Abstract
Environmental change can simultaneously cause abiotic stress and alter biological communities, yet adaptation of natural populations to co-changing environmental factors is poorly understood. We studied adaptation to acid and predator stress in six moor frog (Rana arvalis) populations along an acidification gradient, where abundance of invertebrate predators increases with increasing acidity of R. arvalis breeding ponds. First, we quantified divergence among the populations in anti-predator traits (behaviour and morphology) at different rearing conditions in the laboratory (factorial combinations of acid or neutral pH and the presence or the absence of a caged predator). Second, we evaluated relative fitness (survival) of the populations by exposing tadpoles from the different rearing conditions to predation by free-ranging dragonfly larvae. We found that morphological defences (relative tail depth) as well as survival of tadpoles under predation increased with increasing pond acidity (under most experimental conditions). Tail depth and larval size mediated survival differences among populations, but the contribution of trait divergence to survival was strongly dependent on prior rearing conditions. Our results indicate that R. arvalis populations are adapted to the elevated predator pressure in acidified ponds and emphasize the importance of multifarious selection via both direct (here: pH) and indirect (here: predators) environmental changes.
Keywords: acidification, amphibians, environmental stress, predator defences, multiple stressors, natural selection
1. Introduction
Environmental change (e.g. climate change, chemical pollution and exotic species) often results in environmental stress, defined as an environmental condition that lies outside the range of optimal conditions and challenges an organism's ability to maintain function [1]. Environmental stress can threaten population persistence and is an important evolutionary force [1–4]. Temporal and spatial changes in a given stressor are often accompanied by changes in other stressors [5–7], and ecological and evolutionary multiple stress effects are, hence, probably important in nature. While adaptation to a single stressor appears commonplace, simultaneous adaptation to multiple stressors may be prevented by ecological or genetic trade-offs [2,8,9] or facilitated by correlated evolution [4,6]. However, our understanding of adaptation to multiple selective agents in natural populations is still limited [6,10,11].
One source of environmental change that exemplifies the strong potential for stress interactions in nature is natural- and human-induced environmental acidification. Acid stress per se can have strong direct lethal and sub-lethal effects on organisms [12,13] and, subsequently, cause strong natural selection [14–16]. Acidification also alters the composition of biological communities, including the identity and relative abundance of top predators [5,17]. The covariation between pH and predators along acidity gradients provides a powerful system for investigating local adaptation to simultaneous selection by abiotic and biotic stressors [17].
Predation is a major selective force in natural systems and can impose strong selection on anti-predator traits (e.g. behavioural or morphological defenses). Depending on spatial and temporal environmental heterogeneity and costs of plasticity, predator-mediated selection may drive the evolution of either inducible (plastic) or constitutive (genetically fixed) defences (e.g. [18,19]). As environmental stressors can increase the costs [20–22] or decrease the investment in defences [23–25], adaptive responses to predation risk may be modified by other simultaneously occurring stressors. However, the extent of divergence among populations in the expression of anti-predator defences is rarely studied along stress gradients [26,27].
Amphibians provide a good model system for studying simultaneous adaptation to pH and predation. First, pH-mediated divergent selection can drive adaptive divergence in embryonic survival and larval life-history traits, whereby the extent of trait divergence among populations can depend on the magnitude of differences in breeding pond pH (and, hence, strength of divergent selection [14,16,28]). Second, amphibian larvae possess a range of predator defence traits (i.e. alterations in behaviour, growth and morphology) that can vary among populations inhabiting different predator environments (e.g. [26,29,30]). Third, environmental acidification typically results in the increase of invertebrate predators [5,17] that can be ferocious predators of amphibian tadpoles. Hence, amphibians should experience simultaneous divergent selection via both acid stress and predation risk, and adaptation to both stressors should be highly beneficial to local populations inhabiting acidic wetlands.
Here, we study local adaptation of the moor frog Rana arvalis to covariation between pH and predation risk in six populations along an acidification gradient in Sweden [28]. In R. arvalis, exposure of tadpoles in the presence of predatory dragonfly larvae induces reduced activity, deeper tail fins and deeper tail muscles, and the expression of these plastic responses may be suppressed by acidic pH in a population-specific manner [25,30]. The six populations studied here ranged from those inhabiting acid/predator-rich (populations at pH 4) to those inhabiting neutral/predator-poor (populations at pH 7) environments (table 1).
Table 1.
Descriptive information on study sites: coordinates (N, E), mean ± s.d. of pond pH and density of gape-unlimited predators (number of Aeshna sp. and Dytiscus sp. individuals per five sweeps). For further details and a map, see [28].
| population | coordinates | pond pH | pond predator density |
||
|---|---|---|---|---|---|
| May | June | average | |||
| Tottajärn (T) | 57°60′ N, 12°60′ E | 4.0 ± 0.2 | 4.0 ± 2.6 | 5.0 ± 2.0 | 4.5 ± 2.2 |
| Lomsjö (L) | 57°76′ N, 12°88′ E | 4.1 ± 0.2 | 5.7 ± 3.2 | 5.7 ± 3.5 | 5.7 ± 3.0 |
| Kungsbacka (K) | 57°50′ N, 12°06′ E | 4.8 ± 0.2 | 1.7 ± 0.6 | 6.3 ± 1.5 | 4.0 ± 2.8 |
| Nitta (N) | 57°87′ N, 13°21′ E | 6.0 ± 0.3 | n.a. | 1.7 ± 2.1 | 1.7 ± 2.1 |
| Rud (R) | 58°59′ N, 13°79′ E | 7.1 ± 0.2 | 2.7 ± 3.1 | 4.0 ± 2.6 | 3.3 ± 2.7 |
| Stubberud (S) | 58°46′ N, 13°76′ E | 7.3 ± 0.2 | 0.7 ± 1.2 | 2.7 ± 0.6 | 1.7 ± 1.4 |
We conducted laboratory experiments to study: (1) among-population divergence in anti-predator defences (behaviour, size and morphology) (Experiment 1) and how their expression depends on environmental pH (acid versus neutral pH treatment), and (2) the relative fitness of the populations (tadpole survival under predation by dragonfly larvae) (Experiment 2). We made three main predictions. First, assuming adaptive divergence in response to pH, and costly expression of inducible defenses, tadpoles originating from neutral ponds should be more constrained in the expression of inducible defences under acid stress. Second, assuming adaptive divergence in response to predation risk, tadpoles originating from acidic ponds should invest more in inducible defences or show stronger constitutive defences than tadpoles from neutral ponds. Third, assuming populations are locally adapted to both pH and predation, tadpole survival under acid stress and in the presence of predators should increase with pond acidity—but this outcome may be altered if trait expression is pH-dependent.
