Abstract
Metal complexes represent today an attractive class of experimental anti-Alzheimer agents with the potential of blocking β-amyloid 1–42 aggregation and scavenging its toxicity. Three representative ruthenium(III) complexes, namely NAMI A, KP1019, and PMRU20, were specifically evaluated to this end in an established in vitro model of AD relying on primary cortical neurons. Notably, PMRU20 turned out to be highly effective in protecting cortical neurons against Aβ 1–42 toxicity, while the other tested ruthenium compounds were poorly active or even inactive; we also found that PMRU20 is virtually devoid of any significant toxicity in vitro at the applied concentrations. Interestingly, PMRU20 was neuroprotective even against the toxicity induced by Aβ 25–35. The direct reaction of PMRU20 with Aβ 1–42 was explored through ESI MS analysis and some adduct formation evidenced. In addition, thioflavin T assays revealed that PMRU20 greatly reduces Aβ 1–42 aggregation. The implications of these findings are discussed in relation to emerging treatment strategies for the Alzheimer’s disease.
Keywords: Alzheimer’s disease, β-amyloid, ruthenium(III) complex
Alzheimer’s disease (AD) is a major and incurable neurodegenerative disorder with a very high social impact.1 Its etiopathogenesis is unclear, and no effective treatment is yet available even to slow down disease progression. Among the several mechanistic theories proposed so far, the so-called “amyloid cascade hypothesis” is one of the most credited. This theory postulates that amyloid peptides are the “true” causative agent of AD; more precisely, low molecular weight “oligomers” formed in the early stages of β-amyloid (Aβ) aggregation would be the actual neurotoxic species.2,3 The “amyloid cascade hypothesis” has stimulated new drug discovery strategies and methods to identify substances capable of blocking amyloid aggregation and preventing its neurotoxicity; a number of compounds with these characteristics were indeed discovered during the last few years.4
Within this frame, Barnham et al.5,6 recently proposed that a few metal based agents might be effective in inhibiting Aβ aggregation and thus contrasting its neuropathological actions. In particular, these authors found that some platinum–phenanthroline complexes are able to bind tightly Aβ 1–42, block its aggregation, and confer an appreciable protection to cortical neurons against Aβ toxicity. Other examples of this kind of approach have appeared afterward.7 This