Abstract
Many species use tools, but the mechanisms underpinning the behaviour differ between species and even among individuals within species, depending on the variants performed. When considering tool use ‘as adaptation’, an important first step is to understand the contribution made by fixed phenotypes as compared to flexible mechanisms, for instance learning. Social learning of tool use is sometimes inferred based on variation between populations of the same species but this approach is questionable. Specifically, alternative explanations cannot be ruled out because population differences are also driven by genetic and/or environmental factors. To better understand the mechanisms underlying routine but non-universal (i.e. habitual) tool use, we suggest focusing on the ontogeny of tool use and individual variation within populations. For example, if tool-using competence emerges late during ontogeny and improves with practice or varies with exposure to social cues, then a role for learning can be inferred. Experimental studies help identify the cognitive and developmental mechanisms used when tools are used to solve problems. The mechanisms underlying the route to tool-use acquisition have important consequences for our understanding of the accumulation in technological skill complexity over the life course of an individual, across generations and over evolutionary time.
Keywords: habitual tool use, ontogeny, social learning, cognition, inhibition, phenotypic plasticity
1. Introduction
Research over the past two decades has shown that tool use (for definition see: [1, p. 5]) is not as rare among non-human animals as we once thought [1]. Tool use in natural settings nevertheless remains restricted to only a minority of animals that mostly express the behaviour incidentally rather than routinely. Although studies on incidental tool users can be useful in determining factors influencing behavioural innovations, they would provide us with little insight on the adaptive value, evolution or cognitive underpinnings of tool use. Routine tool users, on the other hand, provide us with opportunities to study ontogeny and individual variation which can help to elucidate the level of phenotypic plasticity and cognition underlying the behaviour.
Routine tool users are often classified as either customary (or universal) or habitual, based on geographical variation in the trait. Although habitual tool use is often considered to be the product of social learning, this inference is usually based on the problematic exclusion method (i.e. elimination of environmental or genetic causes of variation). However, we propose that both longitudinal and experimental studies on tool-use development and individual variation can assist in identifying underlying mechanisms and cognitive underpinnings of habitual tool use. As such, we confine ourselves to the cases of habitual tool-assisted foraging as reported by Shumaker et al. [1]. We advocate similar lines of study for other cases of routine tool use (i.e. more customary/universally prevalent forms of tool use, such as we find in humans), before we can try to unravel potential selective pressures on tool use and cognitive evolution.
(a). Habitual tool use
In cataloguing variation in tool repertoires of wild chimpanzee populations, McGrew went beyond the simplistic categorization of ‘present’ versus ‘absent’ tool variants by additionally distinguishing between ‘habitual’ versus ‘rare, idiosyncratic or questionable’ tool use. He defined habitual tool use as tool-use patterns shown repeatedly by several members of a group; excluding single instances by one or several individuals, several instances by only one individual and all instances of insufficient data or involving released animals [2, p. 180]. Subsequently, Whiten et al. distinguished between ‘customary’, ‘habitual’, ‘present’, ‘absent for ecological reasons’, ‘absent for no apparent ecological reason’, and ‘unknown’ and redefined ‘habitual’ as ‘behaviour that is not customary (i.e. occurring in all or most able-bodied members of at least one age–sex class) but has occurred repeatedly in several individuals, consistent with some degree of social transmission’ [3, p. 682]. This definition also applies to our use of the term ‘habitual tool use’. McGrew emphasized that the definition of ‘habitual’ leads to an incomplete tool catalogue of habitual tool variants for most populations owing to the positive correlation between study length and number of identified habitual tool-use variants. Whiten et al. [3] thus focused on the importance of: (i) making tool catalogues more complete; (ii) clarifying the extent to which a variant is habitual; and (iii) systematically documenting behavioural variants absent in a particular population but present elsewhere.
In view of this geographically based categorization, habitual tool use has been set aside from other forms of (routine) tool use that are, for example, customary, idiosyncratic or absent in some sites owing to ecological or genetic variation. Accordingly, in contrast to these other forms of (routine) tool use, habitual tool use has been suggested to depend on cognitive flexibility that enables animals to solve disparate problems and use social cues, rather than rely on predisposed action patterns that are comparatively fixed [3,4]. However, the supporting data that these tool users invent and other individuals within the population then socially learn their techniques is rarely definitive and open to alternative explanation [4–6]. First, the distinction between habitual and ‘absent due to no apparent ecological reasons’ is problematic. Not only is it logically impossible to demonstrate the absence of a cause, in the end only a small portion of the potentially relevant factors can be realistically considered, even without including possible interaction effects. Second, to some extent, ecological factors always influence the expression of behavioural phenotypes, so why bother to exclude them at all? When trying to exclude genetic factors or individual learning, similar problems arise. Langergraber et al., for instance, showed that, for chimpanzees, geographical variation correlated strongly to genetic variation, leaving only a few behaviours (ca 13%) for which expression varied geographically among genetically similar groups [6]. Third, when no geographical variation is found, behaviours might still be socially learned. Tool-use preferences in sea otters (Enhydra lutis), in terms of type of prey, tool-use method and foraging strategy seem, for example, to be vertically transmitted even though the use of rocks to open or dislodge hard-shelled food is common for all sea otters and does not seem to require social learning [7]. Fourth, when behaviours can be acquired exclusively by individual learning, this does not mean that they are. Simple Pandanus and twig tool use in New Caledonian crows is, for instance, influenced by social input even though correct performance can also be acquired without social cues [8,9].