2. Material and methods
(a). Study system
Rana arvalis is a western Palaearctic brown frog that occurs in a wide range of habitats [31]. Our six study populations inhabit an acidification gradient in southwest Sweden (table 1), where R. arvalis inhabits permanent ponds and small lakes that vary in pH from 4.0 to 7.3 because of natural- and human-induced acidification and lime stone bedrock [28]. The study populations have diverged in embryonic acid stress tolerance and larval life-history traits, facilitated via direct genetic and maternal effects [16,32]. The pairwise geographical distances between these six populations range from 15 to 160 km, and all population pairs for which genetic data are available (no data available for population Nitta, table 1) are distinct in neutral markers (FST = 0.012–0.044) [16].
(b). Quantification of the selective environment
Detailed descriptions of the study populations and environmental measurements can be found in the study of Hangartner et al. [28] and we here only shortly summarize measurements of pH and predator density.
To get a proxy for the strength of pH-mediated selection, pH was measured during the larval period in each of the study ponds in May and June 2009 (table 1; for details see [28]). Measurements repeated across years indicate that population differences in pH are consistent across years (K. Räsänen 2007–2012, unpublished data). To get a proxy for the strength of predator-mediated selection, predators were collected at each pond in mid-May and mid-June 2009 using dip net sweeps. The relative abundance (individuals per five sweeps) of Aeshna dragonfly larvae and Dytiscus diving beetles was calculated for each pond [28]. Both of these predators are gape-unlimited, highly efficient predators of amphibian tadpoles [29]. Across eight populations on the study area, there is a significant negative correlation between pond pH and the number of Aeshna and Dytiscus predators (r = −0.791, n = 8, p = 0.019), confirming that invertebrate predators are more abundant in acid ponds. Pond pH and predator density were negatively correlated also across the six populations studied here, albeit only marginally significantly (r = −0.80; n = 6, p = 0.059). Tadpole densities were lower in the three acid most populations (T, L, K) than in the three neutral most populations (N, R, S) (0.2–0.7 versus 2.2–5.7 tadpoles per five sweeps, respectively), indicating that predator densities were higher in acidified ponds also relative to prey densities.
(c). Common garden experiments
Five male and five female R. arvalis in breeding condition were collected at each site and transported to the laboratory at Uppsala University, Sweden (59°50′ N, 17°50′ E). They were artificially crossed to prevent any bias owing to differences in exposure in the early embryonic environment and to assure that the offspring in each clutch are full sibs. The crosses were performed using standard procedures [28]. Embryos from each of the five families per population were reared in a walk in climate room at 16°C and with a 18 L : 6 D photoperiod and in family-specific groups (ca 50 eggs per vial) in three replicate vials (0.9 l plastic vials) that contained 0.5 l of reconstituted soft water (RSW; [33]). Water was changed every 3 days until larvae reached Gosner stage 25 (i.e. complete gill absorption and initiation of independent feeding [34]), at which point Experiment 1 was set up.
(d). Experiment 1. Trait divergence
Experiment 1 was conducted as a 2 × 2 × 6 factorial randomized block design with two pH treatments (acid (A): pH 4.5 and neutral (N): pH 7.5), two predator treatments (presence (PP) or absence (NP) of a caged Aeshna dragonfly larva) and six populations. We henceforth use APP, ANP, NPP and NNP to refer to the four treatment combinations. The experimental units were plastic containers (38 × 28 × 13 cm) containing 10 l treatment water. The containers were arranged over two shelf systems, divided into four vertical blocks to account for a known temperature gradient within the room. Two individuals per each of the five clutches (families) per population were randomly selected and placed in an experimental container (i.e. 10 tadpoles per experimental unit). There were four replicate units for each population–treatment combination (one per block), resulting in 96 experimental units.
At day 25 of Experiment 1 (set-up at Gosner stage 25 = day 0), a randomly selected subset of tadpoles was removed from each replicate container for the survival assay (Experiment 2; see below). Experiment 1 started 5 May 2009 (day 0 of the experiment) and was carried out in a climate room (19°C) under a 18 L : 6 D photoperiod. RSW was used throughout the experiment to maintain stable water quality. pH of the acid treatment water was adjusted in 200 l tanks with 1 M H2SO4 during a 2-day period before use. pH of the neutral treatment water was not adjusted (pH of RSW is 7.2–7.6 [33]). Each experimental container had a plastic filter, with filter wool and peat pellets. In the A treatments, 12 peat pellets were added to the filters to reduce pH fluctuations. In the N treatments, three pellets were added to control for the potential effects of peat presence. Each container was aerated with aquarium pumps, and the filters were sealed within a nylon mesh (250 μm). Water was changed once a week for the first 21 days, and twice a week thereafter. pH was monitored regularly during the experiment (mean ± s.d. acid: pH 4.7 ± 0.4, neutral: pH 7.6 ± 0.2). Tadpoles were fed finely chopped spinach ad libitum every second day.
For the PP treatments, late-instar Aeshna sp. larvae (captured at ponds near Uppsala) were introduced into the experiment on day 1. In each experimental unit, one predator was placed in a cylindrical cage (diameter 11 cm; height 21 cm) made of transparent film with a double mesh bottom (mesh size 1.5 mm) and hung 5 cm above container floor. During the experiment, the caged dragonfly larvae were fed with two R. arvalis tadpoles every second day. This set-up assured visual and chemical cues from the predator to the tadpoles, while preventing direct predation. In the NP treatments (control), the cage was left empty.
(i). Traits measured
Larval activity (i.e. the number of active tadpoles/container) was recorded four times per day (between 10.00 and 16.00 h) during early (days 5, 6 and 7) and later (days 17, 18 and 19) larval period of Experiment 1. Activity was judged visually based on tail movement (moving or not moving). On day 21, five tadpoles were randomly sampled from each container for phenotypic measurements. Tadpole wet mass was measured with an electronic balance (to the nearest 0.1 mg) after dry blotting. For measurements of morphology, each tadpole was placed in a Plexiglas box with a scale, photographed in water from the left side and subsequently returned to the tank of origin. Body length, tail length, maximum body depth, maximum tail muscle depth and maximum tail depth were measured from digital images (to the nearest 0.001 mm) with the software Pro-Plus 4.5.0.29 for Windows (Media Cybernetics, Silver Spring, MD, USA). We here use tail depth and tail muscle depth as descriptors of morphology as PCA analyses (not shown) indicated that these traits had the strongest loading on shape variation.
(ii). Phenotypic variation in the wild
To investigate phenotypic variation in the more complex environment in the wild, we also measured size and morphology of tadpoles collected directly from the study ponds. These methods and details of analyses are presented in the electronic supplementary material, S1, table S1 and figure S1.