strategy fits in the more general frame of targeting intrinsically disordered proteins (IDP) that exist as ensembles of rapidly fluctuating structures.8 Several IDP play key functional roles, including catalysis;9,10 several others are overexpressed in major diseases, where they give rise to couple folding and fibrillar aggregates, thus becoming desirable targets for inhibition.11,12
Inspired by this concept, we started considering a variety of ruthenium compounds as experimental anti-AD agents as an alternative to platinum compounds. Our research is grounded on the observation that ruthenium compounds are, on the whole, less cytotoxic—and arguably less toxic—than platinum compounds; moreover, ruthenium compounds manifest a pronounced reactivity toward histidines and might selectively affect the conformation of Aβ 1–42, bearing three histidine residues in its N-terminal portion.13 In line with these ideas, we recently reported that a ruthenium(II) complex, i.e. fac-[Ru(CO)3Cl2-(N1-thz)] (thz =1,3-thiazole), forms a stable adduct with Aβ 1–28 whereby the ruthenium(II) center is simultaneously coordinated to histidines 13 and 14.14
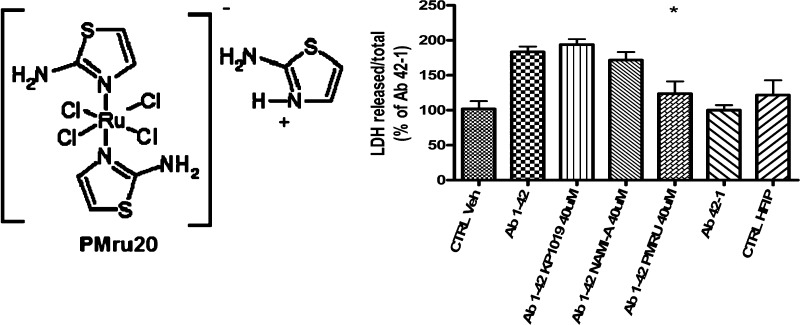
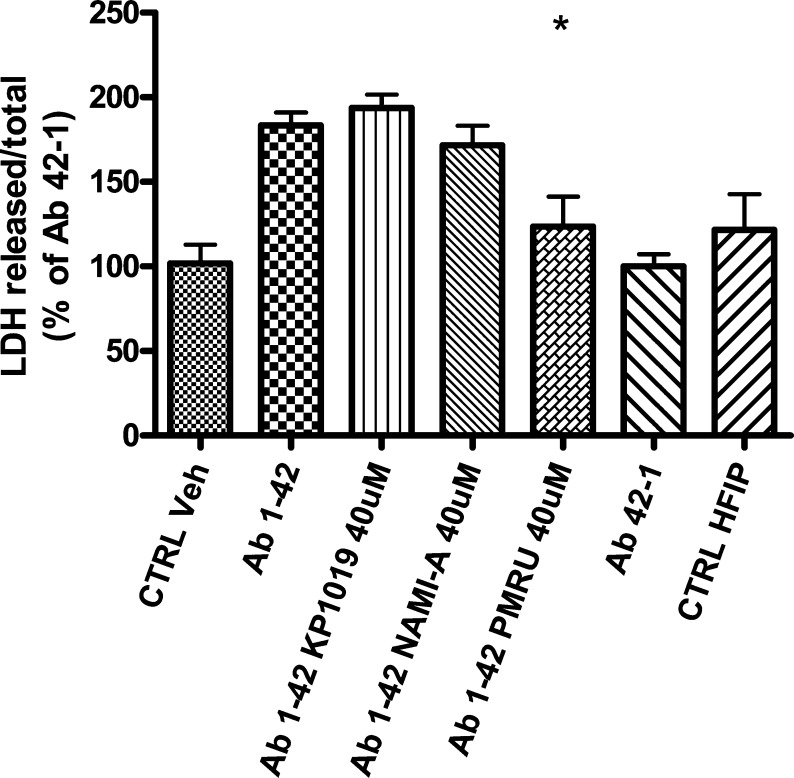
For the present investigation, we have considered three representative ruthenium compounds, namely NAMI A,15 KP1019,16 and PMRU20,17 available in our laboratory (see Chart 1).
Chart 1. Ruthenium Compounds Investigated in This Study.
NAMI A ([ImH][RuCl4(DMSO)(Im)], where DMSO is dimethyl sulfoxide and Im is imidazole), and KP1019 (indazolium trans-[tetrachlorobisindazole ruthenate(III)]) are two popular ruthenium(III) compounds that were extensively investigated and characterized as experimental anticancer agents during the last 20 years. Both are octahedral ruthenium(III) complexes bearing four chloride ions as equatorial ligands and one imidazole and one DMSO as axial ligands in the case of NAMI A and two indazoles in the case of KP1019. Specifically, NAMI A turned out to be very effective as an antimetastatic agent while being acceptably safe and scarcely cytotoxic. At variance, PMRU20 (2-aminothiazolium [trans-tetrachlorobis(2-aminothiazole) ruthenate(III)]) is a relatively newer ruthenium(III) complex of the “Keppler type”, prepared and characterized in our laboratory. The distorted octahedral ruthenium(III) center is equatorially coordinated to four chloride groups while two N-heterocyclic ligands—namely 2-aminothiazole—are metal bound in the axial positions.
