Abstract
Objective
To evaluate the hepatoprotective and antioxidant activity of Clitoria ternatea (C. ternatea) flower extract against acetaminophen-induced liver toxicity.
Methods
The antioxidant property of C. ternatea flower extract was investigated by employing established in vitro antioxidant assay. The C. ternatea flower extract was studied in this work for its hepatoprotective effect against acetaminophen-induced liver toxicity in mice. Activity was measured by monitoring the levels of aspartate aminotransferase, alanine aminotransferase, billirubin and glutathione with histopathological analysis.
Results
The amount of total phenolics and flavonoids were estimated to be 105.40±2.47 mg/g gallic acid equivalent and 72.21±0.05 mg/g catechin equivalent respectively. The antioxidant activity of C. ternatea flower extract was 68.9% at a concentration of 1 mg/mL and was also concentration dependant, with an IC50 value of 327.00 µg/mL. The results of acetaminophen-induced liver toxicity experiment showed that mice treated with the extract (200 mg/kg) showed a significant decrease in alanine aminotransferase, aspartate aminotransferase, and bilirubin levels, which were all elevated in the paracetamol group (P<0.05). Meanwhile, the level of glutathione was found to be restored in extract treated animals compared to the groups treated with acetaminophen alone (P<0.05). Therapy of extract also showed its protective effect on histopathological alterations and supported the biochemical finding.
Conclusion
The present work confirmed the hepatoprotective effect of C. ternatea flower against model hepatotoxicant acetaminophen.
Keywords: Clitoria ternatea flower, Acetaminophen, Hepatoprotective, Antioxidant activity
1. Introduction
Clitoria ternatea L. (C. ternatea) (Family: Fabaceae) an ornamental perennial climber, up to 2-3 m in height, growing wild is a very well known medicnal plant used for different ailments, which has been investigated scientifically in considerable detail[1]. The root, stem and flower are recommended for the treatment of snakebite and scorpion sting in India[2]. In Cuba decoction of roots alone or roots and flowers are considered emmenagogue. This mixture is made by placing a handful of cleaned and macerated roots in a bottle of water. A glass taken in the evening is said to promote menstruation and induce uterine contractions and to aid in el flujoloquial. A stronger dose of the same liquid is used as a vaginal douche. An infusion of the flowers is used against the same problems. Combining a handful each of flowers and roots in a bottle of good wine one makes a magnificent medicine that is taken in one cup a day to treat clorosis (a malady of adolescents involving “impoverishment of the blood,” probably anemia) and against liver and intestinal problems[1]. Due to its attractive flower colours it is also grown as an ornamental plant[3]. The young shoots, leaves, flowers and tender pods are eaten as vegetable in Kerala (India) and in the Philippines. In Malaysia, the leaves are employed to impart a green color to food and the flowers to impart a bright blue color to rice cakes[1]. Kaempferol was isolated and identified from the flowers of C. ternatea and various phytochemicals also detected in this plant such as kaempferol, kaempferol 3-2G-rhamnosylrutinoside, kaempferol 3-neohesperidoside, kaempferol 3-rutinoside, kaempferol 3-glucoside, quercetin, quercetin 3-2G-rhamnosylrutinoside, quercetin 3-neohesperidoside, quercetin 3-rutinoside, quercetin 3-glucoside, myricetin 3-neohesperidoside, myricetin 3-rutinoside and myricetin 3-glucoside[4],[5]. Since various antioxidant kaempferols were isolated from flower, there is good potential to developed C. ternatea flower as an antioxidant and hepatoprotective agents.
Liver, which engages the essential position in the body, plays a vital role in xenobiotic metabolism and in maintaining the biological equilibrium human body. Therefore, any damage to liver can result in many disorders ranging from transient elevation in liver enzymes to life-threatening liver cirrhosis and hepatic failure[6]. Despite remarkable advances in modern medicine, hepatic disease remains a worldwide health problem; thus the search for new medicines is still ongoing[7]. Plant and natural products have been used in traditional remedies worldwide for the prevention and treatment of liver diseases and much scientific research is needed to support such claims[8]. However, to the best of our knowledge, the hepatoprotective effect of C. ternatea flower, against acetaminophen-induced liver injury in mice has not been demonstrated. Hence, the present study focused on evaluating the potential hepatoprotective effect of methanolic extract from C. ternatea leave on acetaminophen-induced liver injury in mice.
