Abstract
Evolutionary radiations, especially adaptive radiations, have been widely studied but mainly for recent events such as in cichlid fish or Anolis lizards. Here, we investigate the radiation of the subfamily Cyprininae, which includes more than 1300 species and is estimated to have originated from Southeast Asia around 55 Ma. In order to decipher a potential adaptive radiation, within a solid phylogenetic framework, we investigated the trophic apparatus, and especially the pharyngeal dentition, as teeth have proved to be important markers of ecological specialization. We compared two tribes within Cyprininae, Poropuntiini and Labeonini, displaying divergent dental patterns, as well as other characters related to their trophic apparatus. Our results suggest that the anatomy of the trophic apparatus and diet are clearly correlated and this explains the difference in dental patterns observed between these two tribes. Our results illustrate the diversity of mechanisms that account for species diversity in this very diverse clade: diversification of dental characters from an ancestral pattern on the one hand, conservation of a basal synapomorphy leading to ecological specialization on the other hand. By integrating morphological, ecological and phylogenetic analyses, it becomes possible to investigate ancient radiation events that have shaped the present diversity of species.
Keywords: Cypriniformes, X-ray microtomography, ecological speciation, dental morphology
1. Introduction
One crucial question in evolution deals with patterns and processes underlying evolutionary radiations involving rapid bursts of speciation [1–3]. Multiple cases of radiation events have been linked to the rapid diversification of adaptive phenotypes [4,5]. Notable examples of recent adaptive radiations are provided by cichlid fish in Eastern African lakes [6], Anolis lizards in the Caribbean Islands [7] and Darwin finches in the Galapagos Islands [8]. These classical cases illustrate how radiations can be related to habitat and diet diversification, and/or to cases of sexual selection, driving rapid speciation [9]. However, evolutionary radiations have not been confined to recent events. Careful examination of phylogenetic trees reveal more ancient radiation events for which factors contributing to species diversification remain more elusive [10,11]. Thus, investigations of more ancient radiations will help to understand cases of rapid diversification of clades recognized as suprageneric taxonomic ranks.
In order to investigate the potential role played by ecological speciation during radiations, whether an evolutionary radiation is adaptive or non-adaptive, it is crucial to focus on characters that provide proxies for assessing biological niches occupied by species. The question is whether or not the diversification of species is correlated to the adaptation to different environments. In this framework, dentition constitutes a major character for assessing adaptive radiation as its shape and arrangement play a key role in food capture and processing. The diversification of the dentition within a diversified clade strongly suggests that this clade has undergone divergent natural selection, leading to ecological speciation. Particularly in mammals, it has been shown that dentition pattern, individual tooth shape and dental interlocking are closely related to various food habits, and tooth crown shape is frequently used as a marker of ecological adaptation [12–14]. Dentition diversification can therefore reliably reflect changes in the life history of species, thus making this character a good indicator for developing and testing hypotheses as to changes in ecological preferences within the evolution of a clade [15].
Here, we aim to examine the factors that could explain the ecomorphological diversification of the Cyprininae from the Mekong Basin, one of the World's most diverse riverine ecosystems. Within the order Cypriniformes, which encompasses about 4000 species [16,17], the subfamily Cyprininae includes about 1300 species [18] mainly distributed in South and East Asia, a region hypothesized as the cradle for the order [19]. All Cypriniformes are characterized by the absence of oral teeth and the presence of two symmetrical sets of pharyngeal teeth located on the right and left parts of the fifth ceratobranchial arch [20]. Right and left sets of pharyngeal teeth do not interlock but together occlude against a keratinized chewing pad (KCP) that covers the pharyngeal plate of the basioccipital [21]. Across the order, there is great diversity in the arrangement, number and shape of pharyngeal teeth [22]. Some species have one row encompassing numerous teeth while others have three rows with fewer teeth on each row. In addition, the pharyngeal tooth crown varies from a simple cone to a complex multicuspid pattern [22].
To carry out this study, it was essential to build a solid phylogenetic framework for the subfamily Cyprininae, encompassing several well-supported monophyletic groups. Among these lineages, we studied two monophyletic tribes that are particularly diverse: Labeonini (400 species) and Poropuntiini (100 species) [18,23]. The Poropuntiini include species mainly restricted to Southeast Asia (Burma, Thailand, Laos, Cambodia, Vietnam, Malaysia and Indonesia), whereas the Labeonini includes species living from East Asia to Africa [24]. We chose to investigate these two tribes because of the influence of geographical extension in species diversification and their ecological diversity in a same geographical region. Our first goal was to evaluate whether the difference in species number between Labeonini and Poropuntiini is a consequence of the difference in geographic distribution between the two tribes. Our second goal was to test whether the radiation in a common geographical area (here, Southeast Asia) is adaptive or not, through adaptation to various food habits, which can be recorded by scrutinizing changes in dental morphology.
2. Results
(a). Phylogeny of the Cyprininae
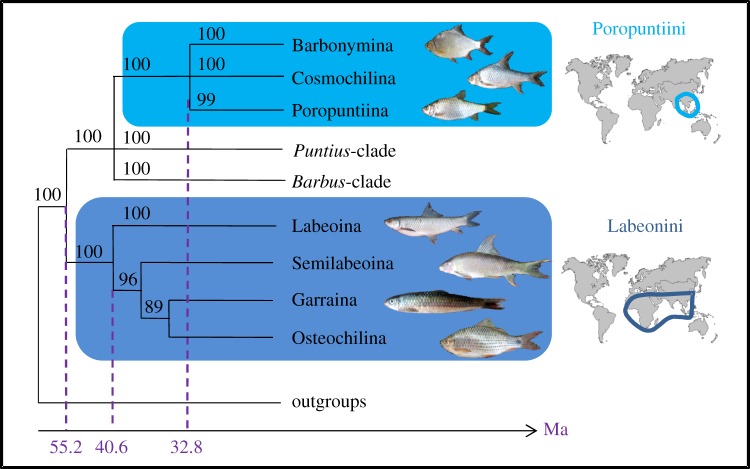
We built a phylogeny of 103 species of Cyprinidae using five mitochondrial genes (electronic supplementary material, table S1 and figure S2). Nuclear markers were not included in this phylogenetic analysis as polyploidy occurs in several cyprinine lineages, thus rendering nuclear markers unreliable [25]. Cyprinidae are divided into nine subfamilies, among which Cyprininae is the most diverse with more than 1300 species [18]. Within the Cyprininae, we identified seven well-supported monophyletic tribes, including Poropuntiini and Labeonini (electronic supplementary material, figure S2). As there are no polyploid species in Poropuntiini and Labeonini, two mitochondrial (Cytb, COI) and two nuclear (Rag1, Rho) markers were used to build a more comprehensive tree for 17 species from 10 genera of Labeonini and 22 species from 16 genera of Poropuntiini (figure 1; electronic supplementary material, figure S3 and table S1). The tribe Poropuntiini splits up into three robust and early diverging monophyletic subtribes that we named Cosmochilina, Poropuntiina and Barbonymina (figure 1). The tribe Labeonini divides up into four early diverging subtribes previously described as Garraina, Labeonina, Osteochilina and Semilabeoina [23].
