Abstract
The appearance of farming, from its inception in the Near East around 12 000 years ago, finally reached the northwestern extremes of Europe by the fourth millennium BC or shortly thereafter. Various models have been invoked to explain the Neolithization of northern Europe; however, resolving these different scenarios has proved problematic due to poor faunal preservation and the lack of specificity achievable for commonly applied proxies. Here, we present new multi-proxy evidence, which qualitatively and quantitatively maps subsistence change in the northeast Atlantic archipelagos from the Late Mesolithic into the Neolithic and beyond. A model involving significant retention of hunter–gatherer–fisher influences was tested against one of the dominant adoptions of farming using a novel suite of lipid biomarkers, including dihydroxy fatty acids, ω-(o-alkylphenyl)alkanoic acids and stable carbon isotope signatures of individual fatty acids preserved in cooking vessels. These new findings, together with archaeozoological and human skeletal collagen bulk stable carbon isotope proxies, unequivocally confirm rejection of marine resources by early farmers coinciding with the adoption of intensive dairy farming. This pattern of Neolithization contrasts markedly to that occurring contemporaneously in the Baltic, suggesting that geographically distinct ecological and cultural influences dictated the evolution of subsistence practices at this critical phase of European prehistory.
Keywords: Neolithic diet, archaeology, pottery, biomarkers, lipids, stable carbon isotopes
1. Introduction
The mechanism of the Neolithization of northwestern Europe has been debated for many years, with some arguing that Mesolithic inhabitants were prime movers, adopting domesticated animals and plants from their Continental neighbours, but retaining much of their own lifestyle [1]. Others argue that these domesticates were introduced as part of a novel package by immigrant farming groups from the Continent, followed by a rapid spread of Neolithic ideas [2,3]. In this ‘colonization’ scenario, a rapid acculturation of indigenous hunter–gatherer–fisher groups is envisaged, with key evidence derived from the stable carbon isotope signatures from Mesolithic and Neolithic human bone collagen. These reveal a marked difference in diet between Mesolithic and Neolithic coastal inhabitants, with enriched δ13C values in the former suggesting a significant marine protein component, while Neolithic individuals display predominantly terrestrial values [4,5].
While the faunal assemblages and strong marine isotopic signature in skeletal remains from coastal Late Mesolithic Britain are unambiguous, criticism has been levelled at the interpretation of low or non-existent contributions of marine products to the Neolithic diet. This is owing, in part, to the lack of sensitivity of the bulk collagen stable isotope approach for low-protein diets combined with possible scrambled routing of dietary carbon, which could render low quantities of marine protein (less than 20%) isotopically invisible [6,7]. Furthermore, it has been argued that possible Neolithic shell middens from Ireland and Scotland point to continued marine resource consumption, and the possibility has been raised that the skeletons investigated isotopically were not representative of the Neolithic population of Britain [6,8]; however, these critiques have been robustly rebutted [9].
In view of this controversy, we sought independent evidence based on the biomolecular and isotopic compositions of lipids preserved in prehistoric pottery from insular and coastal locations from the eastern North Atlantic, including mainland Britain, the Scottish isles and the isles of Man and Ireland (see electronic supplementary material, figure S1). This is a highly sensitive means of investigating the significance of marine product processing in pottery. Although the characteristic long-chain polyunsaturated fatty acid (PUFA) distributions of fresh marine fats and oils are lost from degraded marine lipids owing to rapid oxidation and polymerization, our recent work has identified more stable marine biomarkers, including ω-(o-alkylphenyl)alkanoic acids (APAAs) [10,11] and vicinal dihydroxy acids (DHYAs) [11,12] originating from the degradation of poly- and monounsaturated fatty acids, respectively. While APAAs require heating for formation [11,13], in the case of DHYAs heat is unnecessary because oxidation of monounsaturated fatty acids occurs spontaneously at room temperature [14]. These products preserve the carbon chain length (and, for the latter, double-bond positions) of precursor fatty acids and thus act as reliable proxies for the presence of products containing significant concentrations of long-chain PUFAs (i.e. marine or freshwater fats and oils). Although these products may exist only at low concentrations, operating the gas chromatograph/mass spectrometer (GC/MS) in selected ion-monitoring mode (SIM; see the electronic supplementary material, tables S1 and S2) greatly enhances the sensitivity of the analyses such that picogram per gram concentrations are routinely detectable.
Additionally, the determination of the δ13C values of n-alkanoic acids provides a robust signature of source(s) of dietary carbon and the metabolism of organisms. Specifically, ruminant species (cattle, sheep and goats) are separable from non-ruminants (e.g. pigs); milk fats, moreover, are separable from carcass fats, owing to more depleted δ13C values exhibited by octadecanoic acid in the former, resulting from its different biosynthetic origins [15]. Fats and oils of marine origin would exhibit higher δ13C values than terrestrial species, which is confirmed by our investigation of approximately 100 aquatic organisms from the North Atlantic (see the electronic supplementary material, figure S2) [13]. Together, these molecular and stable carbon isotope methods constitute a powerful multi-proxy approach for testing theories relating to marine and terrestrial resource processing in pottery vessels.