(e). Experiment 2. Survival assay
We tested for the relative fitness (survival) of populations by exposing tadpoles from the different rearing conditions (APP, ANP, NPP and NNP) to a free-ranging predator. The experimental design was a 2 × 2 × 6 factorial design with two pH (A: pH 4.5 and N: 7.5) and two predator (prior rearing at PP or NP) treatments and six populations. There were 17–18 replicates per pH–predation treatment combination, resulting in 69 experimental units.
On day 25 of Experiment 1, five R. arvalis tadpoles were randomly selected from each rearing container. One tadpole from each population was placed in each replicate container (i.e. total of six tadpoles per container) of Experiment 2, representing one of the four larval rearing conditions (APP, ANP, NPP and NNP). Experimental containers (40 × 20 × 24 cm) contained 10 l of either acid (pH 4.5) or neutral (pH 7.5) water, and each tadpole was exposed to the same pH as during Experiment 1. In seven experimental units, only five tadpoles (i.e. tadpoles from five populations) could be used because of mortality prior to day 25.
Each container contained finely chopped spinach as food for tadpoles and 10 g of dried aspen (Populus tremulus) leaves, which provided structural complexity and shelter for the tadpoles. At the start of the Experiment 2, tadpoles were added to the containers and a late-instar Aeshna larva was added 1 h later. To allow the predators to acclimate to the experimental pH, each predator was maintained at either pH 4.5 or pH 7.5 at least 5 days before the start of the experiment. During this time, the dragonfly larvae were fed with two R. arvalis tadpoles every second day. The predators were not fed for 24 h before the start of Experiment 2 to assure a sufficient hunger level.
Tadpoles were exposed to the free-ranging predator for 16 h (18.00–10.00). At the end of this time period, the number of survivors was recorded, and surviving tadpoles stored in 70% alcohol. A total of 256 of the 407 (62.9%) experimental tadpoles survived the predation trial. The population identity of the survivors was later determined with parentage assignment based on microsatellite markers (see the electronic supplementary material, S2). Each dragonfly was used only once, and dragonfly length was recorded at the end of each trial.
(f). Statistical analyses
(i). Experiment 1
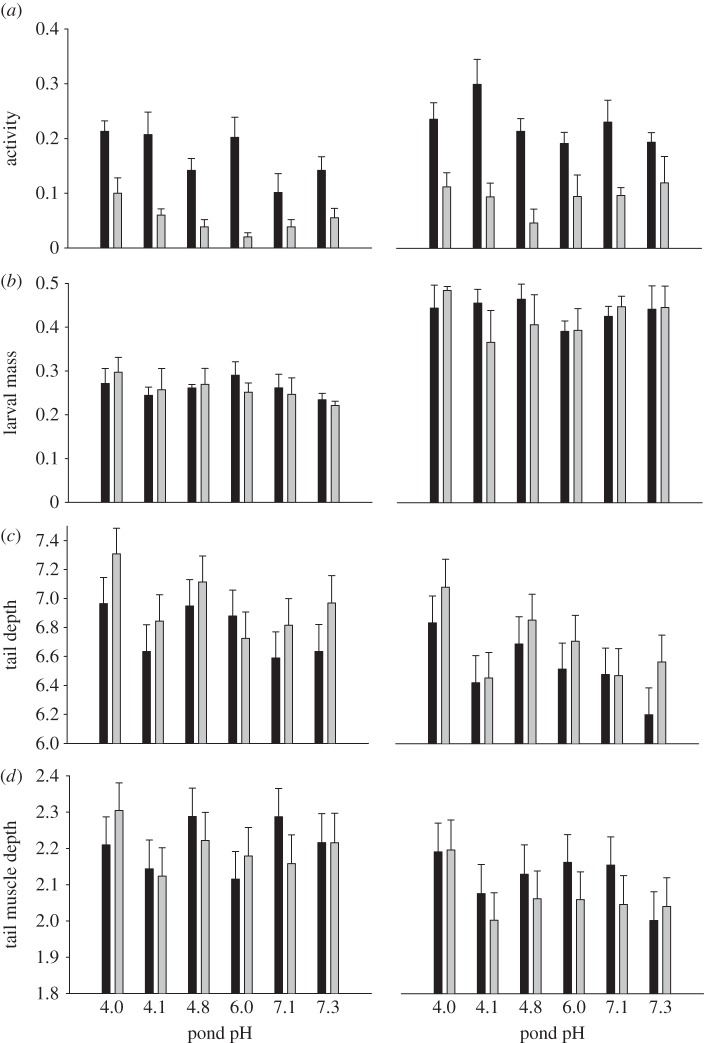
For larval activity, we used mean counts of active larvae within each container and each of the two observation periods (i.e. early and later) in statistical analyses. We calculated the grand mean of each container over each of 12 observations within a given time period (early or late) and then rounded these means up to the nearest full number (i.e. number of active tadpoles). Activity was analysed using a repeated measures generalized linear mixed model (Proc Glimmix in SAS) with logit link and binomial error, autoregressive (AR1) variance structure and Kenward–Roger degrees of freedom. We used time (two levels), pond pH (i.e. population; six levels), pH treatment, predator treatment and their interactions, as well as block (four levels), as fixed factors. Container identity (nested within pH treatment, predator treatment and pond pH) was used as the random effect subject [35]. As there were no significant time × treatment or population interactions, only overall means are presented in the figures.
For larval mass, tail depth and tail muscle depth, container means were used in the statistical analyses. In the analyses of morphological traits, larval mass was used as a covariate to correct for size variation. The morphological analyses were conducted on log-transformed values, but data are presented on original values for ease of interpretation. Morphology was analysed with AN(C)OVAs, with pond pH, pH treatment, predator treatment and their interactions, as well as block (four levels), as fixed factors. The initial models included all two- and three-way interactions, but in subsequent analyses non-significant three-way interactions were removed.
For all response variables, linear orthogonal polynomials [36] were subsequently used to test for a linear trend between a given phenotypic trait and the factor pond pH. We used linear contrasts as the populations represent different levels of breeding pond pH (table 1) and as prior work on trait divergence patterns indicated that the magnitude of pH differences among populations reflects the strength of divergent selection [16,28].
As larval survival was generally high (82.5–100%) and did not differ among populations or treatments (all p > 0.3) in Experiment 1, we do not report survival results further for this part.
(ii). Experiment 2
Tadpole survival in Experiment 2 was analysed with a generalized linear mixed model with REML estimation, logit link function and binomial error structure with Proc Glimmix in SAS v. 9.3. First, a full model was run where pond pH, pH treatment, predator treatment and their interactions were included as fixed factors and container (nested within predator and pH treatment) as a random factor (electronic supplementary material, table S2). As there was a significant pH treatment × predator treatment × pond pH interaction (electronic supplementary material, table S2), these analyses were followed by separate analyses within each pH treatment to allow insight into the nature of the three-way interactions. To account for the effect of predator size on tadpole survival, dragonfly length was included as a covariate in survival analyses.