Geographical variation in tool use is thus an indirect and possibly problematic route to evaluate the degree to which habitual tool use relies on social learning and reflects general cognitive abilities. Instead, it would be more productive to establish whether we can find any direct evidence for social learning and general cognitive abilities. In the case of the woodpecker finch, for example, observational as well as experimental lines of inquiry point toward strong genetic and ecological influence in shaping the form and expression of tool use. Experimental evidence moreover reveals that presence of social cues does not seem to have any effect [10], while cognitive strategies shared with non-tool-using relatives appear to underlie the use of tools [11]. What about the so-called ‘habitual tool users’ as classified by Shumaker et al. [1]: bottlenose dolphins (Tursiops sp.) [12], sea otters (E. lutis), orangutans (Pongo sp.) [4], chimpanzees (Pan troglodytes) [13–15], capuchins (Sapajus sp.) [16,17], Burmese long-tailed macaques (Macaca fascicularis aurea) [18,19],1 New Caledonian crows (Corvus moneduloides) [9,20], and possibly green-backed herons (Butorides sp.) [21]?
In this article, we review studies that directly examine the development of habitual tool use over life history and compare its emergence to other ‘tool-free’ foraging behaviours. We explore three different lines of evidence that contribute to our understanding of habitual tool use: (i) observational data of acquisition patterns; (ii) experimental evidence illustrating cognitive challenges associated with tool use; and (iii) individual differences revealing the role of social input in the wild. We discuss the implications that this analysis of tool ontogeny might have for uncovering the cognitive mechanisms underpinning tool use in different species and some possible directions for future work. For the purpose of this paper, we focus on foraging tools and skills because of their direct link to survival and fitness.
2. Observational field studies reveal typical tool-acquisition patterns
(a). Practice and errors
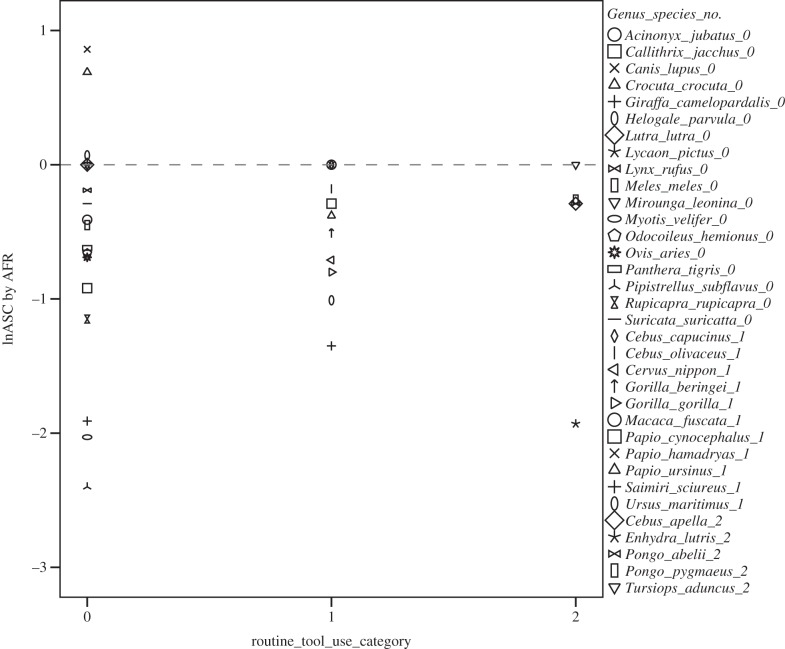
Observational field data indicate that habitual tool users take almost their entire developmental period to acquire tool competence for the relevant tool variant. By competence, we mean regularly succeeding in achieving the goal (here, obtaining food). Table 1 shows that for several species, some behaviours are not acquired until years after the animals are able to forage for themselves without tools. A preliminary comparison of other routine or non-tool users and habitual tool users also suggests a relative late age at skill competence for habitual tool users (figure 1). Sea otters appear to be an outlier among the habitual tool users in acquiring tool competence relatively fast; whereas spotted hyaenas and wolves appear to be outliers among the non-routine tool users because they acquire their skills (i.e. hunting skills) relatively late. Hunting skills are indeed often considered complex, requiring more learning (see also [55]). What are immatures doing during this period? Why the delay? Are they not yet motivated to carry out these possibly more costly foraging skills (in terms of time, and sometimes physical effort), while they are still physically immature and provisioned by their parents? In most observational studies reviewed, immatures spend a good deal of time interacting with tool material before they are competent [17]. What can detailed analysis of their behaviour tell us about the possible development of cognitive adaptations that may underpin the adult behaviour?