Our archaeological investigations focused on 1081 sherds and 142 associated carbonized deposits, yielding roughly 650 sufficiently well-preserved lipid residues for biomolecular and stable carbon isotopic analysis. A significant proportion of the sherds (more than 400; approx. 40%) derived from 48 Neolithic assemblages were chosen to increase sensitivity at this critical time. These sherds included pottery of: (i) Carinated Bowl tradition, before 3700 BC; (ii) the secondary expansion of the Neolithic to insular locations and Middle Neolithic Hebridean, ‘Unstan’ and early Grooved Wares, 3600–2900 BC; and (iii) later Neolithic Grooved and Ronaldsway Ware (2900–2300 BC; table 1; see the electronic supplementary material, figure S1). Post-firing heat discoloration, the incidence of sooting and the vessel shapes are diagnostic that these types of Neolithic vessels were commonly used for cooking, probably boiling. A longer chronological dimension was obtained from coastal and insular northern Britain, through the inclusion of 15 sites from the Bronze Age to Norse period. Existing evidence from faunal assemblages and human stable isotope information was also collated. This allowed three strands of proxy evidence for subsistence patterns to be aligned for this region, and hence chronological trends to be studied over 5000 years of prehistory.
Table 1.
Pottery from coastal and island locations included in this study.
| date | period | region | assemblages (n) | sherds (n) | visible deposits (n) | residues further analysed (n) |
|---|---|---|---|---|---|---|
| >4600 BC | Early Neolithic | Channel Isles | 5 | 22 | 1 | 8 |
| >3700 BC | Early Neolithic | mainland northern Britain, Isle of Man, island of Ireland | 14 | 218 | 12 | 124 |
| 3600–2900 BC | Middle Neolithic | Outer Hebrides, Northern Isles, mainland northern Britain, island of Ireland | 15 | 205 | 26 | 104 |
| 2900–2300 BC | Late Neolithic | Northern Isles, mainland northern Britain, Isle of Man, island of Ireland | 14 | 272 | 28 | 172 |
| 2280–800 BC | Bronze Age | Outer Hebrides, Northern Isles, Isle of Man | 6 | 79 | 8 | 40 |
| 800 BC–AD 800 | Iron Age | Outer Hebrides, Northern Isles | 6 | 162 | 25 | 100 |
| AD 800–1400 | Viking/Norse | Outer Hebrides, Northern Isles | 3 | 123 | 42 | 95 |
| Σ | 63 | 1081 | 142 | 643 |
2. Material and methods
(a). Solvent extraction
Any visible carbonized deposits were first removed using a solvent-cleaned scalpel. Small portions of the external surfaces of sherds were then cleaned using a modelling drill before the piece was removed using a chisel. Carbonized residues and cleaned sherd fragments were crushed in a solvent-washed mortar and pestle, and an internal standard added (10 or 20 μg n-tetratriacontane to the carbonized deposit or sherd fragment, respectively) prior to solvent extraction using 2 × 5 ml (carbonized residue) or 2 × 10 ml (sherd fabric) CHCl3/MeOH (2 : 1 v/v) via sonication (20 min). After centrifugation, the solvent was decanted and blown down to dryness under a gentle stream of N2.
(b). Alkaline extraction
The ‘bound’ fraction from selected sherds was released through the alkaline extraction of solvent-extracted pottery using 5 ml 0.5 M NaOH/MeOH in DCM-extracted double-distilled water (9:1 v/v; 70°, 1 h). After acidification to pH 3 using 3 M aqueous HCl, ‘bound’ lipids were extracted using 3 × 3 ml DCM.
(c). Preparation and analysis of trimethylsilyl ethers and esters
Aliquots of the solvent and alkaline extracts were filtered through a silica column and treated with 40 µl N,O-bis(trimethylsilyl)trifluoroacetamide (BSTFA) containing 1% trimethylsilyl chloride (70°, 1 h). These derivatives were analysed using high-temperature gas chromatography, using a GC fitted with a high-temperature non-polar column (DB1-HT; 100% dimethylpolysiloxane, 15 m × 0.32 mm i.d. 0.1 µm film thickness). The temperature programme comprised a 50°C isothermal hold followed by an increase to 350° at 10° min−1, followed by a 10 min isothermal hold. Alkaline extracts were immediately introduced via a split/splitless injector onto a GC/MS fitted with a non-polar column (100% dimethyl polysiloxane stationary phase; 60 m × 0.25 mm i.d. 0.1 µm film thickness). The instrument was a ThermoFinnigan single quadrupole TraceMS run in EI mode (electron energy 70 eV, scan time of 0.6 s). Samples were first run in full scan mode (m/z 50–650) and then SIM, scanning for the two cleavage fragments and [M-15]+ ions for the most common positional isomers of C18–C22 DHYAs (see electronic supplementary material, table S2) within the appropriate retention time windows. The temperature programme comprised an isothermal hold at 70° for 2 min, ramping to 220° at 10° min−1, followed by the second ramp at 4° min−1 to 300°, with a 10 min isothermal hold.