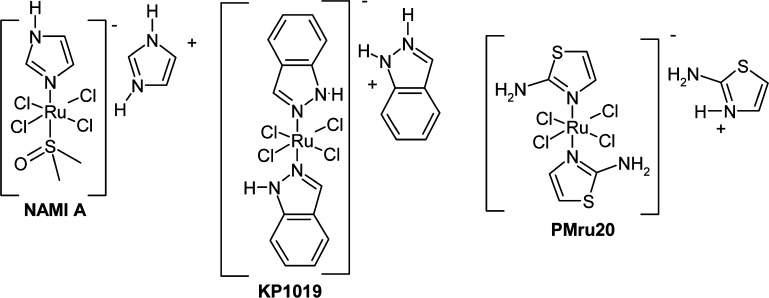
NAMI A, KP1019, and PMRU20 were assayed for their ability to confer protection against Aβ 1–42 toxicity according to an established in vitro cellular model of AD.18 The detailed methodology for preparing primary cortical neurons and for their treatment with Aβ and the various metal compounds is described in the Supporting Information (SI). Briefly, neurons were collected from brain embryos at embryonic age 17, enzymatically dissociated and grown in vitro for 8 days (8 DIV), and then challenged with Aβ for 48 h, in the presence (or absence) of ruthenium compounds. Ru compounds were solubilized in the medium used to further dilute Aβ peptides. This was done to allow compounds to be present when peptide aggregation starts. This step turned out to be very important, as addition of metal compounds after Aβ solubilization in the medium significantly decreased—but did not abolish—neuroprotection (data not shown). Then, neuronal death was checked by measuring released and intracellular Lactic Dehydrogenase (LDH), and it was expressed as external over total LDH. Results obtained in this experiment are shown in Figure 1. Remarkably, PMRU20 showed a significant activity while NAMI was poorly active and KP1019 nearly inactive.
Figure 1.
Effects of ruthenium derivatives on Aβ 1–42 toxicity. In the first test—at high concentration (40 μM)—only PMRU20 was able to counteract the toxic effects of Aβ 1–42. Multiple controls are present to demonstrate the complete inactivity of the reverse Aβ 42–1 or Aβ 35–25 and of other agents used for dissolving the amyloids (see CTR Veh and CTR HFIP; see the Experimental Section (in the SI for explanation). The symbol * indicates a significant difference from Aβ 1–42 treatment, at p < 0.05 (ANOVA plus Tukey Test).
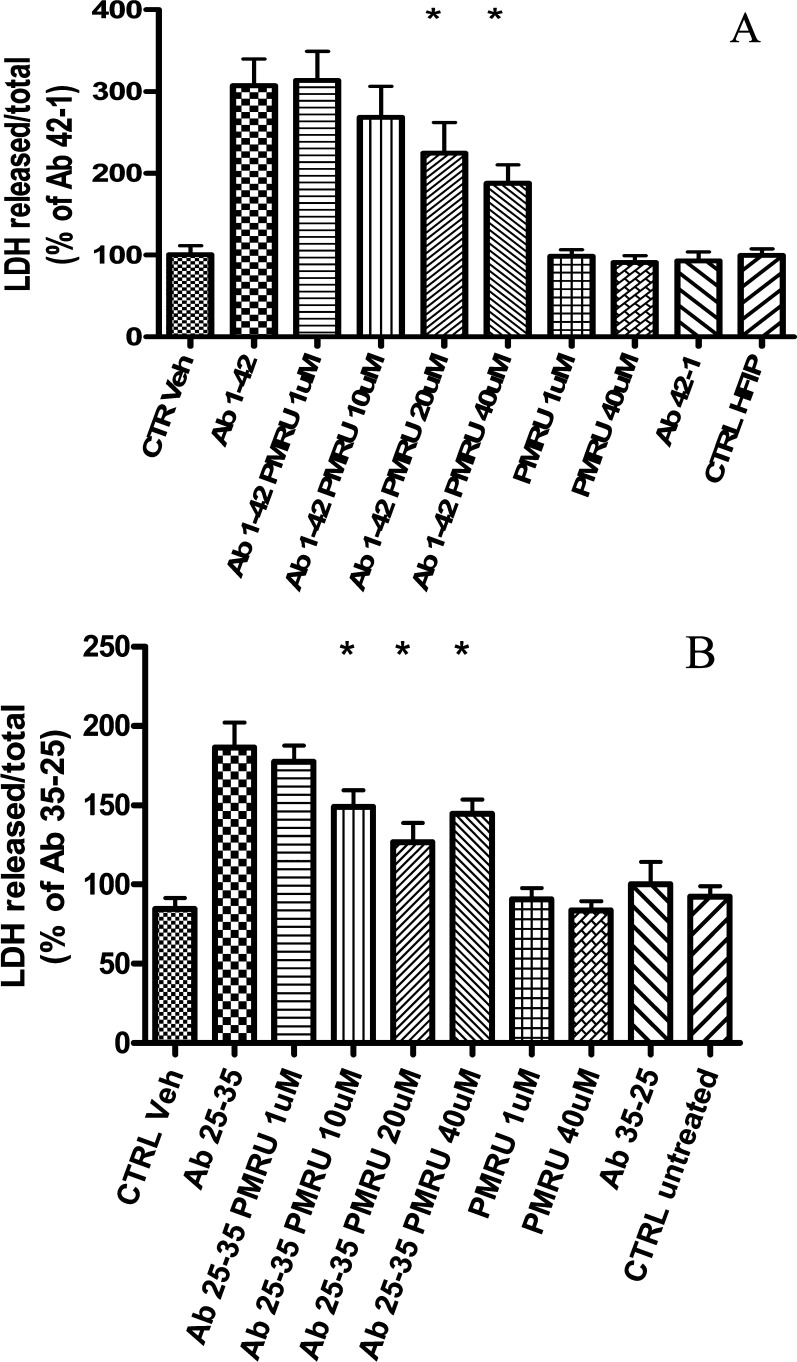