Table 1.
The time course of emergence for several habitual tool behaviours in different species relative to other developmental hallmarks. (Columns 2–8 give the average age (in months)a of sexual maturity, weaning/fledging, first foraging attempts, first object manipulation, competent ‘tool-free’ foraging on simple food, majority of food items and difficult food items. Columns 9–13 gives information on the habitual tool variants: age of competent use (including manufacture), manufacture and use, time (in months) between first object exploration and full competence in tool use and manufacture, age of proficient tool use (incl. manufacture), short description of tool variant, study site from which ontogenetic data originates, tool type: U, use; M, manufacture; S, tool set. The last two columns provide references for tool data and other developmental information. NC crows, New Caledonian crows; B. dolphins, bottlenose dolphins.)
| species | wean/fledge | start explore |
competent feeding w/o tool |
learning period | tool variant (site) | study site | reference |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| sex mat. | food | objects | simple | most | complex | competent TU + M | proficient TU + M | type UMS | tool | −tool | |||||
| sea otters | 48 | 6 | 1 | 1–2.5 | 1–4 | 3–4.5 | 3–4.5 | 3–4.5 | >2 | 4.5–5.5 | rock hammer | all | TU | [7,22,23] | [7,22] |
| NC crows | 24 | 6–12 | 1 | 1–2 | 1–2 | 4 | 6–12 | 2–4 | >1 | 12- | non-hooked stick | Mare | TU | [8,9,24] | [8,25,26] |
| 7 | >6 | 12- | Pandanus-wide | Mare | TM | [9,27] | |||||||||
| ?–12 | <10 | 12- | hooked stick | Grand Terre | TM | [28] | |||||||||
| capuchins | 55 | 12 | 2 | 1 | 6 | 12 | 12–24b | 6–24 | >5 | <24 | stick-weapon | Serra Capivara | TU | [29] | [30,31] |
| >6–48 | >5 | <24 | stone-dig | Serra Capivara | TU | [29,32] | |||||||||
| >6–48 | >5 | >24 | stick-probe | Serra Capivara | TM | [29,32] | |||||||||
| 24–48 | >24 | >48 | stone-pound | Serra Capivara | TU | [29,32] | |||||||||
| 24–48 | >24 | >48 | two tools | Serra Capivara | TS | [29] | |||||||||
| 25–30 | >24 | >30 | stone (nuts, etc.) | Tiete | TS | [33] | |||||||||
| 24–48 | >24 | >24 | termite fish | Mamanguape | TU | [34]c | |||||||||
| chimpanzees | 118 | 60 | 5 | 6 | 24 | 60 | 120 | 24–36 | >18 | 60 | ant dip | Bossou | TM | [35] | [36–40] |
| 29 | >23 | >36 | ant fish | Mahale M | TM | [41] | |||||||||
| 30–66 | >24 | >120 | termite fish | Gombe | TM | [42] | |||||||||
| 36 | >30 | >120 | leaf chew/fold | Bossou | TM | [43] | |||||||||
| 36–42 | >30 | 96–120 | nut cracking | Bossou | TS | [44,45] | |||||||||
| 42 | >36 | >96 | leaf folding | Bossou | TM | [44] | |||||||||
| orangutans | 132 | 84 | 12 | 12 | 18–20 | 34–36 | 36d | 48 | >36 | 120 | tree hole | Suaq | TM | [38]e | [46,47] |
| 84 | >72 | 120 | Neesia | Suaq | TM | [38,39] | |||||||||
| B. dolphins | 120 | 36–96 | 1 | 1 | 3 | 6 | 60-f | 20 | >20 | >36 | sponge | Shark Bay | TM | [12,48,49] | [12,49–51] |
| humans | 198 | 24–30 | 6 | 1 | 6 | 24 | 180 | 18 | >17 | 300 | out-of-reach | Hunter–gatherers | TU | [52] | [37,39,53,54] |
aIn case of doubt or sex differences, the younger alternative was used.
bFeeding Luehea candida by Cebus capuchinus (slower life history).
cLimited observational data.
dFeeding on water or honey from tree holes without the use of tools (E. Meulman 2013, unpublished data).
eE. Meulman 2013, unpublished data.
fBeach hunting (also influenced by body size).