(d). Preparation and analysis of fatty acid methyl esters
Aliquots of the total lipid extract were hydrolysed (0.5 M NaOH/MeOH; 70°, 1 h). The neutral fraction was removed (3 × 3 ml hexane) followed by acidification to pH 3 using 1 M aqueous HCl and the extraction of free fatty acids (3 × 3 ml CHCl3). Fatty acids were methylated using 100 µl BF3/MeOH (14% w/v, 75°, 1 h) and extracted (3 × 2 ml CHCl3). Fatty acid methyl esters were analysed using a GC/MS fitted with a polar column, with the MS operated in full scan (m/z 70–650) and SIM (m/z 105, 262, 290, 318 and 346 to determine APAAs) modes.
The δ13C values of individual fatty acids were determined using GC-combustion-isotope ratio MS (GC-C-IRMS). Analyses were performed using a Varian 3400 GC coupled to a Finnigan MAT Delta-S IRMS with a modified Finnegan MAT interface, Cu and Pt wires (0.1 mm o.d.) in an alumina reactor (0.5 mm i.d.). Samples were injected via an SPI injector onto a non-polar column (CP-Sil CB, 100% dimethylpolysiloxane, 50 m × 0.32 i.d. 0.1 µm film thickness). The temperature programme consisted of a 2 min isothermal hold at 50° and then ramped at 10° min−1 to 300°C followed by a 10 min isothermal hold. Results were calibrated against reference CO2, which was injected directly into the source three times at the beginning and end of the run. All samples were run in duplicate with external standards every four runs; any runs of unacceptable integrity were discarded and repeated. The δ13C values were derived according to the following expression and are relative to the international standard vPDB: δ13C‰ = ((Rsample – Rstandard)/Rstandard) × 1000, where R = 13C/12C. The δ13C values were corrected for the carbon atoms added during methylation using a mass balance equation [16].
3. Results and discussions
(a). Marine biomarkers
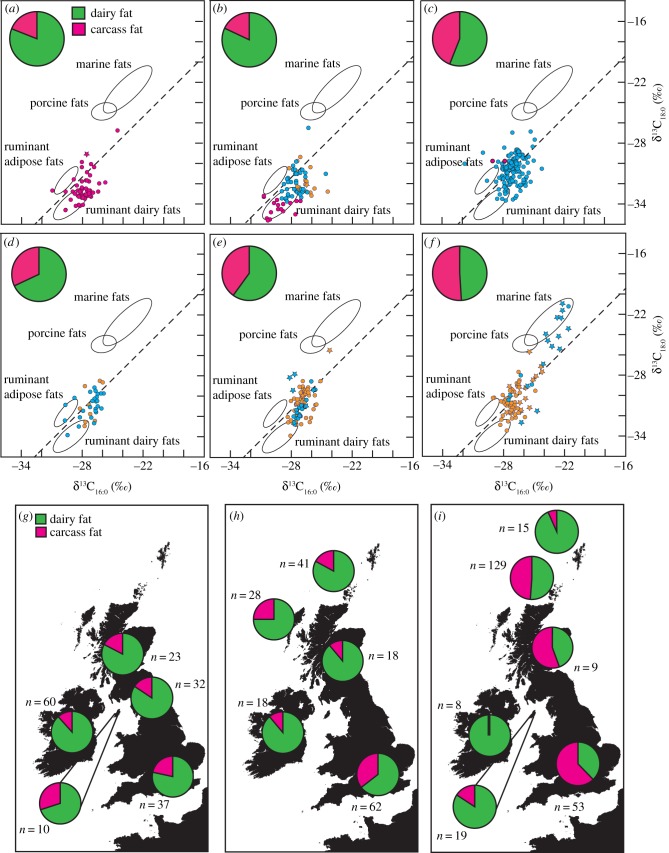
Over 300 Neolithic residues from coastal locations on northern Britain and the smaller isles of Orkney, Shetland, North and South Uist, Lewis and Man were investigated using high-sensitivity GC/MS-SIM. Lipid residues typically displayed compositions of saturated carboxylic acids consistent with degraded animal fats (see the electronic supplementary material, figure S3), with the incidence of a distinctive series of mid-chain ketones (e.g. approx. 30% of Early Neolithic residues) indicating that the fats extracted were regularly reaching temperatures in excess of 270°C [17,18]. Nonetheless, long-chain DHYAs and APAAs were detected in just two sherds, one from Moray on the Scottish mainland and one from South Uist on the Outer Hebrides (figure 1). The near-complete absence of aquatic biomarkers in the Neolithic pottery residues was supported by stable carbon isotope signatures of n-alkanoic acids, which reflect a predominantly ruminant origin for the majority of fats (figures 1a–c and 2; electronic supplementary material, table S3).
Figure 1.