The efficacy of PMRU20 was then confirmed through an additional experiment where increasing PMRU20 concentrations (ranging from 1 μM to 40 μM) were applied. Results are shown in Figure 2. Upon inspection of Figure 2A, it clearly emerges that PMRU20 is highly effective in protecting cortical neurons against Aβ 1–42 toxicity. We also analyzed whether PMRU20 is capable of contrasting Aβ 25–35 toxicity (Figure 2B);18 again, an appreciable protection was evidenced, significantly different than that of controls (p < 0.05), though lower than that observed in the case of Aβ 1–42. The lower efficacy observed versus Aβ 25–35 might derive from lack of histidines in the latter peptide.
Figure 2.
PMRU20 turned out to be effective in protecting cultured rat cortical neurons against both Aβ 1–42 (A) and Aβ 25–35 injury (B). The toxicity of both Aβ’s is described as percent of the reverse peptide. The symbol * indicates a significant difference, of related treatments, from Aβ 1–42 (A) or Aβ 25–35 (B) at p < 0.05.
Interestingly, PMRU20 alone did not affect neuronal viability. Results in Figure 2 are comprehensive of PMRU20 alone, at two concentrations. A larger concentration–response curve was also analyzed, and PMRU20—again—did not manifest any evident toxicity at all applied concentrations (Figure S1 of the SI). As a further control, the PMRU20 counterion, 2-aminothiazole, was assayed in the presence of Aβ 1–42 and Aβ 25–35 (or alone) to rule out that the observed neuroprotective activity might arise from its known antioxidant properties.19 2-Aminothiazole showed effects neither in the presence of Aβ 1–42 or Aβ 25–35 nor by itself (Figure S2 of the SI). This makes us confident that the observed biological effects are to be ascribed—primarily—to the metal center. Confirmatory results on PMRU20 neuroprotective effects against both Aβs, obtained through imaging analysis, are shown in Figure S3 of the SI.