Figure 1.
Summarized phylogeny of Cyprininae focusing on Poropuntiini and Labeonini. Phylogeny of Cyprininae, including Poropuntiini and Labeonini, based on ML and Bayesian analyses (electronic supplementary material, figure S2), using mitochondrial (Cytb, COI) and nuclear (Rag1, Rho) markers. Bootstrap values from ML analyses are provided above nodes. Molecular dating of clades is also provided and is based on the electronic supplementary material, figure S3. Geographical extension is shown for the two tribes.
Molecular dating analyses for the phylogeny of Cyprininae were performed using mitochondrial data (see the electronic supplementary material, text S10 and figure S4). They were calibrated using four different fossil taxa: Labeo (17.0 Ma), Cyprinus (33.9 Ma), Puntius (28.4–37.2 Ma) and the oldest cyprinid fossil (Parabarbus, 48.6 Ma). Divergence time estimation, conducted using Bayesian analyses (see Material and methods), clearly supported that the subfamily Cyprininae diverged at 55 Ma (95% CI: 47–66 Ma). The tribe Labeonini diverged at 40 Ma (95% CI: 33–49 Ma), whereas the tribe Poropuntiini diverged more recently at 33 Ma (95% CI: 26–40 Ma; see figure 1 and the electronic supplementary material, figure S4).
(b). Comparative anatomy of the pharyngeal feeding apparatus
The anatomy of the pharyngeal dental system was investigated using X-ray microtomography for each species of Poropuntiini and Labeonini included in the electronic supplementary material, figure S2 phylogenetic analysis. Microtomography provides high-resolution three-dimensional reconstructions of mineralized tissues [22,26], allowing the virtual segmentation and investigation of both pharyngeal bones and teeth, but not the KCP. Thus, it was possible to scrutinize the number and arrangement of pharyngeal teeth, as well as individual shape of tooth crowns. Two to four specimens were examined for each species in order to avoid potential intraspecific polymorphisms although previous studies have shown that cyprinid intraspecific polymorphism in tooth number is low [27].
(i). Tooth number
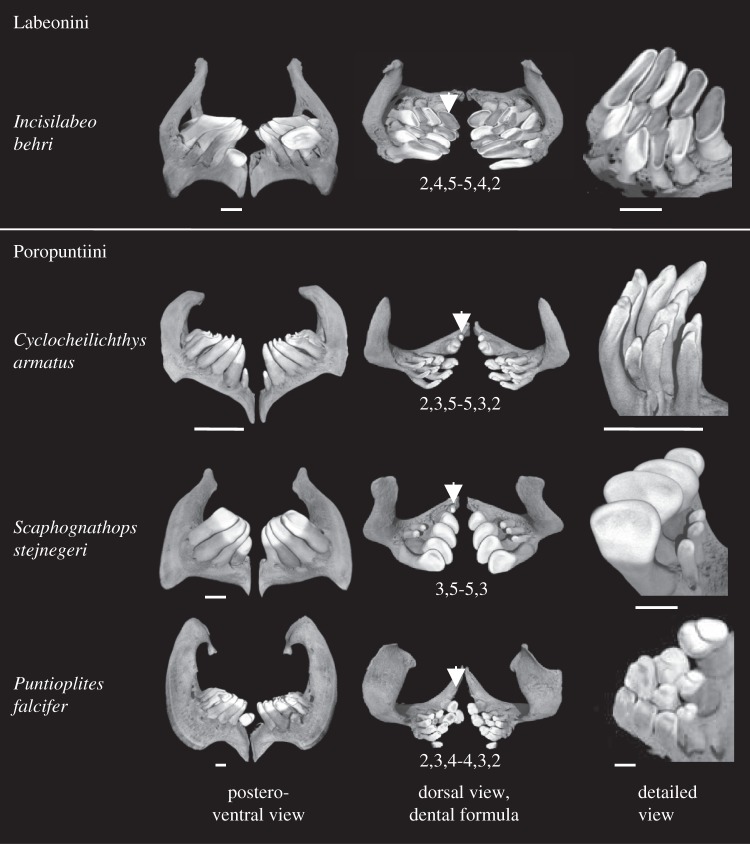
Pharyngeal teeth on both left and right pharyngeal bones are organized in several rows, from one to three tooth rows in Cyprininae [22,28]. Most species have three dental rows [22], named internal, medial and external rows. The internal row, or main row, always comprises the highest number of teeth. In each row, the number of teeth is variable (see figure 2). We recorded neither left versus right asymmetry in tooth number contrary to what is reported in other cyprinid clades [22], nor intraspecific polymorphism. Thus, the dental formula can be established from one set of pharyngeal teeth and considered as symmetrical. Both number of tooth rows and number of teeth per row are consistent at the generic level, but they may vary between various genera (see figure 2).
Figure 2.
Comparison of pharyngeal dentition between Labeonini and Poropuntiini. Pharyngeal bones bearing pharyngeal teeth are shown in three different views: postero-ventral, dorsal and detailed view of teeth. In the dorsal view, the dental formula is indicated, as well as the position of the first tooth on the main row (indicated by arrows), which can be small or absent in Poropuntiini (see text). Scale bar is 1 mm.
All investigated Labeonini have the same number of tooth rows and the same number of teeth per row, recapitulated in the dental formula [5,4,2] (figure 2). On the contrary, the dental formula was variable between Poropuntiini genera (figure 2). The most common dental formula is [5,3,2] but we observed genera with only one or two tooth rows (see Scaphognathops in figure 2) as well as genera with only four teeth on the internal row (see Puntioplites in figure 2). Although half of studied genera display a [5,3,2] dental formula, the other half show substantial variations leading to the following dental formula for Poropuntiini: [4-5,0-3,0-2]. Thus, there is a striking contrast between the stability of dental formula in Labeonini as opposed in intergeneric diversity of this character in Poropuntiini.