Prevalence of marine and dairy fats in prehistoric pottery determined from lipid residues. (a–f) Scatter plots show δ13C values determined from C16:0 and C18:0 fatty acids preserved in pottery from northern Britain (red circles), the Outer Hebrides (yellow circles) and the Northern Isles of Scotland (blue circles), dating to (a) Early Neolithic, (b) Mid/Secondary expansion Neolithic, (c) Late Neolithic, (d) Bronze Age, (e) Iron Age and (f) Viking/Norse. Star symbol indicates where aquatic biomarkers were also detected. Ellipses show 1 s.d. confidence ellipses from modern reference terrestrial species from the UK [19] and aquatic species from North Atlantic waters [13]. (g–i) Maps show the frequency of dairy fats in residues from Neolithic pottery from (g) Early Neolithic, (h) the Middle Neolithic/Secondary expansion and (i) Late Neolithic. Additional data from isotopic analysis of residues from Neolithic southern Britain (n = 152) and Scotland (n = 104) are included [19,20].
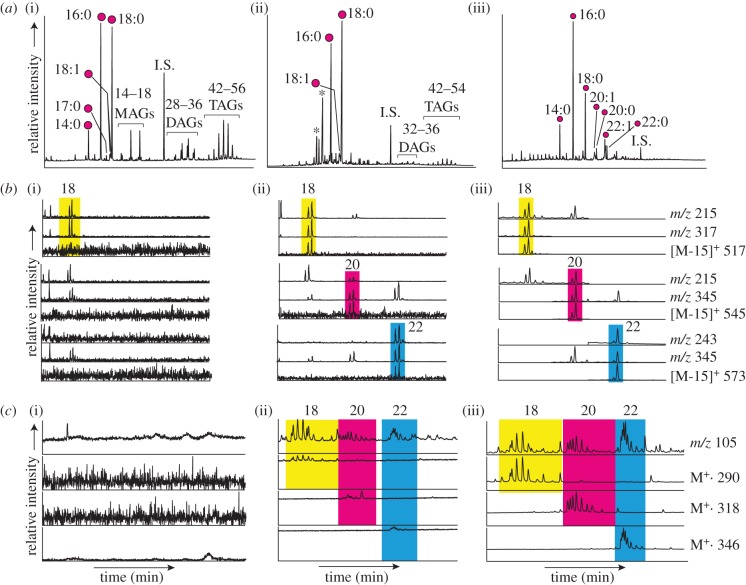
Figure 2.
(a) Partial high-temperature gas chromatograms showing total lipid extracts from three sherds above mass chromatograms from aliquots analysed using GC/MS operated in SIM mode for the detection of (b) C18–C22 DHYAs (as tris-trimethylsilyl derivatives) and (c) C18–C22 APAAs (as methyl esters; electronic supplementary material, tables S1 and S2). Components were identified based upon the presence of identical chromatographic peaks for the characteristic fragment ions and molecular ions at the expected retention times. Residue (i) is from the Neolithic settlement at Braes o'Habreck on Wyre, the Orkney Isles; (ii) is from Cille Pheadair, South Uist; and (iii) is from Jarlshof, Shetland; only (ii) and (iii) contain detectable marine biomarkers, with (iii) displaying the strongest signatures. Filled circles represent free fatty acids with carbon number x and degree of unsaturation y. Retention window: (a) 5–35 min, (b) 23–30 min and (c) 24–36 min.
Such low prevalence of marine biomarkers in these pottery residues, less than 1% from over 40 sites, is inconsistent with significant exploitation of aquatic resources throughout the Neolithic. Moreover, the composition of organic residues from the post-Neolithic pottery demonstrates that biomarkers of aquatic origin remain rare over the subsequent 2 millennia. No evidence for marine product processing, based on long-chain DHYAs and APAAs, was identified from 40 sherds of Bronze Age pottery, and marine biomarkers and more enriched stable carbon isotope signatures only re-emerge by the Late Iron Age (figure 1d,e). By the Viking and Norse period, from which nearly 100 sherds were investigated, marine biomarkers are considerably more widely detected in pottery (approx. 40% sherds; figures 1–3).
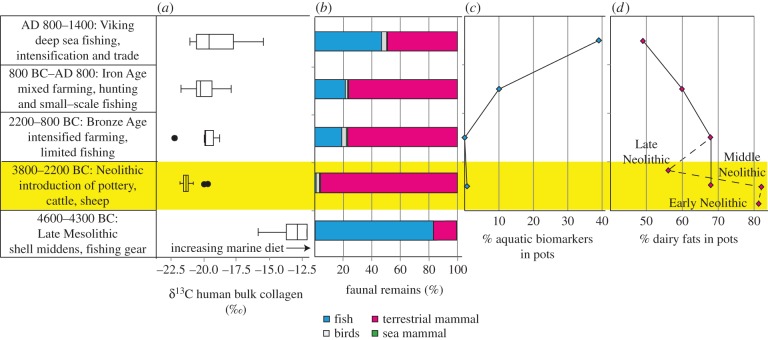
Figure 3.