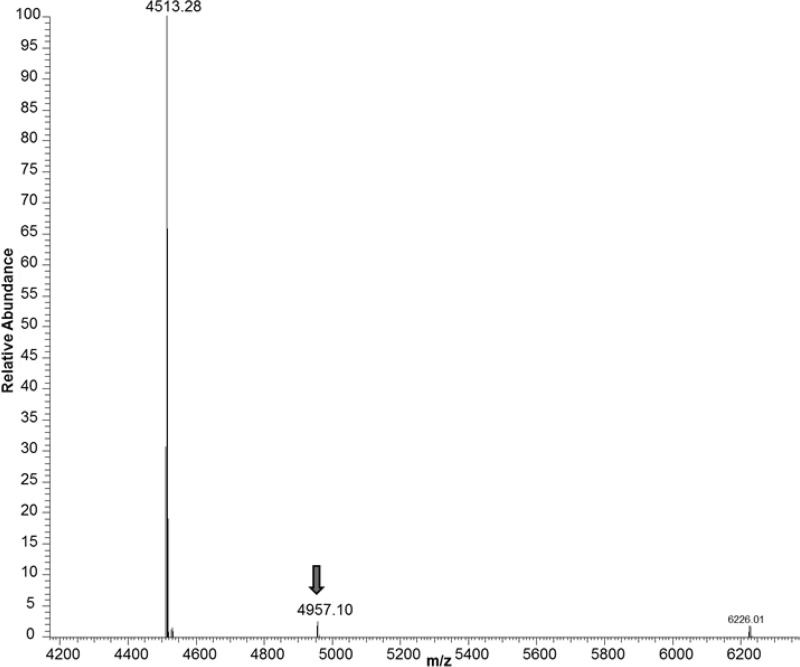
To gain more detailed insight into the ability of PMRU20 to react directly with Aβ 1–42 and modify it, Aβ1–42 was treated with a stoichiometric amount of PMRU20 and the reaction products analyzed through ESI MS spectrometry after 12 h of incubation according to established procedures.14 Some relevant ESI MS results are shown in Figure 3; it is observed that an adduct is formed between the peptide and PMRU20, though in tiny amount.
Figure 3.
Deconvoluted ESI-MS spectra of Aβ 1–42 reacted with PMRU20. The aqueous mixture of Aβ 1–42 plus PMRU20 was prepared by mixing equivalent amounts of Aβ 1–42 and PMRU20. The mixture was incubated at 37 °C for 1 h.
Analysis of the mass shift of this adduct compared to the free peptide suggests that the peptide bound molecular fragment exactly matches the molecular mass of the PMRU20 anion. The amount of formed adduct is indeed very low, suggesting that this adduct just corresponds to an ionic pair and is subject to facile cleavage under the harsh conditions of the ESI MS experiment. At variance, no evidence was obtained for ruthenium coordination to peptide side chains that might occur upon release of at least one chloride ligand. No adduct formation was observed in the ESI MS spectra upon reacting Aβ1–42 with NAMI A or KP1019.
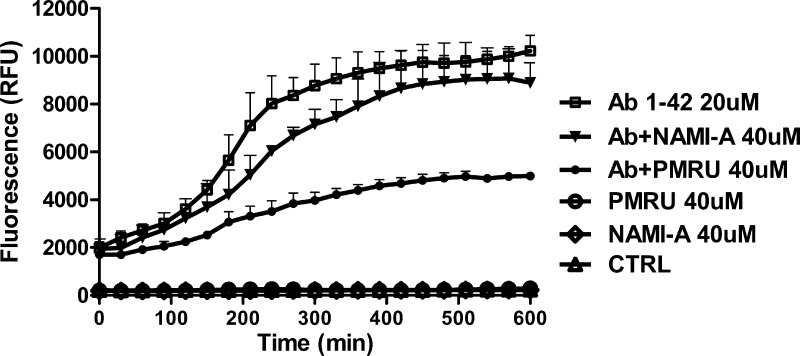
Further, we tested comparatively whether PMRU20 and NAMI A might inhibit Aβ 1–42 aggregation through the established thioflavin T assay that is commonly used to quantify formation and inhibition of amyloid aggregates.20 Experimental details are given in the SI; the obtained profiles are shown in Figure 4. It is evident that addition of PMRU20, at a 2:1 molar ratio, causes a large decrease in aggregate formation during 10 h of incubation while NAMI A turns out to be far less effective. No similar profiles could be obtained for KP1019 due to a direct reaction with the dye and precipitation. Thus, the thioflavin T assay nicely confirms occurrence of a strong interaction between PMRU20 and Aβ 1–42 that effectively reduces oligomer formation and associated neurotoxictiy.