(ii). Tooth arrangement
In Labeonini, tooth crowns of each pharyngeal bone are very close to one another (see Labeonini dentitions on the electronic supplementary material, figure S5) so that they together form two compact pavements which act like grinders by occluding against the KCP [29]. The grinding function is demonstrated by the abraded crown tips and by the occurrence of exposed dentine on all flattened occluding surfaces, except those of neoformed replacement teeth (see figure 2 and the electronic supplementary material, figure S5). Exceptions to this pattern can be seen in Garra and Mekongina, in which tooth crowns are more spaced (see the electronic supplementary material, figure S5). On the contrary, Poropuntiini display well-separated rows of pharyngeal teeth whose crowns are usually pointed and weakly abraded (figure 2 and the electronic supplementary material, figure S5). An exception to this pattern is Amblyrhynchichthys in which teeth are closer to one another and crown tips slightly abraded, similar to Labeonini (see the electronic supplementary material, figure S5). Moreover, in Poropuntiini, the first dental position of the main row is always occupied by a tiny tooth, much smaller than other teeth of the same row (see arrows in figure 2). This tooth is even lacking in seven out of 16 poropuntiine genera, so that the internal row only counts four teeth. This suggests that the first internal tooth, which will never occlude against the chewing pad given its size, could be lost by relaxed constraints applied on this position. On the contrary, the first tooth of the internal row is always present in Labeonini, and it fully participates in the grinding pavement.
(iii). Tooth crown shape
Each pharyngeal dental set of Cyprinidae is composed of teeth that have neither exactly the same size nor the same shape. In this regard, all Cyprinidae are clearly heterodont. Shape and size of teeth can feebly vary from one position to another as in Poropuntius (see the electronic supplementary material, figure S5), but this variation can also be slightly more substantial as in Discherodontus (electronic supplementary material, figure S5). In order to circumvent this problem of individual dental variation, we established the representative crown phenotype for each species based only on the shape of the two first distal replacement teeth of the internal row, as replacement teeth do not show any sign of wear (electronic supplementary material, figure S5).
We hypothesized in a previous work [22] that the likely basal condition of tooth crown in Cyprinidae is a hooked-bicrested spoon (HBS) pattern, in which the crown is basically made of a single conical curved cusp that bears an internal concave surface laterally flanked by two crests running from the tip of the cusp to the bottom of the concave surface. This phenotype is well documented in Cyclocheilichthys (figure 2).
Our morphological investigations showed that crown shape is highly diversified in Poropuntiini. Their crown shape likely radiated from the HBS pattern, which is the most common phenotype across the tribe (see figure 2 and the electronic supplementary material, figure S5). Balantiocheilos, Cyclocheilichthys, Poropuntius and Hypsibarbus have a crown phenotype very close to the typical HBS shape. ‘Barbonymus’ gonionotus and Puntioplites show a trend of the HBS pattern toward a strong reinforcement of the crests as well as of the curvature of the crown tip, resulting in a ‘Phrygian hat’ pattern. Barbonymus altus, Cyclocheilos and Mystacoleucus display a HBS pattern with the addition of a second lesser developed cusp, which is located at the base of the concave surface. Scaphognathops shows a great enlargement of the concave surface to the detriment of both cusp tip and lateral crests. The crown phenotype results in a shovel pattern perfectly documented in this genus. Cosmochilus and Albulichthys display shovel crowns with the addition of cingular cusps at the basis of the concave surface, whereas Discherodontus and Sabwa display a shovel pattern with conservation of a tiny pointed cusp at the crown tip. Two genera, Sikukia and Amblyrhynchichthys, display more derived tooth shapes that are difficult to interpret in terms of evolutionary trends. Sikukia displays teeth with much reinforced crests along with a central groove and very abraded crowns. Amblyrhynchichthys displays teeth that have kept a small tip cusp but the general shape and arrangement makes it convergent with the dentition in Labeonini. Most Labeonini display flat tooth crowns. Neoformed replacement teeth show that unworn crown morphologies derive from the HBS pattern, from which the concave surface substantially expanded transversally. Occlusal surfaces are rapidly worn out so that the whole set constitutes a unique pavement parallel to the KCP (see figure 3). Exceptions to this general pattern are Garra and Mekongina that likely conserved the basal characteristics for the tribe, in which crowns are moderately expanded and less abraded, so that they do not constitute a compact pavement. Finally, crown shape evolution of Labeonini shows a trend in the reinforcement of the lateral crests, which results in the bilophid crown pattern of Cirrhinus, Henicorhynchus, Labiobarbus and Osteochilus. To sum up, although crown shape of Poropuntiini and Labeonini are likely to both derive from the HBS pattern, Poropuntiini display a radiative diversity including multiple cases of convergence while Labeonini mainly evolved towards one direction, which seems highly constrained by the pavement arrangement of the dentition.
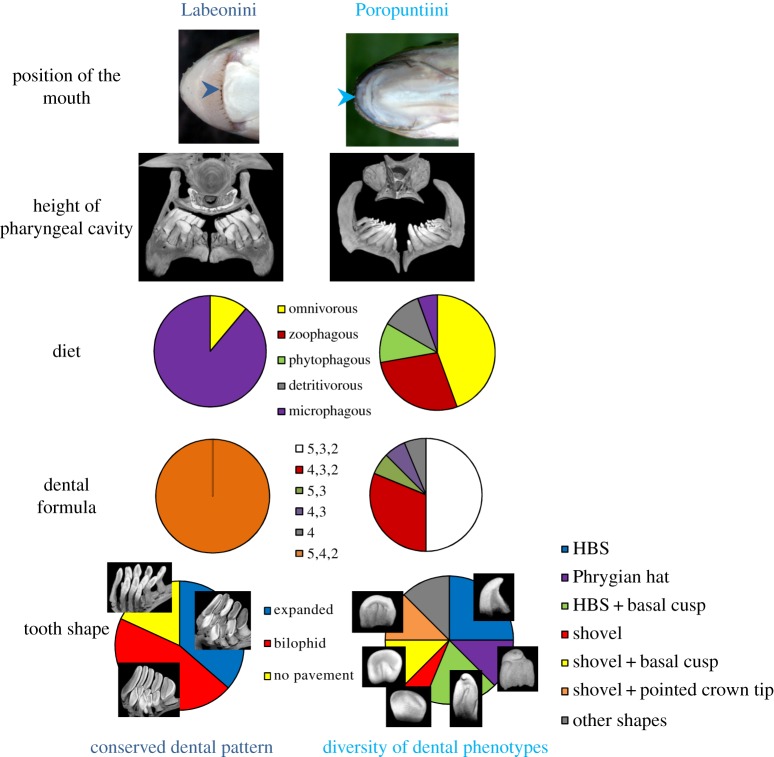
Figure 3.