Multi-proxy palaeodietary information from the Western and Northern Isles of Scotland and mainland northern Britain. (a) Mean δ13C values from bulk human bone collagen [21–27] with error bars showing 1 s.d. (b) Proportions of fauna bones in prehistoric assemblages determined from NISP. Owing to the over-representation of shellfish, this class has been excluded; however, shellfish comprise just 1.3% of Neolithic assemblages. Faunal skeletal assemblages from the Mesolithic from this region are sparse, and comparable published datasets almost non-existent; the Mesolithic bar here therefore represents the faunal data from a single site of Northton, Isle of Harris [28]. Although no marine mammals were recorded at this site, a high number were recorded at Oronsay [29]. (c) The percentage of pot lipid residues containing components diagnostic of aquatic fat processing and (d) the percentage of pot residues that were classified as dairy fats.
(b). Dairy product processing
While aquatic commodities were rarely identified in the lipid residues of Neolithic pottery of the northeast Atlantic archipelagos, the intact triacylglycerols often exhibited a wide acyl carbon number distribution. This distribution is characteristic of dairy-derived fats, arising from the higher abundance of lower carbon number (C12–C14) fatty acids in milk fats compared with adipose fats. The stable carbon isotope signatures confirmed an overwhelming predominance of dairy products associated with Neolithic pottery throughout the northeast archipelago (figure 1a–c,g–i). During the earliest Neolithic, dairy fats comprised some 80% of lipid residues identified in pottery vessels (figures 1 and 3) and thus strong evidence now associates the introduction of the earliest pottery with the exploitation of secondary (liquid) animal products. At more northerly and westerly locations in the northeast Atlantic archipelago, this pattern was maintained into the Late Neolithic; however, on mainland England and Scotland the prevalence of dairy fats dropped by the later part of the third millennium BC, with terrestrial carcass products starting to play a more predominant role (figures 1i and 3d). Contributions from wild terrestrial fauna (notably deer) can be discounted, since they typically make up less than 10% of faunal assemblages on the Western Isles of Scotland [30] and are sparse on Ireland (1–3%) until the Later Neolithic [31].
(c). Environmental effects
The non-parametric Mann–Whitney U-test (SPSS v. 21) was run to determine whether there was any significant difference between the δ13C values of Neolithic milk (Δ13C ≤ −3.4‰) fatty acids from island compared with mainland coastal locations. Analysis of milk residues only was chosen in order to remove any possibility of different types of fat affecting the analysis. Distributions of δ13C values from island and mainland locations were similar, as assessed by visual inspection, for both C16:0 and C18:0 fatty acids, and the dataset did not deviate significantly from the assumptions required for a Mann–Whitney U-test. Interestingly, an offset is observed between the range of compound-specific δ13C values from the smaller and larger island masses. The median δ13C value was statistically significantly higher for both fatty acids from island locations (n1 = 129, C16:0 = −27.0‰, C18:0 = −32.0‰) compared with mainland locations (n2 = 67, C16:0 = −28.1‰, C18:0 = −33.3‰; C16:0 U = 1454.5, z = −7.612, p < 0.001; C18:0 U = 1446.5, z = −7.633, p < 0.001).
These differences between the absolute values of fatty acids from milk fats can probably be explained by the greater relative expanse of coastline. More saline conditions, which are encountered at shoreline locations, are known to cause stomatal closure and hence higher stable carbon isotope signatures in the terrestrial non-halophytic plants [32,33]. This would be incorporated into the tissues of herbivore consumers, thus explaining the higher δ13C signatures in the herbivore fats in Hebridean, Shetlandic and Orcadian pottery.
(d). Intercomparison of pottery lipid, human stable isotope and faunal proxies
Comparing these lipid biomarker proxies with our synthesis of faunal and human skeletal stable carbon isotope data from the northern island and coastal sites further confirms that marine products were of low importance during the Neolithic compared with those of terrestrial origin (figure 3). Indeed, significant proportions of aquatic biomarkers and more positive δ13C values are only observed in Late Iron Age and Viking/Norse residues. When viewed together with the archaeozoological evidence and human collagen δ13C values from these later periods (figures 1d–f, 2 and 3), our findings reveal that the immediate shift in subsistence patterns in the earliest Neolithic was followed by a gradual return to the inclusion of marine products over subsequent millennia, reaching its highest levels by the Late Norse period. By this point, greater regional variability in resource exploitation is also observed, with a strong emphasis on processing of aquatic resources detected on Shetland, where it is likely that intensive, possibly commercial fishing was undertaken [34].
4. Conclusion
To summarize, our findings provide unequivocal evidence that marine products were of little overall importance to the Neolithic farmers of the northeast Atlantic archipelagos, as evidenced by (i) almost non-existent evidence for aquatic product processing in pottery, (ii) low presence of aquatic species in faunal assemblages from southern Britain [35] and the Western and Northern Isles of Scotland (figure 3b), and (iii) collagen stable carbon isotope signatures of Mesolithic and Neolithic humans from Britain showing a terrestrial-based diet of coastal-dwellers in the Neolithic (figure 3a) [4,5,21]. While interpretations based on the stable carbon isotope values have been challenged, our new evidence for a widespread intensive dairy economy across the region provides an entirely plausible explanation for the high terrestrial carbon isotope signal recorded in human collagen and seemingly negates any arguments for these farmers consuming low-protein diets that would be required to mask a marine dietary contribution. Interestingly, stable carbon isotope evidence from Middle Neolithic humans from the Channel Isles [36] and our investigations of Early/Early–Mid Neolithic pottery from Jersey, which are significantly earlier and related to a northwest French Neolithic tradition (table 1), produce much the same picture to the northerly sites.