Figure 1.
A preliminary dataset including 34 species (Artiodactyla: n = 5, Carnivora: n = 13, Cetacea: n = 1, Chiroptera: n = 2, Primates: n = 13) suggests that most habitual tool users do seem to acquire their skills relatively late during ontogeny compared with other species that were not qualified as habitual tool users by Shumaker et al. [1] (although differences are not significant). Legend numbers refer to tool-use category as displayed on the x-axis. Sea otters appear to be an outlier among the habitual tool users (acquiring their tool use relatively fast); whereas spotted hyaenas and wolves appear to be outliers among the non-routine tool users because they acquire their skills (i.e. hunting skills) relatively late. Data on age at skill competence (ASC) and age at first reproduction (AFR) were taken from Schuppli et al. [55]. Data on routine tool use come from Shumaker et al. [1].
Many, though by no means all, habitually used tool behaviours are associated with a long period during which immatures interact with the tools and the goal objects, but use a characteristic pattern of non-random ‘errors’: either the wrong action or tool material, an incomplete action sequence, action sequences performed in the wrong order, or the correct complete and ordered action sequence applied towards the wrong goal or substrate. For example, Pandanus tool competence in New Caledonian crows progresses according to four probing techniques and five manufacturing techniques, of which only the fourth probing and fifth manufacturing technique resemble adult-like competence, which takes on average seven months to master. Adult-like proficiency (i.e. efficiency, speed, etc.) is acquired even later (ca. 12 months). All other probing and manufacturing techniques include errors that result in faulty detachment or dysfunction of the tool [9]. Capuchins in Tiete (Sapajus apella) go through eight developmental stages across 2.5 years before mastering their nut-cracking skills, from simple manipulation, to rubbing or hitting objects, to inserting in and hitting against substrates, striking objects against anvils and eventually placing nuts on anvils, followed by ineffective nut cracking before effective nut cracking. On rare occasions, individuals bang two detached objects together [56]. Gombe chimpanzees start with pressing a tool to the termite mound or swiping the mound (at 3.5 years), and gradually change this into haphazard, rapid tool insertion without the required depth (4.5 years), to successful termite fishing (5.5 years) [42]. Although evidence for such ‘errors’ is still missing for bottlenose dolphins, orangutans, sea otters and long-tailed macaques, anecdotal evidence and studies on macaque stone handling suggest similar paths of development [22,57,58].
As to how far the ‘errors’ observed during the developmental period actually represent goal-directed attempts instead of random play or exploration is difficult to establish. However, errors diminish over time while tool-using skills improve, until eventually adult-like competence is reached before or around weaning age [9,42,44,59,60]. Nonetheless, compared to adults, competent weaned immatures often still show inadequate skills by persisting at unrewarding locations, using tools at successful locations less often, having shorter or longer lasting tool sessions than adults, using more tools per session, modifying tools more frequently, using tools with different features (material, size and shape) than adults or—only relevant in some contexts—lacking hand preferences [9,18,35,41,44,59,60]. Hence, although we might have mischaracterized play and exploration as ‘errors’, the ‘error-filled’ period of practice does seem to eventually result in skill improvement, whether actively goal-directed or facilitated by exposure to ecological and/or social factors.
Of course, development, physical maturation and changing motivation coincide (e.g. with regard to foraging strategies, social interactions, perception and coordination). Physical maturation thus probably contributes to, but cannot fully explain the delays and errors in tool-using competence. For example, the random instead of routine tool use at younger ages suggests lack of systematic coordination rather than physical strength. Given that individual variation in competence often varies with learning opportunity (with some immatures performing even better than some adults, see §4), we suggest that opportunities for learning, not maturation, is the primary limiting factor. Age might even constrain learning ability if a sensitive period of exposure has passed [10,61].