2. Materials and methods
2.1. Sample collection
Fresh flower of C. ternatea were collected from various areas in AIMST University, Kedah, Malaysia in January 2012. The flower were separated and cut into small pieces, which were first washed with tap water and then with distilled water. The flowers were then dried in an oven at 60 °C for 7 d, after which the dried flowers were ground into fine powder using a grinder.
2.2. Extraction procedure
The powdered sample (approximately 100 g) was added to methanol (300 mL) and soaked for 4 d at room temperature (30±2 °C). The suspension was stirred from time to time to allow the flower powder to fully dissolve in the methanol. Removal of the sample from the solvents was done by filtration through cheesecloth followed by filter paper (Whatman No. 1); the filtrate was concentrated under vacuum to one-fifth its volume using a rotary evaporator at 60 °C and then sterilized by filtration using a 0.22-mm membrane. The thick paste obtained was further dried in an oven at 40 °C. The resultant extract was kept at 4 °C for further analysis. The methanol was used for the extraction in this study to mimic the usage of water by the traditional healers to prepared plant extract as decoction. Water and methanol have the highest polarity in the group of polar protic solvents. Moreover the usage of water makes easier the process of evaporation compared to water.
2.3. Antioxidant activity assays
2,2-Diphenyl-1-picrylhydrazyl radical (DPPH) scavenging assay quantitative measurement of radical scavenging activity was carried out in a universal bottle. The reaction mixture obtained test sample (50 µL) ranging in concentration from 0.5 mg/mL to 6 mg/mL and 0.004 w/v % DPPH solution in methanol (5 mL, 80% v/v). The mixture without test sample was used as blank and spiked with 50 µL of blank methanol. The commercial antioxidant butylated hydroxyl toluene (BHT, Sigma) and vitamin E were used for comparison or as a positive control. Discoloration was measured at 517 nm after being incubated for 30 min. Measurements were taken in triplicates. DPPH radical's concentration was calculated using the following equation: DPPH scavenging effect(%)= Ao-A1/Ao×100 where Ao is control absorbance and A1 is the absorbance in the presence of the sample (extract of the flower)[9]. The actual decrease in the absorbance induced by the test sample was compared with the positive controls.
2.4. IC50 determination
The IC50 was defined as the amount of the sample that is sufficient to elicit 50% reduction of the initial DPPH radical concentration. This was calculated from the linear part of the inhibition of DPPH radical[10].
2.5. Determinations of total phenolic contents
Total phenolic content of C. ternatea flower extract was determined using the Folin-Ciocalteau assay according to the method previously described[11]. Total phenolic content was calculated from the calibration curve of a gallic acid standard solution. Results were expressed as gallic acid equivalents, in mg/g dry extract.
2.6. Determination of total flavonoid content
The amount of total flavonoid in the C. ternatea flower extract was measured spectrophotometrically following method described by Bothon et al[12]. Catechin was used as standard to construct a calibration curve. Various concentrations of C. ternatea flower extract (1.5 mL) were mixed with 1.5 mL of 2 % AlCl3 methanolic solution. After incubation at room temperature for 15 min, the absorbance of the reaction mixture was measured at 430 nm. The flavonoids content was expressed as mg catechin equivalents in 1 g extract (mg CE/g).
2.7. Hepatoprotective activity of C. ternatea flower extract
2.7.1. Animals
Eighteen specific pathogen free and age-matched (7- to 10-week-old) male Wister albino mice were used to study the hepatoprotective activity of the C. ternatea flower extract. The Institution Animal Ethics Committee of AIMST University has approved the animal study for this project (AUHAEC 62/FAS/2011). The animals were kept at 27±2 °C, relative humidity 44-56% and light and dark cycles of 10 and 14 h respectively, for a week before and during the experiments. Animals were provided with standard diet (Lipton, India) and water ad libitum. The food was withdrawn 18-24 h before starting the experiment. All experiments were performed in the morning according to current guidelines for the care of the laboratory animals and the ethical guidelines for the investigation of experimental pain in conscious animals[13].