This contrasts markedly with recent findings from Late Mesolithic and Neolithic human bone stable isotopes and organic residues from pottery from the Baltic region [37–39], which indicate significant continuation of hunter–fisher–forager activities alongside the adoption of domesticates. Considering the broader impact of our findings, it should be emphasized that: (i) the contrasting Neolithization trajectories occurring contemporaneously in different regions (e.g. northeast Atlantic archipelagos versus the Baltic) point to geographically distinct ecological, demographic and cultural influences dictating the patterns of adoption of subsistence practices at this key phase of European prehistory; (ii) the rapid shift to an intensive dairy economy, persisting for several millennia, is consistent with current theories concerning the high abundance of lactase persistence among the modern inhabitants of northwest European archipelagos, which are predicated on high milk consumption driving the evolution of the -13910*T allele, and would have been enhanced by the practices introduced by the milk-producing dairy farming populations identified herein [40–43]; and (iii) these findings are relevant to the debated calcium absorption hypothesis, which emphasizes milk consumption to be of particular importance for high latitude populations where low UV light exposure can result in vitamin D deficiency and thus poor calcium absorption [44]. Given the absence of any indication of the consumption of vitamin D-rich marine resources, the suggested calcium absorption-stimulating effect of milk consumption may have been critical in maintaining the fitness of these prehistoric farming populations. Notwithstanding the need for further work to confirm the genetic characteristics and bone health of the early dairying populations of the northwest European archipelago, their continued success exploiting such marginal zones is indisputable.
Supplementary Material
Acknowledgements
We are grateful to the following for providing samples and advice: Mike Parker Pearson; Tim Darvill; Tom Dawson; Shetland Museum and Archives; UHI, Orkney College; National Museums, Scotland; Manx National Heritage; Museum nan Eilean (Stornoway); Clive Waddington and Archaeological Research Services Ltd; and Jersey Heritage. We are grateful to Ian Armit, Fiona Shapland and Historic Scotland for stable isotope data and radiocarbon dates obtained for Iron Age Scotland. We are grateful to Alison Kuhl and James Williams for technical support. We also thank Mark Thomas for very useful discussions and the anonymous reviewers for constructive comments on the manuscript.
Data accessibility
— Archaeological GC and GC/MS data: Bristol University Research Data Repository (doi:10.5523/bris.13kidnrls4jnl1m806eyfd8h6z).
— Archaeological single compound stable isotope data: Bristol University Research Data Repository (doi:10.5523/bris.upjtf9os1dzr154phmgvrupib).
Funding statement
This research was funded by the UK Natural Environment Research Council (NE/F021054/1), with research on the Irish material funded by EU FP7 (Marie Curie Actions) under REA grant agreement no. 273462.
References
- 1.Thomas JS. 2008. The Mesolithic–Neolithic transition in Britain. In Prehistoric Britain (ed. Pollard J.), pp. 58–89. Oxford, UK: Blackwell. [Google Scholar]
- 2.Sheridan SA. 2010. The Neolithisation of Britain and Ireland: the big picture. In Landscapes in transition (eds Finlayson B, Warren G.), pp. 89–105. Oxford, UK: Oxbow Books. [Google Scholar]
- 3.Whittle A, Healy F, Bayliss A. 2011. Gathering time: dating the Early Neolithic enclosures of Southern Britain and Ireland. Oxford, UK: Oxbow Books. [Google Scholar]
- 4.Richards MP, Schulting RJ, Hedges REM. 2003. Sharp shift in diet at onset of Neolithic. Nature 425, 366 ( 10.1038/425366a) [DOI] [PubMed] [Google Scholar]
- 5.Richards MP, Hedges REM. 1999. A Neolithic revolution? New evidence of diet in the British Neolithic. Antiquity 73, 891–897. [Google Scholar]
- 6.Milner NJ, Craig OE, Bailey G, Pedersen K, Andersen SH. 2004. Something fishy in the Neolithic? A re-evaluation of stable isotope analysis of Mesolithic and Neolithic coastal populations. Antiquity 78, 9–22. [Google Scholar]
- 7.Hedges REM. 2004. Isotopes and red herrings: comments on Milner et al. and Lidén et al. Antiquity 78, 34–37. [Google Scholar]