(b). Phenotypic biases
The discrepancy between adult and immature tool behaviour described above (or see table 1) may also tell us something about the predisposed phenotypic biases a species may have that may either promote or constrain innovation and/or acquisition of tool behaviours. Both physiological traits (e.g. lack of appendages for manipulation) and behavioural biases are informative, especially when contrasted with closely related species (see §3a). Such phenotypic biases provide guidance with respect to which tool-mediated behaviours are relatively fixed (i.e. genetically hard-wired), as opposed to those that require extensive learning and social input, assuming ecological learning opportunities are present. For instance, North American badgers (Taxidea taxus) frequently capture hibernating squirrels underground and are morphologically and behaviourally specialized to excavate burrow systems by the movement of soil [62]. Hence, the use of soil to plug openings into burrow systems occupied by ground squirrels may be considered an idiosyncratic expression of their normal behaviour, also because opportunities for social transmission are rare. Similarly, sea otters show a strong genetic predispositions for increased tactile sensitivity of the hand [7] and object-carrying pouches [63], which might contribute to the lack of geographical variation exhibited in terms of presence of tool use—although variation in frequency and preferences exists—and their relatively young age at competence (figure 1). At the other extreme, we have the habitual tool-using bottlenose dolphins that are not well designed for object manipulation, which perhaps explains their small repertoire of tool variants so far [12]. The sponging dolphins are moreover tasked with searching for prey in an entirely new way, where vision and sonar become secondary to the sponge tool use itself [48]. They may even need to inhibit a likely predisposed resistance to put something over the beak and face, which interferes with echolocation and grasping prey. Other phenotypic biases are more subtle. Thumb morphology, for instance, allows for complex object manipulation in capuchins, chimpanzees [64] and precision grip in humans [65], but capuchins initially tend to strike or rub objects, whereas chimpanzees tend to stack them [45,56,66]. Thus, in acquiring nut-cracking skills, capuchins must learn to place a nut on the anvil before striking it, suggesting that striking is more fixed than stacking, whereas the contrary seems to apply to chimpanzees. Actions that are less fixed may therefore require more time to master and perform in a routine fashion than tool variants involving a more fixed action pattern, for which expression seems to be less variable and dependent on ecological contexts and learning opportunities.
3. Experimental evidence: cognition and tool use
The discrepancy between adult and immature tool behaviour described above, which does not seem to be owing to physical size or strength, thus suggests a role for cognitive skills to adjust or overcome predisposed action patterns, or to master behaviours that are not in the inherited repertoire. An ability to innovate, knowledge of object properties and observational learning has been suggested to be important [67]. All of these have been shown to correlate with slow life histories and brain encephalization [68–71]. In this section, we examine evidence from captive studies on problem solving with objects and tools, and consider how studying cognitive underpinnings and developmental change might help us to identify candidate cognitive adaptations underpinning habitual adult tool use.
(a). Adjusting phenotypic biases
If the tool-using action is not in the inherited repertoire, the animal may need to inhibit or change performance of other predisposed actions in that context. The errors described in the previous section may indicate an inability to inhibit such actions. Work in the laboratory shows that inhibiting so-called ‘pre-potent’ responses can indeed be a significant hurdle to problem solving in immature humans and mature primates (but see [72]). For example, chimpanzees performance on trap problems reveals that using a tool to rake-in the reward is easier for them than using a tool to push it away, in which they are less successful (see [73] for a review). Looking-time experiments showed that human infants, as young as four months, and mature monkeys are capable of anticipating that a dropped object will not pass through a hidden shelf, as revealed by longer looking when the dropped object is revealed below the shelf rather than resting on top of it [74,75]. However, when the object was dropped behind a screen onto an occluded shelf, both groups show a bias for searching beneath the shelf, perhaps owing to experience inducing an overgeneralized expectation for objects to be located at ground level. Older children (2.5–3 years) and mature apes show evidence of being able to overcome this bias and search in the correct location [74,76]. In other problem-solving contexts, both monkeys and infants show perseverative reaching (i.e. repeatedly searching in one location) and fail to use action flexibly depending on the context to solve the task [77]. Interpreting the dissociation between positive evidence from looking measures, and negative evidence from action, has generated a good deal of controversy [78]. However, the notion that integration between object knowledge, memory, and planning and executing goal-direct actions requires maturation of the pre-frontal cortex [79] and therefore a period of development, is an interesting one, that invokes the need for further studies. In toddlers, the ability to solve the ramp task, in which they need to open a door to locate a ball that was rolled down a ramp and should have come to rest in front of a partially obscured wall, has been shown to correlate with success on tasks measuring inhibitory control [80]. There is considerable variation in inhibitory skills across primate species [81]. Investigating how this relates to tool use and problem-solving competence will, therefore, be an interesting question for future work.