2.7.2. Acetaminophen dose regimen
Acetaminophen tablets were obtained from a nearby clinic. Each tablet contains 500 mg of acetaminophen. The dose administered to the mice was set as 1 g/kg. The acetaminophen was made into fine powder using a mortar and pestle to increase the dissolution. The powdered acetaminophen was suspended in saline and was administered orally according to the body weight of mice.
2.7.3. Grouping of mice and treatments
Eighteen mice (25-30 g) were randomly divided into three groups and each group consists of 6 mice. The first group received a single daily dose of 1 mL/kg of saline orally (control group). Group II was given a single daily dose of acetaminophen (1.0 g/kg) orally (induced group) and group III received orally a single daily dose of both 1.0 g/kg acetaminophen[14] and 200 mg/kg of C. ternatea flower extract (treated group) respectively. C. ternatea flower extract was administered 3 h after the administration of acetaminophen. Acetaminophen 1 g/kg was given to mice to induce hepatotoxicity. The treatments were continued for 7 d and on the 8th day of the experiment; all animals were anesthetized and dissected[15].
2.7.4. Sacrifice and organ harvesting
The liver was removed carefully after euthanizing and killing the animals by cervical dislocation. The livers were fixed in 10% buffered formalin. After fixation, the livers were dehydrated in a graded series of alcohol, cleared in xylene and embedded in paraffin wax. Multiple 5 µm sections from each block were mounted on slides and stained with hematoxylin and eosin. The remaining liver was quickly frozen in dry ice and stored at -80 °C for further analysis.
2.7.5. Liver function tests
The mice of each group were anaesthetized with ether, and blood was collected directly from the heart. It was centrifuged at 2 000 g for 10 min at 4 °C to separate the serum and kept at 4 °C to assay the activities of serum enzymes. Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were determined by the method described by Reitman and Frankel[16]. Serum bilirubin level was estimated according to Malloy and Evelyn[17].
2.7.6. Determination of reduced glutathione
The liver glutathione determination was performed according to the method of Hamid et al. using 5,5,-dithiobis-2-nitrobenzoic as substrate[18]. The liver homogenate was then prepared using Ultra Turax where liver tissue was homogenized in 0.01 mol/L phosphate buffer (pH 7.4) at 4 °C. The absorbance was read at 420 nm and the concentration of glutathione is calculated and expressed in µmol/g protein.
2.7.7. Statistical analysis
All values are mean±SEM obtained from six animals. For statistical analysis, One-way ANOVA with Duncan's variance (SPSS 15) was used to compare the groups. In all the cases a difference was considered significant when P<0.05.
3. Results
3.1. Radical scavenging assay and IC50 determination
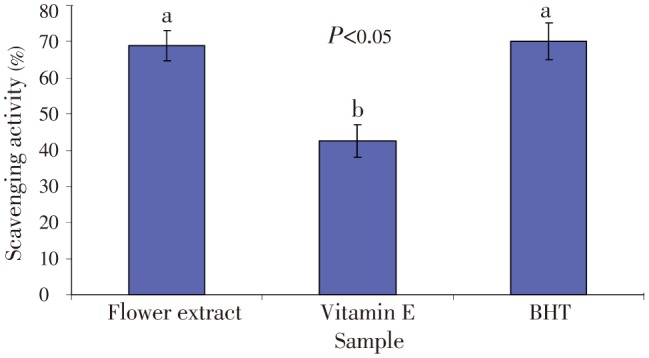
The antioxidant potential of C. ternatea leave extract was investigated in this work for new antioxidant bioactive medicinal plant extract from medicinal plant. The measured DPPH radical scavenging activity is summarised in Figure 1. The percentage of free radical scavenging activities was 68.9%, 42.5% and 70.2% for the C. ternatea flower extract, vitamin E and BHT respectively. The IC50 value is 327.00 µg/mL which is the concentration of the flower extract that decreases the initial DPPH radical concentration by 50%.
Figure 1. Scavenging effect (%) of flower extract of C. ternatea, and standard antioxidants, butylated hydroxyl toluene (BHT) and vitamin E at 1.0 mg/mL.