We used linear orthogonal polynomials to test for a linear trend between pond pH and survival under predation. To then test to what extent phenotypic divergence correlates with among-population differences in survival, population pairwise differences (i.e. phenotypic distances) in larval trait means (activity, larval mass, tail depth or tail muscle depth from Experiment 1, see above) were calculated within the four treatments and analysed with a multiple regression on distance matrices (MRM analyses [37,38], electronic supplementary material, S3 and table S3). The MRM analyses were conducted using the Ecodist package in R (v. 3.0.2). All other statistical analyses were conducted in SAS v. 9.3 (SAS Insitute, Inc.).
3. Results
(a). Experiment 1. Trait divergence
Both stressors strongly affected trait expression of tadpoles. Acid stress reduced activity and mass of tadpoles, but increased relative tail depth and relative tail muscle depth (table 2 and figure 1a–d). Predator stress strongly reduced tadpole activity and increased relative tail depth (table 2 and figure 1a,c), but did not affect larval mass or relative tail muscle depth (table 2 and figure 1b,d). pH treatment × predator treatment interactions were non-significant in all traits, indicating that the expression of anti-predator traits is independent of acid stress (table 2 and figure 1a–d).
Table 2.
Summary statistics for analysis of (a) repeated measures generalized linear mixed model on activity and analyses of (co)variance on (b) tadpole mass, (c) relative tail depth and (d) relative tail muscle depth under two predation (presence and absence) and two pH (pH 4.5 and 7.5) treatments in six R. arvalis populations occurring along a pH gradient. Activity was estimated during early and late larval period. Significant values (p < 0.05) are shown in bold, and marginally significant (p < 0.06) trends in italics.
| (a) activity |
(b) mass |
(c) tail depth |
(d) tail muscle depth |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| random effects | Var ± s.e. | — | — | — | ||||||||||||
| container | 0.11 ± 0.11 | — | — | — | ||||||||||||
| ndf | ddf | F | p-value | ndf | ddf | F | p-value | ndf | ddf | F | p-value | ndf | ddf | F | p-value | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| fixed effects | ||||||||||||||||
| time | 1 | 99.0 | 70.5 | <0.001 | — | — | — | — | — | — | — | — | — | — | — | — |
| block | 3 | 78.4 | 0.9 | 0.424 | 3 | 74 | 2.9 | 0.040 | 3 | 73 | 8.6 | <0.001 | 3 | 73 | 10.5 | <0.001 |
| pH treatment | 1 | 80.2 | 16.8 | <0.001 | 1 | 74 | 131.1 | <0.001 | 1 | 73 | 5.6 | 0.020 | 1 | 73 | 4.9 | 0.031 |
| predator treatment | 1 | 80.3 | 85.9 | <0.001 | 1 | 74 | 0.3 | 0.589 | 1 | 73 | 6.3 | 0.014 | 1 | 73 | 1.0 | 0.313 |
| pH treatment × predator treatment |
1 | 80 | 1.3 | 0.256 | 1 | 74 | 0.1 | 0.755 | 1 | 73 | 0.0 | 0.870 | 1 | 73 | 0.5 | 0.488 |
| pond pH | 5 | 80.1 | 2.8 | 0.023 | 5 | 74 | 0.8 | 0.525 | 5 | 73 | 4.9 | 0.001 | 5 | 73 | 1.7 | 0.147 |
| pH treatment × pond pH | 5 | 78.7 | 1.1 | 0.355 | 5 | 74 | 0.8 | 0.582 | 5 | 73 | 0.3 | 0.929 | 5 | 73 | 0.6 | 0.680 |
| predator treatment × pond pH |
5 | 79.9 | 1.2 | 0.330 | 5 | 74 | 0.5 | 0.797 | 5 | 73 | 0.5 | 0.768 | 5 | 73 | 0.7 | 0.637 |
| larval mass | — | — | — | — | — | — | — | — | 1 | 73 | 232.9 | <0.001 | 1 | 73 | 83.0 | <0.001 |
| linear contrasts on pond pH | ||||||||||||||||
| pond pH | 1 | 79.9 | 3.8 | 0.054 | 1 | 74 | 0.7 | 0.400 | 1 | 73 | 9.64 | 0.003 | 1 | 73 | 0.5 | 0.468 |
| predator treatment × pond pH |
1 | 79.6 | 0.3 | 0.605 | 1 | 74 | 0.4 | 0.512 | 1 | 73 | 0.22 | 0.642 | 1 | 73 | 0.7 | 0.420 |
| pH treatment × pond pH | 1 | 78.8 | 2.2 | 0.140 | 1 | 74 | 0 | 0.907 | 1 | 73 | 0.01 | 0.940 | 1 | 73 | 0.3 | 0.622 |
Figure 1.
Mean + s.e. of (a) activity (proportion of active tadpoles) and least square mean ± s.e. of (b) tadpole mass (g), (c) relative tail depth (mm) and (d) relative tail muscle depth (mm) of tadpoles from six R. arvalis populations from acid (pH 4) to neutral (pH 7) breeding ponds. Pond predator density increases with pond acidity. Tadpoles were reared in predator absence (black bars) or predator presence (grey bars) and at acid (left panel) and neutral (right panel) pH.
With regard to among-population differences, populations differed in activity (table 2 and figure 1a), whereby activity increased with decreasing pond pH in the NP treatments (pond pH linear contrast b: –0.394, p = 0.029) but not in the PP treatments (pond pH linear contrast: p = 0.635). Populations did not differ significantly in larval mass (table 2 and figure 1b). However, relative tail depth increased with decreasing pond pH (linear contrast: p = 0.003; table 2 and figure 1c), whereas populations did not differ significantly in relative tail muscle depth (table 2 and figure 1d). There were no significant treatment × pond pH interactions in any of the traits (table 2 and figure 1), indicating that effects of the stressors on trait expression were similar in all populations.
(b). Wild collected tadpoles
In the wild, populations differed significantly in larval size, developmental stage, relative tail depth and relative tail muscle depth (electronic supplementary material, table S1). Linear contrast indicated that all trait values increased with decreasing pond pH (electronic supplementary material, table S1 and figure S1).
(c). Experiment 2. Survival assay
Under direct predation risk, tadpole survival was, on average, lower in the acid (mean ± s.e. = 53.1 ± 4.7%) than in the neutral (65.6 ± 4.3%, figure 2) treatment. Among populations, survival increased, on average, with decreasing pond pH (figure 2; electronic supplementary material, table S2). However, the survival differences among populations depended on the treatment combination (i.e. pH treatment × predator treatment × pond pH interaction: p = 0.048; electronic supplementary material, table S2; figure 2).