Contrasts between species that routinely use tools in the wild with closely related species that do not, can also inform on phenotypic biases. For example, experimental studies comparing tool-using woodpecker finches to the non-tool using but closely related tree finches indicate that both species possess flexible cognitive adaptations considered foundational for tool use [11]. Likewise, both the tool-using New Caledonian crows and the non-tool-using common ravens start off with similar frequencies of object manipulations, considered a precursor for tool use, possibly originating from their shared propensity for food caching. Naive New Caledonian crows do show higher motivation for continued performance of object combinations, facilitating learning, whereas this decreases over time in common ravens, possibly owing to a higher probability of social interruption for ravens [82]. Such evidence suggests that the cognitive traits underpinning tool use preceded rather than evolved with tool use. Tool use in these species may therefore be better conceived as a manifestation of cognitive traits, rather than a selective force on cognition (see also Discussion).
(b). Problem solving with and without tools
Studies exploring the relationship between problem solving and executive control (e.g. inhibition) can not only be explored from onto- and phylogenetic perspectives, but also by comparing mature performance, focusing on the influence of including a tool relative to performing a similar task without a tool. Trap problems, for example, demonstrate that chimpanzees are more successful in choosing which way to move a reward with one tool than choosing one of two pre-positioned tools. Moving the reward with merely the fingers is easiest of all [83]. Similarly, two-and-a-half-year old children performed much better on a non-tool-using variant of the trap problem than they did when they had to use a tool (A. M. Seed 2013, unpublished data). Learning to solve a new problem with a tool may be more cognitively demanding, and seemingly small differences in the required action can have large effects on performance. This may be because of demands on executive function, for example, splitting attention between the novel action and the physical task at hand (see [73] for a review). Additionally, reduced visual feedback, such as when the food is hidden or out of reach, or when visual attention is taken away from the movement of the goal (e.g. when focusing on the tool rather than the food reward) may make the acquisition of a new tool-using action more difficult, as revealed by new work on both New Caledonian crows [84] and chimpanzees [85]. For chimpanzees, individuals that had already acquired the solution did not suffer any impairment from the removal of visual feedback, suggesting that feedback is most important during learning.
(c). Innovation and social learning
What is unclear, and contentious, from the pattern of slow, error-prone acquisition of habitual tool use is the extent to which social learning is required. The performance of tool-using animals such as New Caledonian crows and apes, presented with novel multi-step problems in the laboratory, allows individual learning and problem-solving abilities to be isolated from social influence. One approach has been to present naive captive adult apes with the same problems they solve by using tools in the wild. Several problems were solved by these apes without social input. Although this does not preclude the idea that in the wild social influence plays a role in shaping the behaviour, it does falsify any argument that posits a need for social input based solely on the perceived level of difficulty or complexity of the task [86]. Other studies use artificial tasks designed to probe the extents and limits of innovative problem solving. These reveal that both New Caledonian crows and chimpanzees can solve novel problems that involve up to three tools to be used in sequence [87–89], although this does seem to require more practice (see also [66]). Both apes and New Caledonian crows (as well as kea) can also solve problems that involve finding a novel solution, which becomes obsolete after a time, requiring that solution to be abandoned and another to be found [90,91]. The precise cognitive mechanisms behind these impressive problem-solving skills are a matter of dispute (see [92] for a review). Nevertheless, it is clear that it is within the capability of these species to solve new problems involving unfamiliar materials and using novel behavioural sequences. One point to note is that to date there has been no evidence that tool users outperform non-tool-using relatives in the arena of innovation or problem-solving involving tools (see [93] and [94] for examples of innovation and sequential tool use in non-tool-using rooks). Interestingly, performance in these studies is often characterized by large individual differences, with some experiments showing a minority of individuals completing the most difficult conditions [89,95,96]. Of those solutions to natural problems which require innovations, social influence is likely to reduce intrapopulation variation in tool use. Can developmental studies support this?
4. Developmental evidence for the role of social input
Observational field studies on individual variation within a population provide some of the clearest evidence that social-learning opportunities can have an impact on tool acquisition. Among mammals, nursing young associate more with their mother than with any other individual and she is often more tolerant of immatures than others. A correlation between individual variation in tool use among mothers and offspring (e.g. in terms of time spent using tools, preferences for a certain type or technique, etc.) can be used to provide evidence for vertical transmission. Indeed, variation in percentage of ant-dipping time among chimpanzee mothers at Bossou was shown to correlate positively with ant-dipping time and duration of ant-dipping sessions in offspring, and negatively to infant age of competence and number of dipping errors [35]. A study on termite fishing in chimpanzees at Gombe revealed similar patterns, although here the relationship was not always that straightforward [42]. Preferences for type of prey, method of tool use and foraging tactics strongly correlate between mother and offspring in sea otters and dolphins [7,48]. Juvenile New Caledonian crows show a preference for either Pandanus or stick use, and possibly tool-manufacturing techniques dependent on tool preferences of their parents, although for the latter larger sample sizes are required [27]. Individual variation among parents may also be present in the form of different association patterns affecting the number of social-learning opportunities. Van Schaik et al. [97] demonstrated a strong relationship between tool-use competence and mean female party size in orangutans. An analysis of social networks among tool-using dolphins, showed that after weaning, spongers preferred to associate with other spongers [61], which may be crucial for them to be able to find the best sites for tools and prey. Such social-learning opportunities may also affect tool repertoires on a wider scale (i.e. at population or species level). For instance, New Caledonian crows have one of the longest known periods of regular extended parental provisioning in birds [25]. Evidence for habitual tool use in orangutans comes from a site inhabiting the densest population [98]. Idem for Goualougo chimpanzees [99], who have a rich tool repertoire including various tool sets [13]. A comparative analysis on emergence of skill acquisition among mammals and birds also indicated an effect of gregariousness, slow conservative development, and post-weaning provisioning and sharing of resources, on age at skill competence [55]. But how do such increased opportunities for social learning serve individual skill acquisition?