Model of evolution of pharyngeal dentition in correlation with the trophic apparatus and diet. Labeonini and Poropuntiini differ in their mouth position, as well as the height of the pharyngeal cavity. For the position of the mouth, Labeonini is illustrated with Mekongina erythropsila, and Poropuntiini with Poropuntius carinatus. The arrows indicate the position of the mouth opening. These observations are congruent with data available on the diet of these species: in Labeonini, eight species are recognized as microphagous and one species as omnivorous, whereas in Poropuntiini, most species are omnivorous or zoophagous. All these factors can be correlated with the difference in dental patterns (both tooth number and tooth shape) between the two groups. All categories of tooth shapes are described in the text and detailed in the electronic supplementary material, figure S5.
(iv). Morphology of the fifth ceratobranchials
Pharyngeal teeth of all Cypriniformes are attached to the right and left fifth ceratobranchial bones (FCB). Each FCB is composed of a dorsal branch, stretching perpendicularly to the mesio-distal axis, and a ventral branch, pointing toward mesial direction. Teeth are located at the angle of these two branches. As in most clades of Cyprinidae, Poropuntiini display falciform FCB, with a curved dorsal branch. However, in Labeonini, FCB display a different shape, the ventral and dorsal branches being rather straight and perpendicular (see figure 2).
Outline morphometric analyses of FCB (electronic supplementary material, figure S6) show that Poropuntiini share a same morpho-space together with other species of Cyprininae, Cultrinae and Danioninae, whereas Labeonini occupy a separated morpho-space. Labeonini are discriminated from other clades by the first axis of variation, which represents 66.9% of the total variation. This latter axis opposes the slender and falciform FCB of Poropuntiini and other Cyprinidae to the robust and perpendicular FCB of Labeonini. Exceptions to this discrimination are the FCB of Garra and Mekongina, which plot among Poropuntiini. This would suggest that Garra and Mekongina likely conserved some basal characteristics.
(v). Height of the pharyngeal cavity
A significant difference was detected in the relative size of the pharyngeal cavity between Labeonini and Poropuntiini (figure 3 and the electronic supplementary material, figure S7). The height of the pharyngeal cavity of Labeonini is low, meaning that tooth tips are close to the KCP. This would suggest that Labeonini feed on small particles, for example planktonic elements. On the contrary, the pharyngeal cavity of Poropuntiini is high, allowing them to feed on larger prey items. These anatomical observations were confirmed by significant biometrical data: the pch/(pch + pdh) ratio calculated between pharyngeal cavity height (pch) and pharyngeal dentition height (pdh) was equal to 0.30 for Poropuntiini and to 0.17 for Labeonini (n = 20 for Poropuntiini, n = 15 for Labeonini, p-value < 0.001; see the electronic supplementary material, figure S7). Sikukia is the exception for Poropuntiini by having a low cavity (ratio equal to the mean ratio for Labeonini), and Garra and Mekongina have a higher pharyngeal cavity than other Labeonini (ratio equal to 0.25 for both genera).
(c). Mouth position
The mouth of Labeonini is located on the ventral part of the head and opens inferiorly, whereas Poropuntiini have a terminal mouth, located at the rostral end of the fish and opening towards the mesial direction (see figure 3). The position of the mouth is an important character, which is directly linked to diet and trophic position within the food web. A mouth located ventrally suggests that Labeonini are either demersal or benthic bottom-feeders, mainly taking nutrients from the substratum [30]. On the contrary, a terminal mouth indicates that Poropuntiini are benthopelagic swimmers, feeding throughout the water column as well as on the bottom. Mouth position thus involves that Labeonini mostly feed on epibenthic organisms and organic remains while Poropuntiini have access to more diverse food resources, including nektonic and planktonic organisms.
With respect to these observations, it was interesting to note that two genera of Poropuntiini, namely Amblyrhynchichthys and Sikukia, have a ventral mouth, as well as similarities of the feeding apparatus with Labeonini (as mentioned previously, the dental arrangement for Amblyrhynchichthys and the narrow pharyngeal cavity for Sikukia). This implies that changes in mouth positioning are associated with changes in pharyngeal pattern. Given the phylogenetic position of these two genera within Poropuntiini (cf. electronic supplementary material, figure S3), those changes have occurred twice independently. It appears therefore that the position of the mouth and the pharyngeal dentition display correlated evolution.
(d). Diet
Diet is well known to be intimately linked to dental morphology. However, the diet of Cyprininae in Southeast Asia is poorly known. Poropuntiini are mostly recognized as omnivorous, with some species preferentially feeding on plant matter, for example Puntioplites, and others on animal matter (insects, crustaceans or even fish), for example Cyclocheilichthys [31,32]. Labeonini are mostly reported as phytophagous and microphagous, mostly feeding on algae, periphyton, phytoplankton and, in lesser proportion, zooplankton [30–32]. By exploiting the whole benthopelagic zone, Poropuntiini thus display a wider range of food habits than Labeonini. These differences are likely linked to the position of the mouth and the height of the pharyngeal cavity (figure 3). The low pharyngeal cavity of Labeonini is consistent with detritivory and plankton microphagy, whereas the high pharyngeal cavity of Poropuntiini gives the possibility to feed on larger preys. In that perspective, it is interesting to note that Sikukia and Amblyrhinchichthys, the two poropuntiine genera which show some similarities with Labeonini regarding their pharyngeal feeding apparatus, also display a diet similar to Labeonini mainly composed of plankton and detritus [31].
δ15N and δ13C isotopic analyses were performed on Poropuntiini and Labeonini that we sampled in Laos, in order to decipher their position within the trophic web. The range values for both tribes are similar (electronic supplementary material, figure S8), indicating that Labeonini and Poropuntiini both feed on plant and animal matters. This is not inconsistent with previous observations, given that Labeonini are planktivorous and detritivorous, which includes both plant and animal matters. These results however do not allow a deeper comparison without complementary detailed studies on diet for each species.
3. Discussion
The stability versus diversity of the pharyngeal dentition in Labeonini and Poroputiini, respectively, can be explained by their ecological strategies. However, the species number in these two tribes, 100 for Poropuntiini against 400 for Labeonini, raises the question of the evolutionary success of these two clades in relation to the diversity of their ecological specialization.