Figure 2.
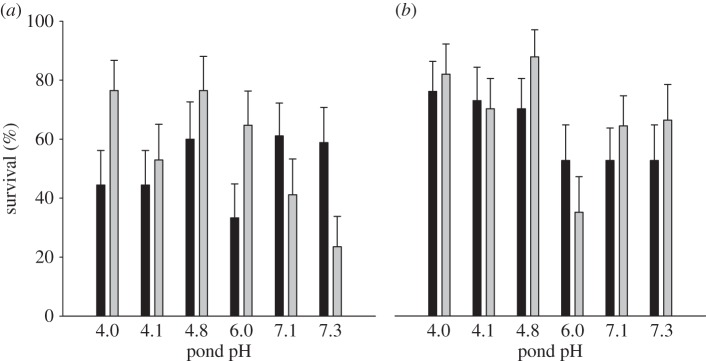
Mean + s.e. survival (%) of tadpoles from six R. arvalis populations from acid (pH 4) to neutral (pH 7) breeding ponds when exposed to a free-hunting dragonfly predator at two pH treatments ((a) acid and (b) neutral). Tadpoles had been previously reared in predator absence (black bars) or predator presence (grey bars) and at acid (a) and neutral pH (b). Pond predator density increases with pond acidity.
Separate analyses within the pH treatments further showed that in the acid treatment, tadpole survival under predation increased with decreasing pond pH in the presence of predators (APP treatment: survival of tadpoles from acidic ponds ranged from 55 to 78% and that of tadpoles from neutral ponds from 25 to 50%), but not in the absence of predators (predator treatment × pond pH interaction: p = 0.026; table 3 and figure 2). In neutral treatments, predator treatment had no effect and tadpole survival always increased with decreasing pond pH (table 3 and figure 2).
Table 3.
Generalized linear mixed model of tadpole survival in six R. arvalis populations along an acidification gradient and within two pH treatments (acid and neutral) when exposed to free-hunting predators. Tadpoles were prior reared at a combination of two pH and two predator treatments (acid/predator present, neutral/predator present, acid/predator absent or neutral/predator absent). Significant effects (p < 0.05) are shown in bold. (For full model, see the electronic supplementary material, table S2).
| acid treatment |
neutral treatment |
|||||||
|---|---|---|---|---|---|---|---|---|
| Var ± s.e. |
Z | p-value | Var ± s.e. |
Z | p-value | |||
| random effects | ||||||||
| tank (predator treatment) | 0.26 ± 0.26 | 1.0 | 0.159 | 0 | — | — | ||
| ndf | ddf | F | p-value | ndf | ddf | F | p-value | |
|---|---|---|---|---|---|---|---|---|
| fixed effects | ||||||||
| predator treatment | 1 | 32 | 0.3 | 0.609 | 1 | 32 | 1.5 | 0.230 |
| pond pH | 5 | 161 | 1.3 | 0.286 | 5 | 155 | 2.9 | 0.017 |
| predator treatment × pond pH | 5 | 161 | 2.6 | 0.026 | 5 | 155 | 0.7 | 0.621 |
| predator size | 1 | 161 | 1.8 | 0.186 | 1 | 155 | 9.3 | 0.003 |
| linear contrasts pond pH | ||||||||
| pond pH | 1 | 161 | 2.6 | 0.112 | 1 | 155 | 7.4 | 0.007 |
| predator treatment × pond pH | 1 | 161 | 8.0 | 0.005 | 1 | 155 | 0.1 | 0.818 |
(i). Trait–survival associations
The relative contribution of trait divergence to survival under predation depended strongly on experimental conditions. In the APP treatment, survival differences were related jointly to larval mass and tail depth (mass: b = 0.049, tail depth: b = 0.042; both p < 0.02; model: R2 = 0.151, p = 0.01, electronic supplementary material, table S3). In the NNP treatment, survival differences between the populations were significantly related only to tail depth (b = 0.032, p = 0.013; electronic supplementary material, table S3). In the ANP and NPP treatments, survival differences between the populations were not significantly related to any of the traits (electronic supplementary material, table S3).
4. Discussion
We found that R. arvalis tadpoles from relatively acidic/predator-rich populations survived better under predation risk than tadpoles from relatively neutral/predator-poor populations in most treatments. Moreover, constitutive morphological defences (relative tail depth) increased with decreasing pond pH, and tail depth mediated survival differences among the populations in two of the treatments. These results suggest that the higher predation pressure in acidic ponds imposes selection on constitutive morphological defences of tadpoles. Together with our previous findings that acidity drives divergence in embryonic acid stress tolerance and larval life-history traits [14,16,28,32], our results provide strong evidence for simultaneous adaptation to acidic pH and increased invertebrate predator pressure in acidified ponds, whereby changes in the abiotic environment (pH) increase strength of selection via biotic interactions (predation).
(a). Relative fitness of populations under direct predation
The direction of evolutionary responses of natural populations may depend on the collinearity of selective agents in nature [10], and the effects of combined stressors may depend on the selective history of populations (e.g. [13,39]). We predicted that—given increased predator abundance in acidic ponds and putative adaptation to both acid and predator stress—tadpole survival under predation should increase with pond acidity. Our results for most of experimental conditions are in agreement with this prediction. Overall, survival of tadpoles under predation ranged from 85% in the most acid population to only a 20% in the most neutral population, but the rank order of populations was strongly dependent on larval rearing conditions (figure 2). In particular, the effect of prior predator treatment altered the survival differences of the populations—but only within the acid treatment: survival increased with pond acidity when tadpoles had been reared in the presence of predators, but not when they had been reared in the absence of predators. These results suggest that R. arvalis populations inhabiting acidic environments are adapted to the combined stress of acidity and invertebrate predators, and that conditions experienced during the larval stage influenced relative fitness of populations. The latter is unlikely owing to phenotype-dependent survival prior to predation experiment (as survival was high in Experiment 1). Instead, among treatments variation in relative fitness is probably because of phenotypic plasticity in morphology, behaviour and/or physiology. The core candidate traits mediating fitness differences in our study are anti-predator defences and physiological acid stress tolerance.
(b). Divergence in behavioural and morphological defences
Environmental stress may influence the ability of individuals to express adaptive plasticity [23–25], and this propensity can depend on the selective history of populations (e.g. [11]). We predicted that populations inhabiting neutral environments would be more constrained in the expression of inducible defences under acid stress. However, we found no strong or consistent pH dependent differences among the study populations in the expression of inducible defences, indicating that acid stress did not compromise their capacity of mounting defences. In fact, we found that tadpoles generally had relatively deeper tails and tail muscles (i.e. when statistically corrected for size effect) under acid than neutral conditions. In this regard, the present findings are in contrast with our previous study, where tadpoles from a neutral population did not express inducible defences (deeper tails) under acid stress [25]. This discrepancy could be owing to higher sensitivity of acidic conditions in that specific neutral population or differences in experimental methods between the two studies.