- 8.Milner NJ, Craig O, Bailey G, Andersen SH. 2006. A response to Richards and Schulting. Antiquity 80, 456–458. [Google Scholar]
- 9.Richards MP, Schulting RJ. 2006. Against the grain? A response to Milner et al. (2004). Antiquity 80, 444–456. [Google Scholar]
- 10.Hansel FA, Copley MS, Madureira LAS, Evershed RP. 2004. Thermally produced ω-(o-alkylphenyl)alkanoic acids provide evidence for the processing of marine products in archaeological pottery vessels. Tetrahedron Lett. 45, 2999–3002. ( 10.1016/j.tetlet.2004.01.111) [DOI] [Google Scholar]
- 11.Evershed RP, Copley MS, Dickson L, Hansel FA. 2008. Experimental evidence for the processing of marine animal products and other commodities containing polyunsaturated fatty acids in pottery vessels. Archaeometry 50, 101–113. ( 10.1111/j.1475-4754.2007.00368.x) [DOI] [Google Scholar]
- 12.Hansel FA, Bull ID, Evershed RP. 2011. Gas chromatographic mass spectrometric detection of dihydroxy fatty acids preserved in the ‘bound’ phase of organic residues of archaeological pottery vessels. Rapid Commun. Mass Spectrom. 25, 1893–1898. ( 10.1002/rcm.5038) [DOI] [PubMed] [Google Scholar]
- 13.Cramp LJE, Evershed RP. 2014. Reconstructing aquatic resource exploitation in human prehistory using lipid biomarkers and stable isotopes. In Treatise on Geochemistry, 2nd edn, vol. 14 (eds Holland HD, Turekian KK.), pp. 319–339. Oxford, UK: Elsevier. [Google Scholar]
- 14.Frankel EN, Neff WE, Rohwedder WK, Khambay BP, Garwood RF, Weedon BC. 1977. Analysis of autoxidized fats by gas chromatography-mass spectrometry: I. Methyl oleate. Lipids 12, 901–907. ( 10.1007/BF02533309) [DOI] [PubMed] [Google Scholar]
- 15.Dudd SN, Evershed RP. 1998. Direct demonstration of milk as an element of archaeological economies. Science 282, 1478–1481. ( 10.1126/science.282.5393.1478) [DOI] [PubMed] [Google Scholar]
- 16.Rieley G. 1994. Derivatization of organic-compounds prior to gas-chromatographic combustion-isotope ratio mass-spectrometric analysis—identification of isotope fractionation processes. Analyst 119, 915–919. ( 10.1039/an9941900915) [DOI] [Google Scholar]
- 17.Evershed RP, Stott AW, Dudd SN, Charters S, Leyden A. 1995. Formation of long-chain ketones in ancient pottery vessels by pyrolysis of acyl lipids. Tetrahedron Lett. 36, 8875–8887. ( 10.1016/0040-4039(95)01844-8) [DOI] [Google Scholar]
- 18.Raven AM, van Bergen P, Stott AW, Dudd SN, Evershed RP. 1997. Formation of long-chain ketones in archaeological pottery vessels by pyrolysis and acyl lipids. J. Appl. Pyrolysis 40–41, 267–285. ( 10.1016/S0165-2370(97)00036-3) [DOI] [Google Scholar]
- 19.Copley MS, Berstan R, Dudd SN, Docherty G, Mukherjee AJ, Straker V, Payne S, Evershed RP. 2003. Direct chemical evidence for widespread dairying in prehistoric Britain. Proc. Natl Acad. Sci. USA 100, 1524–1529. ( 10.1073/pnas.0335955100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mukherjee AJ, Gibson AM, Evershed RP. 2008. Trends in pig product processing at British Neolithic Grooved Ware sites traced through organic residues in potsherds. J. Archaeol. Sci. 35, 2059–2073. ( 10.1016/j.jas.2008.01.010) [DOI] [Google Scholar]
- 21.Schulting RJ, Richards MP. 2002. The wet, the wild and the domesticated: the Mesolithic–Neolithic transition on the west coast of Scotland. Eur. J. Archaeol. 5, 147–189. [Google Scholar]
- 22.Richards MP, Mellars P. 1998. Stable isotopes and the seasonality of the Oronsay middens. Antiquity 72, 178–184. [Google Scholar]
- 23.Pearson MP, et al. 2005. Evidence for mummification in Bronze Age Britain. Antiquity 79, 529–546. [Google Scholar]
- 24.Schulting RJ, Richards MP. 2009. Radiocarbon dates and stable isotope values on human remains. In On the fringe of Neolithic Europe (ed. Ritchie A.), pp. 67–74. Edinburgh, UK: Society of Antiquaries of Scotland. [Google Scholar]
- 25.Richards MP, Fuller B, Molleson T. 2006. Stable isotope palaeodiet study of humans and fauna from the multi-period (Iron Age, Viking and Late Medieval) site of Newark Bay, Orkney. J. Archaeol. Sci. 33, 122–131. ( 10.1016/j.jas.2005.07.003) [DOI] [Google Scholar]
- 26.Barrett J, Richards MP. 2004. Identity, gender, religion and economy: new isotope and radiocarbon evidence for marine resource intensification in early historic Orkney, Scotland. Eur. J. Archaeol. 7, 249–271. ( 10.1177/1461957104056502) [DOI] [Google Scholar]
