solution containing trypsin or α-chymotrypsin at 37 °C at different time points. (I) Amino acid sequences of the peptides and enzymes used in the present study.
As shown in Figure 2A,E trypsin first made an onslaught on the C terminus of R (arginine) of native-indolicidin. Beyond, it started to attack K (lysine), thus gradually generated ILPWK and WPWWPWR fragments. As the degradation proceeded, the peak corresponding to indolicidin continued to decrease. Almost no typical indolicidin bands were detected on the HPLC chromatograms after 120 min of degradation. Similarly, indolicidin in the presence of α-chymotypsin enzyme was broken down to produce under the main components of ILPW and KWPWWPW fragments (Figure 2C,F). Although there are five W (tryptophan) in the fragment KWPWWPWW sequence, it was not further broken down. Those results suggest that the residue sequences may not completely extend in PBS solution, that WPWWPW could be folded, and that the enzyme only selectively acts on the N- and C-terminal parts of such end points. In contrast, ld-indolicidin remained almost unchanged during the degradation period, the peak corresponding to ILPWKWPWWPWRR-NH2 in the HPLC chromatograms of both enzymes remains at very high levels by 120 min (Figure 2B,D,G,H).
Overall speaking, the ld-indolicidin design involves the concepts of stereoselectivity and stereospecificity. ld-Indolicidin peptide, but not native-indolicidin, has high purity from stepwise synthesis, indicating the sequential addition of α-Fmoc protected amino acid residues undergoes a stereoselective reaction, i.e., one specific stereoisomer is loaded onto peptide resin preferentially. We also confirmed enzymatic degradation is highly stereospecific, this means an enzyme (trypsin or α-chymotrypsin, for example) recognizes the stereochemistry of indolicidin and only catalyzes its reaction if the stereochemical sequence is correct (Figure 2I).
To investigate the potential application of the indolicidin stereoisomer for vaccine adjuvant, inactivated H5N1 influenza virus was i.m.-injected into BALB/c mice. Seven days after the boost, single-cell suspensions were prepared from the mouse spleen and restimulated in vitro in the presence of inactivated virus, and we found that the secretion of IFN-γ in the ld-indolicidin-adjuvanted group was significantly higher than that in the nonadjuvanted or indolicidin-adjuvanted group (Figure 3). However, the IL-4 cytokine was found to be at the undetectable level. It should be kept in mind that IFN-γ is a predominant T helper type 1 (Th1) cytokine applicable to virus-specific cytotoxic T lymphocyte (CTL) activity, while IL-4 is a common T helper type 2 (Th2) cytokine relevant to humoral immunity.14 In addition, we found that the antigen-specific IgG antibody responses as well as virus neutralizing titers elicited in mice immunized vaccine candidates formulated either with indolicidin or ld-indolicidin were of no significant difference as compared with those obtained from mice immunized with virus alone (see Supporting Information Figure S3). The finding from IL-4 secretion was in agreement with antibody results. In contrast to the vaccine delivery systems, which mainly function as a depot to ensure the immunoavailability of antigen, the immunomodulatory adjuvants are thought to trigger the immune systems and/or bias the immune response toward Th1 or Th2. Our results suggest that ld-indolicidin, but not the native form of indolicidin, may be a potential adjuvant to modulate/regulate the immune responses.
Figure 3.
IFN-γ secretion in spleen cells following immunization in mice with inactivated H5N1 virus: no adjuvant or formulated with indolicidin/ld-indolicidin.