We further predicted that populations inhabiting acidic ponds should show stronger inducible or constitutive defences—if selection imposed by invertebrate predators in acidic ponds is stronger. These results were supported by the contrast analyses: relative tail depth of laboratory-reared and field-collected tadpoles increased with pond acidity. However, although all study populations increased tail depth when reared in predator presence, there was no evidence for divergence in the extent of morphological plasticity (i.e. all populations showed similar degree of plasticity, figure 1). As the degree of plasticity is expected to reflect the extent of environmental heterogeneity (e.g. [18,40]), the observed divergence in constitutive—rather than plastic—defences indicates that temporal variation in predator-mediated selection is uniform across our study area [41,42]. However, temporally replicated sampling of predators is clearly needed to confirm this.
We also found that larval activity increased with increasing pond acidity when larvae were reared in the absence of predators. Although low activity is a common adaptive response to high predation risk at both genetic and phenotypic levels (e.g. [26,30,43]), higher activity could be selected for, for instance, if it allows for higher ingestion and growth rates and, ultimately, decreases predation risk under size-limited predation [44,45]. In line with this hypothesis, we found that survival differences among populations under predation were positively correlated with differences in body size (and tail depth) in the APP treatment. Although we did not find significant among-population differences in tadpole body mass here, we note that tadpoles from acidic ponds were larger in the wild (electronic supplementary material, table S1 and figure S1) and have genetically higher growth rates (both in acid and neutral rearing conditions) when reared individually in the laboratory and fed ad libitum [16,28,35]. The higher activity rate in tadpoles from more acidic ponds may also indicate divergence owing to non-predator mediated selection if higher activity indicates higher feeding (and growth) rates and thereby compensates for the commonly observed negative effects of acid stress on growth and developmental rates [28].
With regard to putative mechanisms relating responses of tadpoles to simultaneous acid and predator stress, corticosterone hormone is a key candidate. First, elevated corticosterone levels may facilitate predator-induced increases in tail depth and its levels can correlate positively with pond predator densities [46]. Second, as acidic conditions may increase corticosterone expression [47], the relatively deeper tails in the acidic populations, as well as within the acidic treatment, seen here may reflect higher corticosterone expression. Third, corticosterone also mediates locomotor and feeding activity of tadpoles [48]—possibly providing a link between the observed increased activity (in the absence of predators) and increased tail depth in acidic ponds. Experimental manipulations of corticosterone levels of tadpoles across acidification gradients would shed light on this hypothesis.
(c). Trait divergence: survival association
With regard to trait divergence–fitness relationships, we found that survival differences among populations were related primarily to larval mass (in APP) and tail depth (in APP and NNP), whereas in the ANP and NPP treatments, none of the traits made a significant contribution. These context-dependent patterns may reflect two mutually non-exclusive effects: on the one hand, differences among treatments in trait expression (and hence the extent of among-population phenotypic divergence) or, on the other hand, differences in the relative importance of different traits for survival under predation under acid versus neutral conditions. Moreover, individual level (rather than population means as used here) would allow more rigorous inferences on trait-fitness associations.
Nevertheless, the results for the APP treatment are in line with the general observations that deeper tails and tail muscles (e.g. [49]) and larger larval size [44,45] increase chance of survival under predation. The lack of body size effects under neutral conditions may indicate that tadpoles had passed a size where the influence of body size on survival is strongest (tadpoles in the neutral treatments were roughly twice as large as those in the acid treatment; figure 1). The lack of any activity effects on survival under predation are not surprising given that tadpoles from all populations reduced their activity to very low levels in the presence of predators (figure 1).
The context-dependent ‘trait–relative fitness’ relationships here are in agreement with general observations that trait divergence may not correlate with the degree of local adaptation (i.e. measured as fitness differences) [50], possibly owing to complexity of traits mediating adaptation. In our case, the observed strong survival differences among populations in the APP treatment were probably influenced simultaneously by anti-predator and (unmeasured) acid stress tolerance traits. It is possible that the relative role of defence traits in evading predation may have been stronger under acid stress, and/or that acid origin tadpoles are better able to cope with physiological acid stress [16,28,32]. Moreover, the particular combination of traits mediating adaptation may differ among populations owing to underlying genetic variation or contribution of additional selective factors. Future studies should aim to disentangle the relative importance of single traits versus the composite multivariate phenotype (e.g. [51]) in adaptation to acidification and to multiple stressors in general.
(d). General implications and conclusions
Multifarious selection by covarying environmental factors may enhance the potential for local adaptation even in networks of local populations connected with migration (e.g. [10]) and partly explain the strong local adaptation found in amphibian metapopulations (e.g. [29,52,53]). For the case here, selection against immigrant genotypes from neutral ponds may be strong in acidic ponds because of their low embryonic acid stress tolerance and high risk of predation during the larval stage [32]. Although current evidence indicates that acidic pond genotypes are not selected against (or may even be favoured) in neutral environments in these same traits [32], selection against acidic genotypes may act via slow developmental rates of tadpoles [16,28,32] and low fecundity of females [54].
In conclusion, together with previous work, the results here indicate that R. arvalis populations are adapted simultaneously to both acid and predator stress. Adaptive divergence was apparent as higher relative fitness of populations originating from acid/predator-rich environments under predation—at least in part mediated by variation in larval size and defence morphology. Our study reinforces the view that combined selection by multiple stressors is an important driver of phenotypic diversification in natural populations, and highlights the need to study responses to direct (here pH) and indirect (here predation) environmental changes, as well as understanding selection on the composite phenotype in nature.
Supplementary Material
Acknowledgements
We thank Beatrice Lindgren, Claudio Brunold and Nicole Hatt for invaluable help with the field and laboratory work, Rajwinder Sohal for help in the molecular laboratory and all landowners for permission to use their sites. The molecular analyses were conducted in the Genetic Diversity Centre of ETH Zurich.
The experiments were conducted under the permissions from the county boards in Halland and Västra Götaland counties and from the ethical committee for animal experiments in Uppsala County.
Data accessibility
The data are available at the public repository dryad.
Funding statement
This study was supported by grants from Swiss National Science foundation (to K.R.) and Formas (A.L. and K.R.).