The tribe Poropuntiini encompasses 100 species, which almost exclusively live in Southeast Asia, at the exception of 20 species present in the adjacent areas of China and India. The tribe Labeonini is more diverse, with 400 species living from Southeast Asia to Africa, including India and Middle East [24,31]. The geographical range of Labeonini is thus much wider than that of Poropuntiini (figure 1). The broad geographical extension of Labeonini could be a primary reason for their higher species diversity considering that Labeonini have been exposed to a greater array of naturally occurring isolation events that promote allopatric speciation. Among the 400 species of Labeonini, 105 species are restricted to Southeast Asia [24], as compared with the 75 species of Poropuntiini present in the same geographical area (electronic supplementary material, figure S9). From the divergence dates obtained with molecular analyses, the Labeonini diverged 40 Ma, whereas the Poropuntiini diverged 33 Ma (cf. electronic supplementary material, figure S4). Thus, the relative divergence time estimations for these two clades are roughly proportional to their differential species number in Southeast Asia. Consequently, our results highlight a significant difference in the total number of species between Labeonini (400 species) and Poropuntiini (100 species) that is related to the difference in geographical ranges.
The fact that Labeonini display a much larger geographical range than Poropuntiini could be explained by geological events that occurred during their diversification. Indeed, the collision between the Indian and Eurasian plates, which led to the uplift of the Himalaya Mountains, had multiple consequences in all adjacent areas. In Southeast Asia, there were important movements of the Red River belt between 30 and 23 Ma [33,34]. Those geological movements may have disrupted the freshwater connection from Southeast Asia to India (and then to Africa) that Labeonini followed, which is consistent with the dates of origin of the two groups, as Labeonini appeared almost 10 Ma earlier and had time to migrate through India to Africa before those geological changes of hydrographic basins.
However, it is still necessary to explain the similar levels of species diversity in Southeast Asia between Poropuntiini and Labeonini, whereas only Poropuntiini display a diversity of dental phenotypes that suggests an adaptive radiation.
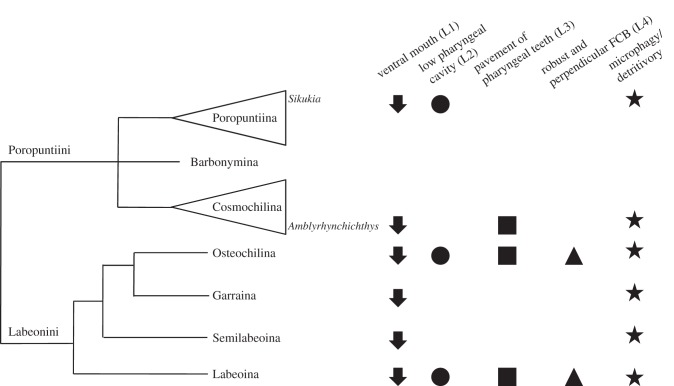
When compared with other subfamilies of Cyprinidae living in Southeast Asia, such as Danioninae and Oxygastrinae, most Labeonini display several specific characters [22,31,35] that are (L1) a ventrally located mouth; (L2) a low pharyngeal cavity; (L3) pharyngeal teeth of each arch arranged in compact pavements and (L4) robust and perpendicular FCB (electronic supplementary material, figure S6). Labeonini all have a ventral mouth, and this character is indisputably a basal synapomorphy of the tribe. However, Garra and Mekongina, which occupy relatively basal positions within the phylogeny of Labeonini, have not acquired the specific pharyngeal apparatus (L2-3-4). Either the acquisition of this pharyngeal apparatus has occurred twice independently in the subtribes Labeoina and Osteochilina, or the four characters are all synapomorphic for the whole labeonine tribe and the pharyngeal apparatus has been lost in Garraina and Semilabeoina. In Labeoina and Osteochilina, the pharyngeal apparatus is thus composed of specific correlated characters, pleading for strong morphofunctional interrelationships, and its acquisition has allowed labeonine fish to acquire a specialized feeding behaviour (figure 3).
On the contrary, Poropuntiini have rather conserved the plesiomorphic conditions for the family such as: (P1) a terminal mouth; (P2) a high pharyngeal cavity; (P3) tooth crowns regularly interspaced and (P4) slender and falciform FCB. In spite of this, Poropuntiini display a substantial diversity in tooth crown shape when compared with other clades of Cyprinidae [22]. Conserving a terminal mouth has enabled Poropuntiini to exploit various freshwater niches, and this variety in potential food resources is probably intimately linked to a high diversity in their tooth number, arrangement and shape (figure 3). Notably, this diversity includes the cases of Sikukia and Amblyrhynchichthys, which have independently evolved toward a labeonine-like feeding behaviour, and their pharyngeal dentition show some convergences with those of Labeonini, such as the pavement arrangement in Amblyrhynchichthys and the low pharyngeal cavity in Sikukia (figure 4).
Figure 4.
Evolution of the pharyngeal feeding apparatus, mouth position and diet in Poropuntiini and Labeonini. From the phylogeny (electronic supplementary material, figure S3), we plotted all the characters that we found specifically in Labeonini: (L1) ventrally located mouth; (L2) low pharyngeal cavity; (L3) pharyngeal teeth of each arch arranged in compact pavements; (L4) robust and perpendicular FCB; as well as the diet mainly composed of plankton, periphyton, algae and detritus. These characters are all present in two subtribes within Labeonini: Labeoina and Osteochilina. The only characters present in all Labeonini are the ventral mouth and the diet. Moreover, two genera in Poropuntiini, Sikukia and Amblyrhinchichthys, have independently acquired labeonine-like characters.
Overall, our results suggest that Labeonini and Poropuntiini have undergone two different histories leading to adaptive radiation. On the one hand, the acquisition in Labeonini of a ventral mouth in relation to intake of specific benthic diet is correlated to the acquisition of a secondary apomorphic pharyngeal apparatus. However, the acquisition of such an amount of structural modifications within the trophic apparatus makes each of its components difficult to be modified, at the risk of no longer being functional. We thus assume that Labeonini is a highly specialized groundfish tribe. The evolutionary success of Labeonini is based on a specialization that has led to the colonization of a new niche. On the other hand, Poroputiini basically possess a rather generalist trophic apparatus and these fish have undergone a wide range of morphological adaptations in relation to the exploitation of various benthopelagic niches, which explains their high diversity in tooth number, shape and arrangement. Thus, Poropuntiini seem to have undergone an adaptive radiation as the high number of species is directly correlated to the diversification of pharyngeal dentition, suggesting ecological speciation. Regarding Labeonini, it seems that the species diversification is correlated to the appearance of a key innovation allowing access to new niches, which would constitute an example of radiation owing to ecological opportunity [4].