3.2. Total phenolic and flavonoid content
Total phenolic content of C. ternatea flower extract was determined to be 105.40±2.47 mg/g gallic acid equivalent. In addition, the total flavonoid content of C. ternatea flower extract was 72.21±0.05 mg/g catechin equivalent.
3.3. Biochemical parameters
The effect of C. ternatea extract on liver marker enzymes, serum bilirubin content and glutathione are given in Table 1. Based on Table 1, it can be observed that, the control mice group showed a normal range of AST, ALT and billirubin levels. However, the acetaminophen treated group showed an elevated level AST, ALT and billirubin. Table 1 also showed the results of extract treated group biochemical parameters were higher than the control group (P<0.05). However, it showed a much lower levels of AST, ALT and billirubin than the acetaminophen treated group. Apart from liver marker enzymes and serum bilirubin content, glutathione activity also studied in this work. As depicts in the Table 1, the level of glutathione was reduced significantly (P<0.05) with acetaminophen treatment at 1.48±0.21 µmol/g. However, when the mice were pretreated with C. ternatea flower extract, the glutathione concentration in the liver significantly elevated compared to group receiving acetaminophen only, at 2.23±1.52 µmol/g.
Table 1. Effect of C. ternatea extract on liver marker enzymes and serum bilirubin content.
| Parameters | Control | Acetaminophen Treated | Extract Treated |
| AST (IU/L) | 39.21±4.18 | 104.22±11.34** | 41.99±3.45* |
| ALT (IU/L) | 33.47±5.12 | 99.42±9.87** | 38.76±4.98* |
| Bilirubin (mg/L) | 1.43±0.60 | 8.63±1.9** | 2.80±1.10* |
| Glutathione (µmol/g) | 2.39±0.02 | 1.48±0.21 | 2.23±1.52 |
Results are expressed as mean±SEM; * Statistically significant compared to acetaminophen treated animals (P<0.05); ** Statistically significant to control animals (P<0.05).
3.4. Histopathology analysis
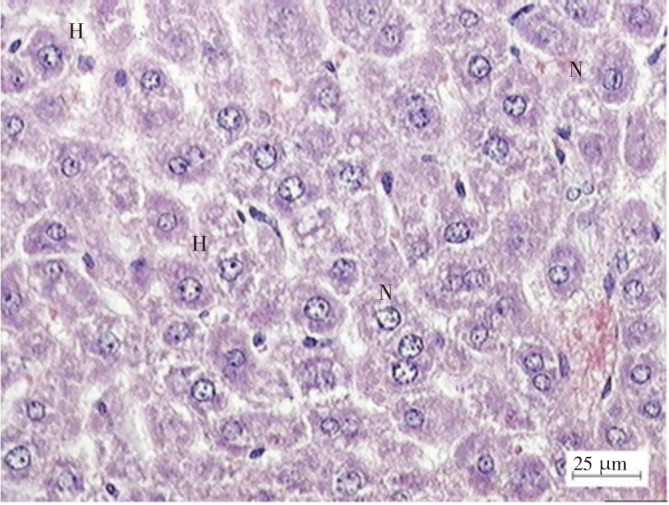
The light microscopy examination of the transverse section of acetaminophen treated and extract treated mice livers were shown in Figures 2 and 3. Figure 2 shows the liver of paracetamol intoxicated mice shows a wide necrosis across the cells. The liver sections of paracetamol intoxicated mice showed necrosis, ballooning and degeneration in hepatic plates and loss of cellular boundaries with karyolysis. There is also accumulation of neutrophils. Figure 3 shows the histological architecture of treated liver sections with mild degree of degeneration and necrosis. The hepatocytes nucleases are at recovery stage and there is very minimal numbers of neutrophils.
Figure 2. Light microphotographs of liver cell of mice exposed to acetaminophen. (lymphocytes and plasma cells (long arrows), neutrophil (short arrows), necrosis (arrow head) and hepatocyte vacuolation is also present (asterisks).