Social-learning opportunities can result from the mere presence of other individuals (local-enhancement), the presence of materials manipulated by other individuals (directly by food and tool transfers, or indirectly by stimulus enhancement through artefacts), and models of the complete action (observational learning). We will briefly discuss some indications for the role of these different kinds of input. The presence of other conspecifics, scrounging and/or food transfers are common for most species and behaviours especially at an early age when individuals still depend on their parent(s) for most of their nutritional intake [100]. This facilitates associating the food reward with the tool, which may provide young with a motivation to persist after repeated failure or reduced visual feedback [9,13,44,101]. Delayed or hidden rewards are commonly encountered in natural tool-use settings (see also [101]) and young naive individuals indeed mainly (attempt to) use tools during sessions when their parents also use a tool (e.g. 100% and 62% for Bossou chimpanzees under 5 years, or from 5 to 10 years, respectively; and at least 40% for juvenile New Caledonian crows) [27,35]. In orangutans, also adult tree-hole tool use often seems to be preceded by another conspecific using a tool or engaging in insect foraging (E. Meulman 2013, unpublished data).
With age, tolerance to scrounging and food transfers gradually declines, and infants start to become interested in the tools used by others, as well as attempting to select and manufacture their own tools. Recycled tools contributed to 80% of the termite-fishing tools used by young naive Goualougo chimpanzees and 95% of the Pandanus tools used by two- to three-month old juvenile New Caledonian crows which decreased to only 5% for seven- to nine-month old crows ([9]; C. M. Sanz 2013, personal communication). Counterparts, that is left-overs from the tool-manufacturing process, may be used as well [9,17]. Most of the first self-made tools are dropped (without use) and replaced by tools made by others to obtain the food reward [9,27,42,43]. Such re-use of tools may facilitate learning of how to use these tools and what kind of tool features may be required for the task, especially when visual feedback is minimal [27,44,101,102]. Indeed, individuals master tool use, often if not always, before mastering tool manufacture [9,42,44,72,103]. Laboratory work supports the social enhancement of objects used as tools: for example, young New Caledonian crows and capuchins showed a preference for handling objects or tools that had been manipulated by demonstrator individuals [8,104]. Also adult ant- or termite-fishing chimpanzees were more successful if they used tools that had just been abandoned by a previous user, rather than self-selected tools [105,106]. An exception appears to be bottlenose dolphins, where calves must always obtain their own sponge tools and have not been observed using a sponge that was previously used by another (J. Mann 2013, unpublished data). Also orangutans rarely re-use tools, probably owing to low levels of social tolerance and arboreal settings [107]. Reuse of tools does seem to occur more often for tool variants that require specific materials and modifications in chimpanzees [8,13,101], or the use of tool sets in primates [108].
Naive individuals may additionally learn through observation of a more experienced or proficient individual [44,109]. Time observing is negatively correlated with the age of successful termite fishing (Gombe [110]) and ant dipping (Bossou [35]) among chimpanzees and positively correlated to nut-cracking proficiency among brown capuchins in Tiete National Park, Brazil [109]. Dolphin calves have ample opportunity to observe their mothers using sponge tools and are attracted to the fish catches by older individuals [48]. Preliminary data on orangutans suggest that there is more object play or feeding attempts after infants watched another individual using a tool (E. Meulman 2013, unpublished data). High-fidelity action copying may provide human children with an alternative and quick route to obtain a material culture [111,112]. In fact, human children are rarely successful at making functional tools without a demonstrator until they are age 7 or older [72]. Although captive studies on apes do suggest that apes are capable of using both sources of information, results are mixed (see [113] for a review) and it is hard to know from observational studies what aspect of the action is attended to and affects learning, that is action imitation or observational learning of object affordances. How such different strategies of observational learning in particular, and socially facilitated learning in general, may impact speed and reliability of the transmission process may provide us with an interesting scope for future experimental studies (see [104,114]).