However, it is clear that this case study will benefit from further investigation on the patterns of diversification [36]. Notably, a natural extension of this study would involve investigating the tempo of diversification within both tribes as well as scrutinizing the phylogeny and the fossil record to check for an early burst of divergence. The main problem with performing such studies is the scarcity of the cyprinid published fossil record. Another aspect concerns the ecological factors at the origin of this diversification: we have identified the potential role of diet but it would be interesting to investigate other factors such as habitat (which is linked to diet, as exemplified by the case of Labeonini) and sexual selection. A last area of study would be the genetic mechanisms underlying the diversification of these groups.
In conclusion, our study demonstrates the clear feasibility of investigating ancient radiation events, thanks to both morphological and ecological studies, under a temporal phylogenetic framework. For that goal, teeth are of high interest as they are known to be correlated with diet, which is an essential aspect of ecological adaptation. Consequently, it is possible to decipher potential adaptive radiation in most toothed vertebrate clades, particularly in actinopterygians that have been probably understudied in that perspective. Indeed, the extant of dentition diversity is very high in this group and little is known about the link between this diversity and potential radiations. We believe that the analyses we have performed in this paper could be applied to many other cases of ancient radiations of teleost fish such as Cypriniformes (4000 species), Characiformes (1600 species) or Perciformes (10 000 species) which all display a high diversity of dentition.
4. Material and methods
(a). Phylogenetic analyses
For the whole Cyprininae tree, a total of 103 species including 74 cyprinines and 29 outgroups were analysed. DNA sequences for the following five mitochondrial genes were downloaded from GenBank: cytochrome oxidase subunit I (COI), cytochrome b (Cyt b), 16S ribosomal RNA (16S), NADH dehydrogenase subunits 4 (ND4) and subunits 5 (ND5). Sequence alignment follows [18] and the final alignment is 5601 bp in length. Partitioned maximum likelihood (ML) search was performed using RAxML v. 7.0.4 [37]. Nucleotide data were partitioned into five (one for each of the three codon positions of protein-coding genes and the stem and loop regions of the 16 s rRNA). The GTR+I+G model was chosen for each partition. A total of 1000 distinct runs were performed based on 1000 random starting trees using the default algorithm of the program. The tree with maximum ML score was chosen as the final tree. ML bootstrap analysis (MLBS) was conducted using RAxML [38,39]. The same partitioning strategy was used as in the initial ML search. The number of non-parametric bootstrap replications was set at 1000, and other parameters were set as default. The resulting trees were imported into PAUP* 4.0.b10 [40] to obtain the 50% majority rule consensus tree. The same dataset was used for molecular dating analyses except that only one outgroup (Acheilognathus typus) was included. Three calibration priors were used for molecular dating analyses: (i) the oldest African Labeo-like fossil from Loperot, Kenya dated from the Early Miocene (17.0 Ma) [41]; (ii) the oldest Cyprinus-like fossil dated from the Eocene (33.9 Ma) [42]; (iii) the fossil of Puntius bussyi from the Sangkarewang Formation in Central Sumatra [43], dates from the Early Oligocene to Late Eocene (28.4–37.2 Ma) [44]. Divergence time estimation was inferred in a Bayesian framework using BEAST v. 1.7. [45] via the CIPRES Science Gateway v. 3.1 [46]. The BEAST input file was created using BEAUti v. 1.7.1 [45]. Further information is provided in the electronic supplementary material, text S1. For the tree focusing on Poropuntiini and Labeonini, two mitochondrial genes and two nuclear genes, including COI, Cyt b, Rag1 (recombination activating gene 1, exon 3) and Rho (rhodopsin), were used in the analyses. The final alignment is 3477 bp in length. The entire dataset was partitioned into nine according to the codon positions of the mitochondrial dataset, Rag1 and Rho. Partitioned ML search and MLBS analyses were conducted using RAxML v. 7.0.4. Partitioned Bayesian analyses were performed using MrBayes v. 3.1.2 [47]. We used jModelTest v. 2.1.1 [48] and the Bayesian information criterion to select best-fit model for each gene partition. The number of substitution schemes was set as three. Eight (one cold chain and seven heated chains) simultaneous Markov chains were run for 20 000 000 generations, with trees being sampled every 200 generations for a total of 100 001 trees in the initial sample. The log-likelihood scores were plotted against generation time to determine when the Markov chains reached stationary. Based on the plotting results (not shown), the first 10 000 trees were discarded as burn-in and the remaining 90 001 trees were imported into PAUP to compute the 50% majority rule consensus trees. Two independent analyses were conducted.
(b). Morphological investigation and morphometric analyses
All species of Poropuntiini and Labeonini included in our phylogenetic study were investigated for dental characters by conventional X-ray microtomography [26], allowing the exploration of mineralized tissues through a non-invasive method, as previously used for Cypriniformes [22]. All samples were scanned using a Phoenix Nanotom (General Eletrics) using the following parameters: 70 kV tensions, 100 mA intensity, 3000 images with time exposure of 500 ms and a voxel size from 3 to 10 µm depending on the size of the specimen. After scanning, three-dimensional images were reconstructed with the software attached to the machine (data rec) and then visualized with VGStudioMax. Images were segmented with VGStudioMax, using the magic wand tool, in order to obtain images of pharyngeal bones and the basioccipital which bears the chewing pad.
Two-dimensional morpometric analyses of pharyngeal bones were carried out on all species of Poropuntiini and Labeonini investigated for dental characters, as well as other species of Cyprinidae. From three-dimensional reconstruction of pharyngeal bones, teeth were virtually clipped out and the pharyngeal bone was imaged from posterior view, the position that gives most information on shape. For each species, two specimens were included in morphometric analyses. For each specimen, both left and right pharyngeal bones were imaged five times independently, to take into account the effect of orientation for imaging. From two-dimensional images of pharyngeal bone outlines, Fourier coefficients were determined using statistical functions on R [49], with normalization of orientation and size. Then, Fourier coefficients were pasted in PAST [50] for Principal Component Analyses, resulting in the determination of the main axes of variation and allowing visualization of two-dimensional plots with two principal components as well as determination of shapes on axis of variation with the Eda deform function in PAST.