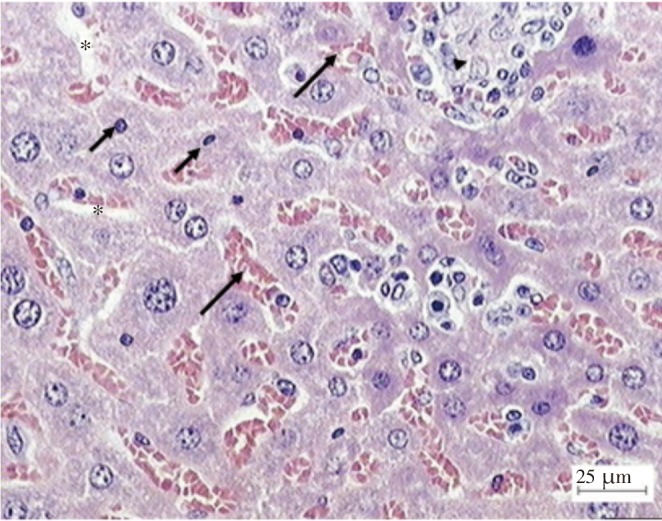
Figure 3. Light microphotographs of liver cells of mice treated with C. ternatea flower extract. (H: hepatocytes; N: nucleus).
4. Discussion
The antioxidant potential of methanolic C. ternatea flower extract was investigated in this study with the objective of identifying potentially new antioxidant bioactive medicinal plant extracts. C. ternatea flower extract exhibited good antioxidant activity in this study. Isolation and identification of various Kaempferol an antioxidant compound from the flowers C. ternatea was previously reported[4], [5]. Therefore the antioxidant kaempferols of C. ternatea flowers may responsible for the observed good antioxidant activity in this study. Phenolic and flavonoid compounds have been reported to be responsible for the antioxidant activity[19] of medicinal plants. The free radical scavenging activity exhibited by C. ternatea flower extract possibly contributed by phenolic and flavonoid compounds existed in the extract. This was confirmed by the observation of high contents of phelonic and flavonoid compounds in the C. ternatea flower extract.
The results of in vitro antioxidant activity were further confirmed with in vivo hepatoprotective study. The acetaminophen treated group showed an elevated level AST, ALT and billirubin indicates that, acetaminophen caused liver injury at higher doses in this study. The rise in serum levels of AST, ALT and billirubin has been attributed to the damaged structural integrity of the liver, because these are normally located in the cytoplasm and are released into the circulation after cellular damage[20]. Bilirubin concentration has been used to evaluate chemically induced hepatic injury. Besides various normal functions, liver excretes the breakdown product of hemoglobin namely bilirubin into bile. It is well known that necrotizing agents like acetaminophen produce sufficient injury to hepatic parenchyma to cause large increases in bilirubin content[21].
On the other hand, the extract treated group showed a very interesting result. Extract treatment significantly reduced the raised levels of AST, ALT and billirubin in hepatotoxic mice. Our results provide strong evidence that C. ternatea extract significantly inhibits the acute liver toxicity induced by high doses of acetaminophen in mice, as shown by a reduction of serum liver enzyme activities and serum bilirubin content. The decrease in the serum levels of these enzymes might possibly be due to the presence of various phenolic and flavonoid compounds in the flower extract that enhanced the regeneration ability of liver. C. ternatea flowers extract was used first time to study its hepatoprotective effect induced by acetaminophen. Moreover, improvement towards normalization of the enzymes following C. ternatea extract pretreatment suggested that the extract have some functions in preserving structural integrity of hepatocellular membrane, thus prevented enzymes leakage into the blood circulation.
In vivo hepatoprotective study proves the liver protective activity of C. ternatea flower extract against acetaminophen mediated liver toxicity. Overdose of acetaminophen results in the generation of free radicals following the depletion of glutathione[22]. Glutathione is a powerful scavenger of these free radicals which also causes self oxidation of glutathione to glutathione disulfide that further depletes glutathione stores in liver cells. It has been shown that preservation of glutathione from being depleted provides direct protection against acetaminophen induced hepatotoxicity[23].
The hepatoprotective activity of C. ternatea flower extract against acetaminophen mediated liver toxicity is then further verified by histopathalogical observations. It was well-established that overdoses of acetaminophen lead to necrosis, fatty infiltration, lymphocytic as well neutrophil infiltration[24]. The possibility of C. ternatea flower extract accelerated recovery of hepatic cells was evidenced from the histopathological observation, which suggests protection against membrane fragility thus decreased the leakage of the marker enzymes into the circulation as observed in this study. The current histopathological examination verified the hepatoprotective effect of C. ternatea against model hepatotoxicant acetaminophen.