Influenza is a highly infectious respiratory disease caused by an influenza virus. Annual influenza vaccination is presently the most effective intervention to prevent seasonal influenza virus infection and its complications; it is also expected to be effective in controlling pandemic influenza. Cross-protective immunity is one of the key challenges in influenza vaccine development. For example, the H5N1 vaccine strain provided by WHO, NIBRG-14, belongs to clade 1; however, the strains that have continued to spread in Southeast Asia are clade 2.15 Moreover, the recommended composition of trivalent influenza virus vaccines for use in the 2011–2012 northern hemisphere influenza season contains a B/Brisbane/60/2008-like virus, but the predominate lineages belong to B/Victoria/2/87 and B/Yamagata/16/88.16 It is commonly believed that protective immunity against influenza virus infection is mediated by neutralizing antibodies, whereas T cells may limit the severity of influenza-associated illness by new strains in the absence of specific antibody responses.17 There is evidence that T cells cannot prevent virus infection but that they can sense infected cells by recognizing epitopes of viral protein complexed to human leukocyte antigen molecules on the surface of infected epithelial cells or antigen-presenting cells (APCs).17 Effective vaccination was also correlated with the induction of the Th1 cytokines (including IFN-γ), especially in the elderly, who undergo a shift toward Th2 cytokine (such as IL-4) production and a relative reduction in CTL activity as they age.14 Therefore, increasing IFN-γ induction via vaccination is thought to be an important strategy for overcoming the age-related influenza susceptibility.
After vaccination, the first step to elicit vaccine responses is to provide sufficient danger/alarm signals through vaccine antigens and/or adjuvant.4 Conventional adjuvants, such as Alum and oil-in-water emulsions, have been reported to favor the recruitment and activation of APCs by creating an immunocomponent-rich environment at the site of injection in muscle tissue.18 Compared with indolicidin, ld-indolicidin helped the antigen to elicit an effective immune response, suggesting that the conformational structure of ld-indolicidin is capable of recognized by the immune cells as signs of danger/alarm. Despite the excitement about how adjuvants work, adjuvanted vaccines probably foster either accepted or hypersensitive autoimmune diseases (boost the immune system too much).2,19 There is no doubt the major regulatory hurdle in the adjuvant development is not to obtain a potent adjuvant but to obtain one adjuvant compound or adjuvant combination having the ability to induce so-called ″appropriate immune responses″. Conferring this, further investigations are under way to optimize the adjuvant efficacy of the combinations of the small host defense peptide analogues together with a vaccine delivery system. Such applications will require further investigations whether the vaccine formulation with a peptidomimetic stereoisomer is beneficial on the host immune cells (e.g., B-cells, T-cells, and APCs) or to skew the immune response toward Th1 or Th2 when compared to vaccine antigen alone.
Here, we reported the first investigation of a peptio-mimetic stereoisomer with alternating l- and d-amino acid substitution of the bovine antimicrobial peptide indolicidin. Our results will be of great interest for the design of future vaccines against the emerging infectious diseases, in particular pandemic influenza. These advances also offer the potential in the immunotherapeutic treatment for cancers, chronic inflammation, and autoimmune diseases.
Acknowledgments
We wish to thank the Laboratory Animal Center of NHRI for technical support in biochemical experiment and the Pathology Core Laboratory of NHRI for histological examination.
Supporting Information Available
Background, terminology, and experimental details. This material is available free of charge via the Internet at http://pubs.acs.org.
Author Contributions
∥ These authors contributed equally to this work.
This work was supported by Department of Health of Taiwan to NIIDV of NHRI under the projects of ″Cell-based H5N1 influenza vaccine development and clinical trials″, and NHRI (101A1-IVPP24-014), and National Science Council of Taiwan (NSC-101-2320-B-400-011) awarded to M.-H.H.
The authors declare no competing financial interest.