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank the following people in Laos for the organization of the travel arrangements and the authorization to bring back specimens: M. Tiengkham Xaisanavong and M. Bounchoo Khenthavong of Science and Technology Department of Savannakhet, Dr Sinthavong Viravong of Living Aquatic Resources Research Center (LARReC). We also thank Fabienne Rogowski and Martine Chapier in France for the administrative organization of the travel arrangements. We thank Joanne Burden for English proofreading and Abderrahman for advice on the manuscript. We finally thank Nima Dehlavi for R function writing.
Data accessibility
DNA sequences: see the electronic supplementary material, table S1 for all GenBank references
Funding statement
This work has been financially supported by the Ministère de l'Enseignement Supérieur et de la Recherche (MESR), the Centre National de la Recherche Scientifique (CNRS) and Ecole Normale Supérieure (ENS) of Lyon as well as by Agence Nationale pour la Recherche (ANR) grant Bouillabaisse (ANR-09-BLAN-0127-02) and the U.S. National Science Foundation funded All Cypriniformes (DEB 1022720) Species Inventory award.
References
- 1.Nee S, Mooers AO, Harvey PH. 1992. Tempo and mode of evolution revealed from molecular phylogenies. Proc. Natl Acad. Sci. USA 89, 8322–8326. ( 10.1073/pnas.89.17.8322) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Glaubrecht M, Schneider H. 2010. Evolution in action case studies in adaptive radiation, speciation and the origin of biodiversity. Heidelberg, NY: Springer. [Google Scholar]
- 3.Gittenberger E. 2008. What about non-adaptive radiation? Biol. J. Linn. Soc. 43, 263–272. ( 10.1111/j.1095-8312.1991.tb00598.x) [DOI] [Google Scholar]
- 4.Schluter D. 2000. The ecology of adaptive radiation. Oxford, UK: Oxford University Press. [Google Scholar]
- 5.Schluter D. 1996. Ecological causes of adaptive radiation. Am. Nat. 148, 40–64. ( 10.1086/285901) [DOI] [Google Scholar]
- 6.Kocher TD. 2004. Adaptive evolution and explosive speciation: the cichlid fish model. Nat. Rev. Genet. 5, 288–298. ( 10.1038/nrg1316) [DOI] [PubMed] [Google Scholar]
- 7.Losos JB, Schneider CJ. 2009. Anolis lizards. Curr. Biol. 19, 316–318. ( 10.1016/j.cub.2009.02.017) [DOI] [PubMed] [Google Scholar]
- 8.Lerner HRL, Meyer M, James HF, Hofreiter M, Fleischer RC. 2011. Multilocus resolution of phylogeny and timescale in the extant adaptive radiation of Hawaiian honeycreepers. Curr. Biol. 21, 1838–1844. ( 10.1016/j.cub.2011.09.039) [DOI] [PubMed] [Google Scholar]
- 9.Yoder JB, et al. 2010. Ecological opportunity and the origin of adaptive radiations. J. Evol. Biol. 23, 1581–1596. ( 10.1111/j.1420-9101.2010.02029.x) [DOI] [PubMed] [Google Scholar]
- 10.Glor RE. 2010. Phylogenetic insights on adaptive radiation. Annu. Rev. Ecol. Evol. Syst. 41, 251–270. ( 10.1146/annurev.ecolsys.39.110707.173447) [DOI] [Google Scholar]
- 11.Whitfield JB, Lockhart PJ. 2007. Deciphering ancient rapid radiations. Trends Ecol. Evol. 22, 258–265. ( 10.1016/j.tree.2007.01.012) [DOI] [PubMed] [Google Scholar]
- 12.Renaud S, Auffray J-C, Michaux J. 2006. Conserved phenotypic variation patterns, evolution along lines of least resistance, and departure due to selection in fossil rodents. Evolution 60, 1701–1717. [PubMed] [Google Scholar]
- 13.Renaud S, Michaux J, Schmidt DN, Aguilar J-P, Mein P, Auffray J-C. 2005. Morphological evolution, ecological diversification and climate change in rodents. Proc. R. Soc. B 272, 609–617. ( 10.1098/rspb.2004.2992) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lazzari V, Charles C, Tafforeau P, Vianey-Liaud M, Aguilar JP, Jaeger JJ, Michaux J, Viriot L. 2008. Mosaic convergence of rodent dentitions. PLoS ONE 3, e3607 ( 10.1371/journal.pone.0003607) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mihlbachler MC, Rivals F, Solounias N, Semprebon GM. 2011. Dietary change and evolution of horses in North America. Science 331, 1178–1181. ( 10.1126/science.1196166) [DOI] [PubMed] [Google Scholar]
- 16.Nelson JS. 2006. Fishes of the world, 4th edn Hoboken, NJ: John Wiley. [Google Scholar]
- 17.Eschmeyer WN, Fong JD. 2013. Species by Family/Subfamily Catalog of Fishes electronic version See http://research.calacademy.org/research/ichthyology/catalog/SpeciesByFamily.asp (accessed 25 March 2013).