In the present study, C. ternatea flower extract possessed good hepatoprotective and antioxidant activity in a mice model of acetaminophen-induced. The hepatoprotective activity of C. ternatea flower may be due to its free radical-scavenging and antioxidant activity, resulting from the presence of phenolic compounds in the flower extracts. Further studies are in progress to isolate the responsible compound for the hepatoprotective and antioxidant activity and to understand the mechanism of actions.
Acknowledgments
Subramanion L. Jothy and Kwan Yuet Ping are supported by the MyPhD fellowship from Ministry of Higher Education of Malaysian Government. This project was partly supported by USM Short Term Grant (304 /CIPPM /6312034) from Universiti Sains Malaysia.
Comments
Background
The objective of this study was to evaluate the hepatoprotective and antioxidant activity of C. ternatea flower extract against acetaminophen-induced liver toxicity
Research frontiers
The current study was intended to assess the in vivo and in vitro antioxidant activity of extracts of C. ternatea using various in vitro assay and in vivo assays.
Related reports
Solanki and Jain (2011) evaluated the hepatoprotective activity of C. ternatea seed and root for hepatoprotective activity. They reported that the C. ternatea possess potent hepatoprotective activity. They also suggested that the hepatoprotective activity of C. ternatea could be attributed to antioxidant properties.
Innovations & breakthroughs
This study has demonstrated that that the C. ternatea extract possesses good in vitro and in vivo antioxidant activity.
Applications
The extract from C. ternatea is a promising candidate for use as natural products based antioxidant for antiaging products development. However this needs more detail studies.
Peer review
This is an excellent work in which the authors evaluated the antioxidant activity of extract of C. ternatea flower which commonly used by traditional healer including. The results are interesting and suggested that C. ternatea flower extract possesses antioxidant activity which can apply for the development of health products.
Footnotes
Foundation Project: Partly supported by USM Short Term Grant (304 /CIPPM /6312034) from Universiti Sains Malaysia.
Conflict of interest statement: The authors declare no conflict of interest.
References
- 1.Mukherjee PK, Kumar V, Kumar NS, Heinrich M. The Ayurvedic medicine Clitoria ternatea from traditional use to scientific assessment. J Ethnopharmacol. 2008;120:291–301. doi: 10.1016/j.jep.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 2.Ponnuswamy S, Wesely JDEG. Comparative study of primary metabolites in different plant parts of Clitoria ternatea Linn. J Chem Pharm Res. 2011;3:614–617. [Google Scholar]
- 3.Mohamed N, Taha RM. Plant regeneration of Clitoria ternatea from leaf explants cultured in vitro. J Food Agric Environ. 2011;9:268–270. [Google Scholar]
- 4.Gupta GK, Chahal J, Bhatia M. Clitoria ternatea (L.): Old and new aspects. J Pharm Res. 2010;3:2610–2614. [Google Scholar]
- 5.Pendbhaje NS, Sudheendra G, Pathan SM, Musmade DS. Ethnopharmacology, pharmacogosy and phytochemical profile of Clitorea ternatea Linn: an overview. Pharmacologyonline. 2011;3:166–175. [Google Scholar]
- 6.Bera TK, Chatterjee K, Jana K, Ali KM, De D, Maiti S, et al. et al. Antihepatotoxic effect of “Livshis,” a polyherbal formulation against carbon tetrachloride-induced hepatotoxicity in male albino rat. J Nat Pharm. 2012;3:17–24. [Google Scholar]
- 7.Nithianantham K, Shyamala M, Chen Y, Latha LY, Jothy SL, Sasidharan S. Hepatoprotective potential of Clitoria ternatea leaf extract against paracetamol induced damage in mice. Molecules. 2011;16(12):10134–10145. doi: 10.3390/molecules161210134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lahon K, Das S. Hepatoprotective activity of Ocimum sanctum alcoholic leaf extract against paracetamol-induced liver damage in albino rats. Pharmacognosy Res. 2011;3:13–8. doi: 10.4103/0974-8490.79110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nehru SS, Zakaria Z, Sreenivasan S, Suryani Sutarjo S. Free radical scavenging activity of Cassia spectabilis and Cassia fistula. Int J Nat Eng Sci. 2008;2:111–112. [Google Scholar]