References
- 1.Ghalambor CK, McKay JK, Carroll SP, Reznick DN. 2007. Adaptive versus non adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments. Funct. Ecol. 21, 394–407. ( 10.1111/j.1365-2435.2007.01283.x) [DOI] [Google Scholar]
- 2.Hoffmann AA, Parsons PA. 1997. Extreme environmental change and evolution. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 3.Reznick DN, Ghalambor CK. 2001. The population ecology of contemporary adaptations: what empirical studies reveal about the conditions that promote adaptive evolution. Genetica 112, 183–198. ( 10.1007/978-94-010-0585-2_12) [DOI] [PubMed] [Google Scholar]
- 4.Bijlsma R, Loeschcke V. 2005. Environmental stress, adaptation and evolution: an overview. J. Evol. Biol. 18, 744–749. ( 10.1111/j.1420-9101.2005.00962.x) [DOI] [PubMed] [Google Scholar]
- 5.Schindler DW, Curtis PJ, Parker BR, Stainton MP. 1996. Consequences of climate warming and lake acidification for UV-B penetration in North American boreal lakes. Nature 379, 705–708. ( 10.1038/379705a0) [DOI] [Google Scholar]
- 6.Crain CM, Kroeker K, Halpern BS. 2008. Interactive and cumulative effects of multiple human stressors in marine systems. Ecol. Lett. 11, 1304–1315. ( 10.1111/j.1461-0248.2008.01253.x) [DOI] [PubMed] [Google Scholar]
- 7.Halpern BS, et al. 2008. A global map of human impact on marine ecosystems. Science 319, 948–952. ( 10.1126/science.1149345) [DOI] [PubMed] [Google Scholar]
- 8.Reznick D, Travis J. 1996. The empirical study of adaptation in natural populations. In Adaptation (eds Rose MR, Lauder GV.), pp. 243–290. San Diego, CA: Academic Press. [Google Scholar]
- 9.Kawecki TJ, Ebert D. 2004. Conceptual issues in local adaptation. Ecol. Lett. 7, 1225–1241. ( 10.1111/j.1461-0248.2004.00684.x) [DOI] [Google Scholar]
- 10.MacColl A. 2011. The ecological causes of evolution. Trends Ecol. Evol. 26, 514–522. ( 10.1016/j.tree.2011.06.009) [DOI] [PubMed] [Google Scholar]
- 11.Rogell B, Hofman M, Eklund M, Laurila A, Höglund J. 2009. The interaction of multiple environmental stressors affects adaptation to a novel habitat in the natterjack toad Bufo calamita. J. Evol. Biol. 22, 2267–2277. ( 10.1111/j.1420-9101.2009.01842.x) [DOI] [PubMed] [Google Scholar]
- 12.Bradford DF, Cooper SD, Jenkins TM, Kratz K, Sarnelle O, Brown AD. 1998. Influences of natural acidity and introduced fish on faunal assemblages in California alpine lakes. Can. J. Fish. Aquat. Sci. 55, 2478–2491. ( 10.1139/cjfas-55-11-2478) [DOI] [Google Scholar]
- 13.Räsänen K, Green DM. 2009. Acidification and its effects on amphibian populations. In Amphibian biology, volume 8. Decline: diseases, parasites, maladies and pollution (ed. Heatwole H.), pp. 3244–3267. Chipping Norton, Australia: Surrey Beatty and Sons. [Google Scholar]
- 14.Räsänen K, Laurila A, Merilä J. 2003. Geographic variation in acid stress tolerance of the moor frog, Rana arvalis. I. Local adaptation. Evolution 57, 352–362. ( 10.1554/0014-3820(2003)057[0352:GVIAST]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 15.Derry AM, Arnott SE. 2007. Adaptive reversals in acid tolerance in copepods from lakes recovering from historical stress. Ecol. Appl. 17, 1116–1126. ( 10.1890/06-1382) [DOI] [PubMed] [Google Scholar]
- 16.Hangartner S, Laurila A, Räsänen K. 2012. Adaptive divergence in moor frog (Rana arvalis) populations along an acidification gradient: inferences from QST–FST correlations. Evolution 66, 867–881. ( 10.1111/j.1558-5646.2011.01472.x) [DOI] [PubMed] [Google Scholar]
- 17.Collier KJ, Ball OJ, Graesser AK, Main MR, Winterbourn MJ. 1990. Do organic and anthropogenic acidity have similar effects on aquatic fauna? Oikos 59, 33–38. ( 10.2307/3545119) [DOI] [Google Scholar]
- 18.Via S, Lande R. 1985. Genotype–environment interaction and the evolution of phenotypic plasticity. Evolution 39, 505–522. ( 10.2307/2408649) [DOI] [PubMed] [Google Scholar]
- 19.Tollrian R, Harvell CD. 1999. The ecology and evolution of inducible defenses. Princeton, NJ: Princeton University Press. [Google Scholar]
- 20.Hanazato T. 2001. Pesticide effects on freshwater zooplankton: an ecological perspective. Environ. Pollut. 112, 1–10. ( 10.1016/S0269-7491(00)00110-X) [DOI] [PubMed] [Google Scholar]
- 21.Huber H, Kane NC, Heschel MS, von Wettberg EJ, Banta J, Leuck AM, Schmitt J. 2004. Frequency and microenvironmental pattern of selection on plastic shade-avoidance traits in a natural population of Impatiens capensis. Am. Nat. 163, 548–563. ( 10.1086/382549) [DOI] [PubMed] [Google Scholar]
- 22.Teplitsky C, Piha H, Laurila A, Merilä J. 2005. Common pesticide increases costs of antipredator defenses in Rana temporaria tadpoles. Environ. Sci. Technol. 39, 6079–6085. ( 10.1021/es050127u) [DOI] [PubMed] [Google Scholar]
- 23.Barry MJ. 2000. Effects of endosulfan on Chaoborus-induced life-history shifts and morphological defenses in Daphnia pulex. J. Plankton Res. 22, 1705–1718. ( 10.1093/plankt/22.9.1705) [DOI] [Google Scholar]
- 24.Relyea RA. 2004. Growth and survival of five amphibian species exposed to combinations of pesticides. Environ. Toxicol. Chem. 23, 1737–1742. ( 10.1897/03-493) [DOI] [PubMed] [Google Scholar]
- 25.Teplitsky C, Räsänen K, Laurila A. 2007. Adaptive plasticity in stressful environments: acidity constrains inducible defences in Rana arvalis. Evol. Ecol. Res. 9, 447–458. [Google Scholar]
- 26.Laurila A, Lindgren B, Laugen AT. 2008. Antipredator defenses along a latitudinal gradient in Rana temporaria. Ecology 89, 1399–1413. ( 10.1890/07-1521.1) [DOI] [PubMed] [Google Scholar]
- 27.Long JD, Mitchell JL, Sotka EE. 2011. Local consumers induce resistance differentially between Spartina populations in the field. Ecology 92, 180–188. ( 10.1890/10-0179.1) [DOI] [PubMed] [Google Scholar]