- 18.Yang L, Mayden RL, Sado T, He S, Saitoh K, Miya M. 2010. Molecular phylogeny of the fishes traditionally referred to Cyprinini sensu stricto (Teleostei: Cypriniformes). Zool. Scr. 39, 527–550. ( 10.1111/j.1463-6409.2010.00443.x) [DOI] [Google Scholar]
- 19.Saitoh K, Sado T, Doosey MH, Bart HL, Jr, Inoue JG, Nishida M, Mayden RL, Miya M. 2011. Evidence from mitochondrial genomics supports the lower Mesozoic of South Asia as the time and place of basal divergence of cypriniform fishes (Actinopterygii: Ostariophysi). Zool. J. Linn. Soc. 161, 633–662. ( 10.1111/j.1096-3642.2010.00651.x) [DOI] [Google Scholar]
- 20.Stock DW. 2007. Zebrafish dentition in comparative context. J. Exp. Zool. B Mol. Dev. Evol. 308B, 523–549. ( 10.1002/jez.b.21187) [DOI] [PubMed] [Google Scholar]
- 21.Sibbing FA. 1982. Pharyngeal mastication and food transport in the carp (Cyprinus carpio L.): a cineradiographic and electromyographic study. J. Morphol. 172, 223–258. ( 10.1002/jmor.1051720208) [DOI] [PubMed] [Google Scholar]
- 22.Pasco-Viel E, Charles C, Chevret P, Semon M, Tafforeau P, Viriot L, Laudet V. 2010. Evolutionary trends of the pharyngeal dentition in Cypriniformes (Actinopterygii: Ostariophysi). PLoS ONE 5, e11293 ( 10.1371/journal.pone.0011293) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang L, et al. 2012. Molecular phylogeny of the cyprinid tribe Labeonini (Teleostei: Cypriniformes). Mol. Phylogenet. Evol. 65, 362–379. ( 10.1016/j.ympev.2012.06.007) [DOI] [PubMed] [Google Scholar]
- 24.Yang L, Mayden RL. 2010. Phylogenetic relationships, subdivision, and biogeography of the cyprinid tribe Labeonini (sensu Rainboth, 1991) (Teleostei: Cypriniformes), with comments on the implications of lips and associated structures in the labeonin classification. Mol. Phylogenet. Evol. 54, 254–265. ( 10.1016/j.ympev.2009.09.027) [DOI] [PubMed] [Google Scholar]
- 25.Leggatt RA, Iwama GK. 2003. Occurrence of polyploidy in the fishes. Rev. Fish Biol. Fish. 13, 237–246. ( 10.1023/B:RFBF.0000033049.00668.fe) [DOI] [Google Scholar]
- 26.Tafforeau P, et al. 2006. Applications of X-ray synchrotron microtomography for non-destructive 3D studies of paleontological specimens. Appl. Phys. A 83, 195–202. ( 10.1007/s00339-006-3507-2) [DOI] [Google Scholar]
- 27.Eastman JT, Underhill JC. 1973. Intraspecific variation in the pharyngeal tooth formulae of some cyprinid fishes. Copeia 1973, 45–53. ( 10.2307/1442356) [DOI] [Google Scholar]
- 28.Yang L, Hirt M, Sado T, Arunachalam M, Manickam R. 2012. Phylogenetic placements of the barbin genera Discherodontus, Chagunius, and Hypselobarbus in the subfamily Cyprininae (Teleostei: Cypriniformes) and their relationships with other barbins. Zootaxa 3586, 26–40. [Google Scholar]
- 29.Kumari U, Yashpal M, Mittal S, Mittal AK. 2009. Surface ultrastructure of gill arches and gill rakers in relation to feeding of an Indian major carp, Cirrhinus mrigala. Tissue Cell 41, 318–325. ( 10.1016/j.tice.2009.01.003) [DOI] [PubMed] [Google Scholar]
- 30.Girgis S. 1952. The bucco-pharyngeal feeding mechanism in an herbivorous bottom-feeding Cyprinoid, Labeo horie (Cuvier). J. Morphol. 90, 281–315. ( 10.1002/jmor.1050900206) [DOI] [Google Scholar]
- 31.Rainboth WJ, Food and Agriculture Organization of the United Nations, Mekong River Commission, DANIDA. 1996. Fishes of the Cambodian Mekong. Rome, Italy: Food and Agriculture Organization of the United Nations. [Google Scholar]
- 32.Siaw-Yang Y. 1988. Food resource utilization partitioning of fifteen fish species at Bukit Merah Reservoir, Malaysia. Hydrobiologia 157, 143–160. ( 10.1007/BF00006967) [DOI] [Google Scholar]
- 33.Schärer U, Lian-Sheng Z, Tapponnier P. 1994. Duration of strike-slip movements in large shear zones: the Red River belt, China. Earth Planet. Sci. Lett. 126, 379–397. ( 10.1016/0012-821X(94)90119-8) [DOI] [Google Scholar]
- 34.Tapponnier P, et al. 1990. The Ailao Shan/Red River metamorphic belt: tertiary left-lateral shear between Indochina and South China. Nature 343, 431–437. ( 10.1038/343431a0) [DOI] [Google Scholar]
- 35.Kottelat M. 2001. Fishes of Laos. Colombo, Sri Lanka: WHT Publications. [Google Scholar]
- 36.Gavrilets S, Losos JB. 2009. Adaptive radiation: contrasting theory with data. Science 323, 732–737. ( 10.1126/science.1157966) [DOI] [PubMed] [Google Scholar]
- 37.Stamatakis A. 2006. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22, 2688–2690. ( 10.1093/bioinformatics/btl446) [DOI] [PubMed] [Google Scholar]
- 38.Felsenstein J. 1985. Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39, 783–791. ( 10.2307/2408678) [DOI] [PubMed] [Google Scholar]
- 39.Stamatakis A, Hoover P, Rougemont J. 2008. A rapid bootstrap algorithm for the RAxML Web servers. Syst. Biol. 57, 758–771. ( 10.1080/10635150802429642) [DOI] [PubMed] [Google Scholar]
- 40.Swofford DL. PAUP*: Phylogenetic analysis using parsimony (*and other methods), version 4. Sunderland, MA: Sinauer Associates. [Google Scholar]
- 41.Van Couvering JAH. 1977. Early records of freshwater fishes in Africa. Copeia 1977, 163–166. ( 10.2307/1443521) [DOI] [Google Scholar]
- 42.Zhou JJ. 1990. The Cyprinidae fossils from middle Miocene of Shanwang Basin. Vertebrata PalAsiatica 28, 95–127. [Google Scholar]
- 43.Sanders M. 1934. Die fossilen Fische der alttertiaren Susswasserablagerungen aus Mittel-Sumatra. Verhandelingen van het geologisch-mijnbouwkundig genoostschap voor Nedeland en kolonien. Geologische Serie 11, 1–44. [Google Scholar]
- 44.Geological Society of London. 2005. Sumatra: geology, resources and tectonic evolution. London, UK: The Geological Society. [Google Scholar]
- 45.Drummond AJ, Rambaut A. 2007. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol. Biol. 7, 214 ( 10.1186/1471-2148-7-214) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miller MA, Pfeiffer W, Schwartz T. 2010. Gateway computing environments workshop (GCE), 2010, pp. 1–8. See http://ieeexplore.ieee.org/xpls/abs_all.jsp?arnumber=5676129 (accessed 9 January 2013). [Google Scholar]
- 47.Ronquist F, Huelsenbeck JP. 2003. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19, 1572–1574. ( 10.1093/bioinformatics/btg180) [DOI] [PubMed] [Google Scholar]
- 48.Darriba D, Taboada GL, Doallo R, Posada D. 2012. jModelTest 2: more models, new heuristics and parallel computing. Nat. Methods 9, 772 ( 10.1038/nmeth.2109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Claude J. 2008. Morphometrics with R. New York, NY: Springer; (http://public.eblib.com/EBLPublic/PublicView.do?ptiID=417711) (accessed 9 January 2013). [Google Scholar]
- 50.Oyving H, Harper David AT, Ryan Paul D. 2001. PAST: paleontological statistics software package for education and data analysis. Paleontol. Electron. 4, 9. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
DNA sequences: see the electronic supplementary material, table S1 for all GenBank references