- 10.Ponnusha BS, Subramaniyam S, Pasupathi P, Subramaniyam B, Virumandy R. Antioxidant and antimicrobial properties of Glycine max-A review. Int J Cur Bio Med Sci. 2011;1:49–62. [Google Scholar]
- 11.Sabir SM, Rocha JBT. Antioxidant and hepatoprotective activity of aqueous extract of Solanum fastigiatum (false “Jurubeba”) against paracetamol-induced liver damage in mice. J Ethnopharmacol. 2008;120:226–232. doi: 10.1016/j.jep.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 12.Bothon FTD, Debiton E, Avlessi F, Forestier C, Teulade JC, Sohounhloue DKC. In vitro biological effects of two antidiabetic medicinal plants used in Benin as folk medicine. BMC Complem Altern Med. 2013;13:51. doi: 10.1186/1472-6882-13-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zimmerman M. Ethical guidelines for investigation of experimental pain in conscious animal. Pain. 1983;16(2):109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
- 14.Jothy SL, Aziz A, Chen Y, Sasidharan S. Antioxidant activity and hepatoprotective potential of Polyalthia longifolia and Cassia spectabilis leaves against paracetamol-induced liver injury. Evid Based Complement Alternat Med. 2012;2012:561284. doi: 10.1155/2012/561284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang TN, Ho YL, Huang GJ, Huang SS, Chen CJ, Hsieh PC, et al. et al. Hepatoprotective effect of Crossostephium chinensis (L.) Makino in rats. Am J Chin Med. 2011;39:503–521. doi: 10.1142/S0192415X11008993. [DOI] [PubMed] [Google Scholar]
- 16.Reitman S, Frankel S. A colorimetric method for the determination of serum glu-tamic oxalacetic and glutamic piruvic transaminases. Am J Clin Pathol. 1957;28:56–63. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 17.Malloy HT, Evelyn KA. The determination of bilirubin with the photochemical colorimeter. J Biol Chem. 1937;119:481–490. [Google Scholar]
- 18.Hamid A, Budin SB, Pakri Mohamed RA, Manaf NA, Yuhana NY, Husain K, et al. et al. Role of oxidative stress in the protective effects of Zingiber zerumbet smith ethyl-acetate extract against paracetamol-induced hepatotoxicity in sprague-dawley rats. Aust J Basic Appl Sci. 2011;5(8):1519–1525. [Google Scholar]
- 19.Huang B, Ban X, He J, Tong J, Tian J, Wang YW. Hepatoprotective and antioxidant activity of ethanolic extracts of edible lotus (Nelumbo nucifera Gaertn.) leaves. Food Chem. 2010;120:873–878. [Google Scholar]
- 20.Zakaria ZA, Rofiee MS, Somchit MN, Zuraini A, Sulaiman MR, Teh LK, et al. et al. Hepatoprotective activity of dried- and fermented-processed virgin coconut oil. Evid Based Complement Alternat Med. 2011;2011:142739. doi: 10.1155/2011/142739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sasidharan S, Aravindran S, Latha LY, Vijenthi R, Saravanan D, Amutha S. In vitro antioxidant activity and hepatoprotective effects of Lentinula edodes against paracetamol-induced hepatotoxicity. Molecules. 2010;15:4478–4489. doi: 10.3390/molecules15064478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yakubu N, Oboh G, Olalekan AA. Antioxidant and hepatoprotective properties of tofu (Curdle Soymilk) against acetaminophen-induced liver damage in rats. Biotechnol Res Int. 2013;2013:230142. doi: 10.1155/2013/230142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fakurazi S, Hairuszah I, Nanthini U. Moringa oleifera Lam prevents acetaminophen induced liver injury through restoration of glutathione level. Food Chem Toxicol. 2008;46:2611–2615. doi: 10.1016/j.fct.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 24.Palani S, Raja S, Senthil Kumar B. Hepatoprotective and antioxidant potential of Chloroxylon swietenia (Rutaceae) on acetaminophen induced toxicity in male Albino rats. Int J Pharm Tech Res. 2010;2:162–170. [Google Scholar]