- 27.Pearson MP, Sharples N, Mulville J, Smith H. 1999. Between land and sea: excavations at the broch of Dun Vulan. Sheffield, UK: Sheffield Academic Press. [Google Scholar]
- 28.Hamilton-Dyer S. 2006. Faunal remains. In Excavations at Northton, Isle of Harris (eds Simpson D, Murphy E, Gregory R.), pp. 33–35. BAR British Series 408. Oxford, UK: Archaeopress. [Google Scholar]
- 29.Mellars P. 1987. Excavations on Oronsay. Edinburgh, UK: Edinburgh University Press. [Google Scholar]
- 30.Mulville J. 2010. Wild things? The prehistory and history of Red Deer on the Hebridean and Northern Isles of Scotland. In Extinctions and invasions: a social history of British Fauna (eds O'Connor T, Sykes N.), pp. 43–50. Oxford, UK: Windgather Press, Oxbow Books. [Google Scholar]
- 31.Carden RF, et al. 2012. Phylogenetic, ancient DNA, fossil and morphometric analyses reveal ancient and modern introductions of a large mammal: the complex case of red deer (Cervus elaphus) in Ireland. Q. Sci. Rev. 42, 74–84. ( 10.1016/j.quascirev.2012.02.012) [DOI] [Google Scholar]
- 32.Heaton THE. 1999. Spatial, species and temporal variations in the 13C/12C ratios of C3 plants: implications for palaeodiet studies. J. Archaeol. Sci. 26, 637–649. ( 10.1006/jasc.1998.0381) [DOI] [Google Scholar]
- 33.Farquhar GD, Ehleringer JR, Hubick KT. 1989. Carbon isotope discrimination and photosynthesis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 40, 503–537. ( 10.1146/annurev.pp.40.060189.002443) [DOI] [Google Scholar]
- 34.Barrett JH. 1997. Fish trade in Norse Orkney and Caithness: a zooarchaeological approach. Antiquity 71, 616–638. [Google Scholar]
- 35.Serjeantson D. 2011. Review of animal remains from the Neolithic and Early Bronze Age of Southern Britain. Research Department Report Series 29. Portsmouth, UK: English Heritage.
- 36.Schulting RJ, Sebire H, Robb JE. 2010. On the road to Paradis: new insights from AMS dates and stable isotopes at Le Déhus, Guernsey, and the Channel Islands Middle Neolithic. Oxf. J. Archaeol. 29, 149–173. ( 10.1111/j.1468-0092.2010.00343.x) [DOI] [Google Scholar]
- 37.Lidén K, Eriksson G, Nordqvist B, Götherström A, Bendixen E. 2004. ‘The wet and the wild followed by the dry and the tame’—or did they occur at the same time? Diet in Mesolithic–Neolithic southern Sweden. Antiquity 78, 23–33. [Google Scholar]
- 38.Craig OE, et al. 2011. Ancient lipids reveal continuity in culinary practices across the transition to agriculture in Northern Europe. Proc. Natl Acad. Sci. USA 108, 17 910–17 915. ( 10.1073/pnas.1107202108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Isaksson S, Hallgren F. 2012. Lipid residue analyses of Early Neolithic funnel-beaker pottery from Skogsmossen, eastern Central Sweden, and the earliest evidence of dairying in Sweden. J. Archaeol. Sci. 39, 3600–3609. ( 10.1016/j.jas.2012.06.018) [DOI] [Google Scholar]
- 40.Smith GD, Lawlor DA, Timpson NJ, Baban J, Kiessling M, Day INM, Ebrahim S. 2009. Lactase persistence-related genetic variant: population substructure and health outcomes. Eur. J. Hum. Genet. 17, 357–367. ( 10.1038/ejhg.2008.156) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Itan Y, Powell A, Beaumont MA, Burger J, Thomas G. 2009. The origins of lactase persistence in Europe. PLoS Comput. Biol. 5, e1000491 ( 10.1371/journal.pcbi.1000491) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gerbault P, Liebert A, Itan Y, Powell A, Currat M, Burger J, Swallow DM, Thomas MG. 2011. Evolution of lactase persistence: an example of human niche construction. Phil. Trans. R. Soc. B 366, 863–877. ( 10.1098/rstb.2010.0268) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gerbault P, Roffet-Salque M, Evershed RP, Thomas MG. 2013. How long have adult humans been consuming milk? Int. U. Biochem. Mol. Biol. J. 65, 983–990. [DOI] [PubMed] [Google Scholar]
- 44.Flatz G, Rotthauwe HW. 1973. Lactose nutrition and natural selection. Lancet 2, 76–77. ( 10.1016/S0140-6736(73)93267-4) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
— Archaeological GC and GC/MS data: Bristol University Research Data Repository (doi:10.5523/bris.13kidnrls4jnl1m806eyfd8h6z).
— Archaeological single compound stable isotope data: Bristol University Research Data Repository (doi:10.5523/bris.upjtf9os1dzr154phmgvrupib).