Figure 4.
Aggregation profiles of Aβ 1–42 analyzed according to the Thioflavin T assay. The effects of PMRU20 and NAMI A on Aβ 1–42 aggregation were investigated versus controls.
In conclusion, we have shown here that a novel ruthenium(III) complex—namely PMRU20—behaves as a very effective neuroprotective agent in vitro. The level of protection afforded to cultured cortical neurons is comparable to that measured for other classical organic compounds.21 Moreover, we have found that PMRU20 is devoid of any significant toxicity in vitro. The biological actions of PMRU20 are tentatively ascribed to its ability to inhibit Aβ aggregation and prevent oligomer formation, most likely as a consequence of direct and tight interactions with the Aβ peptide. Accordingly, substantial reduction of Aβ aggregation by PMRU20 was clearly documented through thioflavin T experiments. In addition, ESI MS measurements provided evidence for the formation, in small amounts, of a stable “noncovalent” adduct between PMRU20 and Aβ 1–42. The molecular bases for the different biological behavior of PMRU20 compared to NAMI A and KP1019 need further investigation. In any case, the results presented here offer a valid support to the concept that a variety of metal based drugs may be successfully exploited for new treatment strategies of AD.
Acknowledgments
We thank Dr. Elena Michelucci for recording ESI-MS spectra.
Supporting Information Available
Experimental procedures and Figures S1, S2, and S3. This material is available free of charge via the Internet at http://pubs.acs.org.
Author Contributions
The manuscript was written through contributions of all authors.
We sincerely acknowledge financial support from Beneficentia Stiftung.
The authors declare no competing financial interest.
Supplementary Material
References
- Selkoe D. J. Alzheimer’s disease: Genes, proteins, and therapy. Physiol. Rev. 2001, 81, 741–766. [DOI] [PubMed] [Google Scholar]
- Hardy J. A.; Higgins G. A. Alzheimer’s disease: the amyloid cascade hypothesis. Science 1992, 256, 184–185. [DOI] [PubMed] [Google Scholar]
- Hardy J.; Selkoe D. J. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 2002, 297, 353–356. [DOI] [PubMed] [Google Scholar]
- Torok B.; Dasgupta S.; Torok M. Chemistry of small molecule inhibitors in self-assembly of Alzheimer’s disease related amyloid-beta peptide. Curr. Bioact. Compd. 2008, 4, 159–174. [Google Scholar]
- Barnham K. J.; Kenche V. B.; Ciccotosto G. D.; Smith D. P.; Tew D. J.; Liu X.; Perez K.; Cranston G. A.; Johanssen T. J.; Volitakis I.; Bush A. I.; Masters C. L.; White A. R.; Smith J. P.; Cherny R. A.; Cappai R. Platinum-based inhibitors of amyloid-beta as therapeutic agents for Alzheimer’s disease. Proc. Natl. Acad. Sci. U.S.A. 2008, 105, 6813–6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenche V. B.; Barnham K. J. Alzheimer’s disease & metals: therapeutic opportunities. Br. J. Pharmacol. 2011, 163, 211–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valensin D.; Gabbiani C.; Messori L. Metal compounds as inhibitors of β-amyloid aggregation. Perspectives for an innovative metallotherapeutics on Alzheimer’s disease. Coord. Chem. Rev. 2012, 256, 2357–2366. [Google Scholar]