Supplementary Material
References
- Huang M. H.; Leng C. H.; Liu S. J.; Chen H. W.; Sia C.; Chong P.. Vaccine Delivery Systems Based on Amphiphilic Bioresorbable Polymers and Their Role in Vaccine Immunogenicity. In Immunogenicity; Villanueva C. J., Ed.; Nova Science Publishers: New York, 2011; pp 61–90. [Google Scholar]
- Schubert C. Boosting our best shot. Nat. Med. 2009, 15, 984–988. [DOI] [PubMed] [Google Scholar]
- O’Hagan D.; De Gregorio E. The path to a successful vaccine adjuvant: ‘The long and winding road’. Drug Discovery Today 2009, 14, 541–551. [DOI] [PubMed] [Google Scholar]
- Hubbell J. A.; Thomas S. N.; Swartz M. A. Materials engineering for immunomodulation. Nature 2009, 462, 449–460. [DOI] [PubMed] [Google Scholar]
- Seder R. A.; Hill A. V. S. Vaccines against intracellular infections requiring cellular immunity. Nature 2000, 406, 793–798. [DOI] [PubMed] [Google Scholar]
- Rudra J. S.; Tian Y. F.; Jung J. P.; Collier J. H. A self-assembling peptide acting as an immune adjuvant. Proc. Natl. Acad. Sci. U.S.A. 2010, 107, 622–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki A.; Medzhitov R. Regulation of adaptive immunity by the innate immune system. Science 2010, 327, 291–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse T.; Kristensen H. H. Using antimicrobial host defense peptides as anti-infective and immunomodulatory agents. Expert Rev. Anti-Infect. Ther. 2008, 6, 887–895. [DOI] [PubMed] [Google Scholar]
- Kovacs-Nolan J.; Latimer L.; Landi A.; Jenssen H.; Hancock R. E. W.; Babiuk L. A.; van Drunen Littel-van den Hurk S. The novel adjuvant combination of CpG ODN indolicidin and polyphosphazene induces potent antibody- and cell-mediated immune responses in mice. Vaccine 2009, 27, 2055–2064. [DOI] [PubMed] [Google Scholar]
- Falla T.; Hancock R. E. W. Improved activity of a synthetic indolicidin analog. Antimicrob. Agents Chemother. 1997, 41, 771–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osapay K.; Tran D.; Ladokhin A. S.; White S. H.; Henschen A. H.; Selsted M. E. Formation and characterization of a single Trp-Trp crosslink in indolicidin that confers protease stability without altering antimicrobial activity. J. Biol. Chem. 2000, 275, 12017–12022. [DOI] [PubMed] [Google Scholar]
- Shepherd N. E.; Abbenante G.; Fairlie D. P. Consecutive cyclic pentapeptide modules form short α-helices that are very stable to water and denaturants. Angew. Chem., Int. Ed. 2004, 43, 2687–2690. [DOI] [PubMed] [Google Scholar]
- Rosenthal-Aizman K.; Svensson G.; Unden A. Self-assembling peptide nanotubes from enantiomeric pairs of cyclic peptides with alternating d and l amino acid residues. J. Am. Chem. Soc. 2004, 126, 3372–3373. [DOI] [PubMed] [Google Scholar]
- McElhaney J. E.; Xie D.; Hager W. D.; Barry M. B.; Wang Y.; Kleppinger A.; Ewen C.; Kane K. P.; Bleackley R. C. T cell responses are better correlates of vaccine protection in the elderly. J. Immunol. 2006, 176, 6333–6339. [DOI] [PubMed] [Google Scholar]
- Webster R. G.; Govorkova E. A. H5N1 Influenza: continuing evolution and spread. N. Engl. J. Med. 2006, 355, 2174–2177. [DOI] [PubMed] [Google Scholar]
- Recommended composition of influenza virus vaccines for use in the 2012–2013 northern hemisphere influenza season. Wkly. Epidemiol. Rec. 2012, 87, 83–96. [PubMed] [Google Scholar]
- Wilkinson T. M.; Li C. K. F.; Chui C. S. C.; Huang A. K. Y.; Perkins M.; Liebner J. C.; Lambkin-Williams R.; Gilbert A.; Oxford J.; Nicholas B.; Staples K. J.; Dong T.; Douek D. C.; McMichael A. J.; Xu X. N. Pre-existing influenza-specific CD4+ T cells correlate with disease protection against influenza challenge in humans. Nat. Med. 2012, 18, 274–281. [DOI] [PubMed] [Google Scholar]
- Mosca F.; Tritto E.; Muzzi A.; Monaci E.; Bagnoli F.; Iavarone C.; O’Hagan D.; Rappuoli R.; de Gregorio E. Molecular and cellular signatures of human vaccine adjuvants. Proc. Natl. Acad. Sci. U.S.A. 2008, 105, 10501–10506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offit P. A.; Jew R. K. Addressing parents’ concerns: do vaccines contain harmful preservatives adjuvants, additives, or residuals?. Pediatrics 2003, 112, 1394–1397. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.