- 28.Hangartner S, Laurila A, Räsänen K. 2011. Adaptive divergence of the moor frog (Rana arvalis) along an acidification gradient. BMC Evol. Biol. 11, 366 ( 10.1186/1471-2148-11-366) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Buskirk J, Arioli M. 2005. Habitat specialization and adaptive phenotypic divergence of anuran populations. J. Evol. Biol. 18, 596–608. ( 10.1111/j.1420-9101.2004.00869.x) [DOI] [PubMed] [Google Scholar]
- 30.Laurila A, Pakkasmaa S, Merilä J. 2006. Population divergence in growth rate and antipredator defenses in Rana arvalis. Oecologia 147, 585–595. ( 10.1007/s00442-005-0301-3) [DOI] [PubMed] [Google Scholar]
- 31.Glandt D. 2008. Der Moorfrosch (Rana arvalis): Erscheinungsvielfalt, Verbreitung, Lebensräume, Verhalten sowie Perspektiven für den Artenschutz. Zeitschrift für Feldherpetologie 13, 11–34. [Google Scholar]
- 32.Hangartner S, Laurila A, Räsänen K. 2012. The quantitative genetic basis of adaptive divergence in the moor frog (Rana arvalis) and its implications for gene flow. J. Evol. Biol. 66, 867–881. ( 10.1111/j.1420-9101.2012.02546.x) [DOI] [PubMed] [Google Scholar]
- 33.APHA. 1985. Standard methods for the examination of water and wastewater, 16th edn Washington, DC: American Public Health Association. [Google Scholar]
- 34.Gosner KL. 1960. A simplified table for staging anuran embryos and larvae with notes on identification. Copeia 1960, 183–190. [Google Scholar]
- 35.Littell RC, Milliken GA, Stroup WW, Wolfinger RD, Schabenberger O. 2006. SAS for mixed models. Cary, NC: SAS Institute Inc. [Google Scholar]
- 36.Quinn GP, Keough MJ. 2002. Experimental design and data analysis for biologists. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 37.Legendre P, Lapointe F-J, Casgrain P. 1994. Modeling brain evolution from behavior: a permutational regression approach. Evolution 48, 1487–1499. ( 10.2307/2410243) [DOI] [PubMed] [Google Scholar]
- 38.Lichstein JW. 2007. Multiple regression on distance matrices: a multivariate spatial analysis tool. Plant Ecol. 188, 117–131. ( 10.1007/s11258-006-9126-3) [DOI] [Google Scholar]
- 39.Espeland EK, Rice KJ. 2007. Facilitation across stress gradients: the importance of local adaptation. Ecology 88, 2404–2409. ( 10.1890/06-1217.1) [DOI] [PubMed] [Google Scholar]
- 40.Lind MI, Johansson F. 2007. The degree of adaptive phenotypic plasticity is correlated with the spatial environmental heterogeneity experienced by island populations of Rana temporaria. J. Evol. Biol. 20, 1288–1297. ( 10.1111/j.1420-9101.2007.01353.x) [DOI] [PubMed] [Google Scholar]
- 41.Edgell TC, Lynch BR, Trussell GC, Palmer AR. 2009. Experimental evidence for the rapid evolution of behavioral canalization in natural populations. Am. Nat. 174, 434–440. ( 10.1086/603639) [DOI] [PubMed] [Google Scholar]
- 42.Bourdeau PE. 2012. Intraspecific trait cospecialization of constitutive and inducible morphological defences in a marine snail from habitats with different predation risk. J. Anim. Ecol. 81, 849–858. ( 10.1111/j.1365-2656.2012.01965.x) [DOI] [PubMed] [Google Scholar]
- 43.Brodin T, Johansson F. 2004. Conflicting selection pressures on the growth/predation risk trade-off in a damselfly. Ecology 85, 2927–2932. ( 10.1890/03-3120) [DOI] [Google Scholar]
- 44.Urban MC. 2007. Risky prey behaviour evolves in risky habitats. Proc. Natl Acad. Sci. USA 104, 14 377–14 382. ( 10.1073/pnas.0704645104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Urban MC. 2007. The growth-predation risk trade-off under a growing gape-limited predation threat. Ecology 88, 2587–2597. ( 10.1890/06-1946.1) [DOI] [PubMed] [Google Scholar]
- 46.Maher JM, Werner EE, Denver RJ. 2013. Stress hormones mediate predator-induced plasticity in amphibian tadpoles. Proc. R. Soc. B 280, 20123075 ( 10.1098/rspb.2012.3075) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chambers DL, Wojdak JM, Du P, Belden LK. 2013. Pond acidification may explain differences in corticosterone among salamander populations. Physiol. Biochem. Zool. 86, 224–232. ( 10.1086/669917) [DOI] [PubMed] [Google Scholar]
- 48.Crespi EJ, Denver RJ. 2005. Roles of stress hormones in food intake regulation in anuran amphibians throughout their life cycle. Comp. Biochem. Physiol. A 141, 381–390. ( 10.1016/j.cbpb.2004.12.007) [DOI] [PubMed] [Google Scholar]
- 49.Van Buskirk J, McCollum SA, Werner EE. 1997. Natural selection for environmentally induced phenotypes in tadpoles. Evolution 51, 1983–1992. ( 10.2307/2411018) [DOI] [PubMed] [Google Scholar]
- 50.Hereford J. 2009. A quantitative survey of local adaptation and fitness trade-offs. Am. Nat. 173, 579–588. ( 10.1086/597611) [DOI] [PubMed] [Google Scholar]
- 51.Kingsolver JG, Huey RB. 2003. Introduction: the evolution of morphology, performance, and fitness. Integr. Comp. Biol. 43, 361–366. ( 10.1093/icb/43.3.361) [DOI] [PubMed] [Google Scholar]
- 52.Skelly DK. 2004. Microgeographic countergradient variation in the wood frog, Rana sylvatica. Evolution 58, 160–165. ( 10.1554/03-425) [DOI] [PubMed] [Google Scholar]
- 53.Richter-Boix A, Quintela M, Kierczak M, Franch M, Laurila A. 2013. Fine-grained adaptive divergence in an amphibian: genetic basis of phenotypic divergence and the role of nonrandom gene flow in restricting effective migration among wetlands. Mol. Ecol. 22, 1322–1340. ( 10.1111/mec.12181) [DOI] [PubMed] [Google Scholar]
- 54.Räsänen K, Söderman F, Laurila A, Merilä J. 2008. Geographic variation in maternal investment: acidity affects egg size and fecundity in Rana arvalis. Ecology 89, 2553–2562. ( 10.1890/07-0168.1) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data are available at the public repository dryad.