- Dyson H. J. Expanding the proteome: disordered and alternatively folded proteins. Q. Rev. Biophys. 2011, 44, 467–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuxreiter M. Fuzziness: linking regulation to protein dynamics. Mol. Biosyst. 2012, 8, 168–77. [DOI] [PubMed] [Google Scholar]
- Zambelli B.; Cremades N.; Neyroz P.; Turano P.; Uversky V. N.; Ciurli S. Insights in the (un)structural organization of B.past.UreG, an intrinsically disordered GTPase enzyme. Mol. Biosyst. 2012, 8, 220–8. [DOI] [PubMed] [Google Scholar]
- Tompa P. Structural disorder in amyloid fibrils: its implication in dynamic interactions of proteins. FEBS J. 2009, 276, 5406–5415. [DOI] [PubMed] [Google Scholar]
- Metallo S. J. Intrinsically disordered proteins are potential drug targets. Curr. Opin. Chem. Biol. 2010, 14, 481–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin D. M.; Chasteen N. D.; Grady J. K. Biochim. Biophys. Acta 1991, 1076, 252–8. [DOI] [PubMed] [Google Scholar]
- Valensin D.; Anzini P.; Gaggelli E.; Gaggelli N.; Tamasi G.; Cini R.; Gabbiani C.; Michelucci E.; Messori L.; Kozlowski H.; Valensin G. fac-{Ru(CO)(3)}(2+) selectively targets the histidine residues of the beta-amyloid peptide 1–28. Inorg. Chem. 2010, 49, 4720–2. [DOI] [PubMed] [Google Scholar]
- Alessio E.; Mestroni G.; Bergamo A.; Sava G. Ruthenium antimetastatic agents. Curr. Top. Med. Chem. 2004, 4, 1525–1535. [DOI] [PubMed] [Google Scholar]
- Hartinger C. G.; Jakupec M. A.; Zorbas-Seifried S.; Groessl M.; Egger A.; Berger W.; Zorbas H.; Dyson P. J.; Keppler B. K. KP1019, a new redox-active anticancer agent--preclinical development and results of a clinical phase I study. Chem. Biodiversity 2008, 5, 2140–55. [DOI] [PubMed] [Google Scholar]
- Mura P.; Piccioli F.; Gabbiani C.; Camalli M.; Messori L. Structure-function relationships within Keppler-type antitumor ruthenium(III) complexes. Inorg. Chem. 2005, 44, 4897–9. [DOI] [PubMed] [Google Scholar]
- Pallitto M. M.; Ghanta J.; Heinzelman P.; Kiessling L. L.; Murphy R. M. Recognition sequence design for peptidyl modulators of beta-amyloid aggregation. Biochemistry 1999, 38, 3570–3578. [DOI] [PubMed] [Google Scholar]
- Uchikawa O.; Fukatsu K.; Suno M.; Aono T.; Doi T. In vivo biological activity of antioxidative aminothiazole derivatives. Chem. Pharm. Bull. 1996, 44, 2070–2077. [DOI] [PubMed] [Google Scholar]
- LeVine H. III. Quantification of beta-sheet amyloid fibril structures with thioflavin T. Methods Enzymol. 1999, 309, 274–284. [DOI] [PubMed] [Google Scholar]
- Michaelis M. L.; Ansar S.; Chen Y.; Reiff E. R.; Seyb K. I.; Himes R. H.; Audus K. L.; Georg G. I. β-Amyloid-induced neurodegeneration and protection by structurally diverse microtubule-stabilizing agents. J. Pharmacol. Exp. Ther. 2005, 3122659–68. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.