In conclusion, the observation that rates of scrounging, object play, feeding attempts, and food and tool transfers, and watching decline with age (and possibly competence) in most, if not all, species [35,100,109], seems to be a further indication for socially facilitated learning during the ontogeny of habitual tool users. Additionally, some rare incidences of opportunity teaching have been reported for chimpanzees [35,115]. Habitual tool users thus seem to profit from socially scaffolded learning environments that facilitate education by master-apprenticeship [27,116].
5. Discussion
To date, habitual tool use and the degree to which it reflects flexible cognitive adaptations remains a controversial and unresolved issue. A review of longitudinal and experimental studies on the ontogeny of tool use and cognition does, however, shed some light on this notorious problem. First, both field and captive studies demonstrate that young animals of habitual tool-using species make a series of errors during (initial) tool-using attempts, which improve over time. These errors help to identify difficult elements of the tool behaviour and illustrate when animals might need to adjust and/or inhibit predisposed action patterns for the correct tool use (e.g. the capuchin's tendency to rub objects or dolphins ‘blinding’ themselves by carrying a sponge). Second, some tool-mediated behaviours emerge relatively late in development compared with most, but not all, other foraging skills that do not involve tools (table 1 and figure 1). Initial attempts suggest that physical strength does not explain the late age of competence, but that these tool-assisted behaviours may be cognitively demanding instead. Captive studies indeed provide support for the idea that inhibiting pre-potent responses and using tools to solve problems (rather than using hand or beak) are cognitively demanding activities that improve over development. To date there is no evidence for cognitive specializations in tool-using species compared to non-tool-using relatives (see [93]), but there has been little exploration of differences in domain-general executive functions such as inhibition and attention between species. Third, ecological- and social-learning opportunities during the early stages of development appear to play an important role in determining later skill levels and thus individual and geographical variation [10,61]. Variation among adults moreover indicates that tool performance is not simply a matter of brain maturation but also (social-) learning opportunities. Social transmission seems to be mainly vertical, through association, tool recycling, food and tool transfers, and watching. Other modes of transmission, although not predominant, might nevertheless be crucial as for example suggested by the finding that habitual tool use only occurs in populations with increased opportunities for social learning owing to enhanced social tolerance [97], prolonged parental feeding or association [25], exposure to artefacts [108] and/or perhaps rare cases of opportunity teaching [35,115]. Re-use of tools may be important for the accumulation of technological complexity [13,108].
The different lines of evidence illustrate two extremes related to the evolution of tool use in animals. At one extreme, only minimal cognitive and social inputs are necessary for the occurrence of tool use, typically because of the presence of an inherent bias to manipulate objects in the first place. The studies on woodpecker finches and North American badgers are good examples, showing that expression of tool innovations mainly depends on ecological factors [10,62] and when flexible cognitive strategies are involved, they appear to be domain-general learning mechanisms shared with non-tool-using relatives [11]. Practice can nevertheless be important, especially for more intermediate forms (e.g. sea otters who specialize2 in using rocks to open snails are more efficient than non-specialists [23]). At another extreme, more flexible cognition may be required to come up with innovations that deviate from more pre-potent action patterns and additionally require long periods of individual practice and social input to use the tool more systematically and habitually. Dolphins, for instance, are not ‘built’ for manipulative tool use, but can readily integrate acoustic and visual inputs to represent objects [117,118], and use their cognitive ability to solve problems with tools in laboratory and field, at least when the conditions call for it. Calves of bottlenose dolphins spend thousands of hours observing maternal tool use before the first instances of tool use are observed [48]. Even then, it still takes them decades to show peak proficiency, that is, if they adopt the skill at all [58]. All the females that do are specialists [48].
For both extremes, there are indications that tool use may be better viewed as a possible manifestation (or by-product) of flexible cognitive abilities rather than acting as a selective force on intelligence itself (see also [119]). Note, however, that tool use may just be one among many other possible ‘tool-free’ manifestations of general intelligence (i.e. one extreme, such as habitual tool users) and, hence, not all tool users need to be characterized by enhanced intelligence (i.e. other extreme, relatively inflexible tool ‘specialists’). Although for the moment, this remains speculative and needs further confirmation, this indeed would explain the flexible cognitive traits that are found in wild tool-using woodpecker finches, New Caledonian crows, robust capuchins, and chimpanzees, as well as their non-habitual tool-using (at least in the wild) close relatives: tree finches, common ravens and rooks, gracile capuchins and bonobos [10,82,93]. Second, it is in line with findings from previous studies that revealed a positive correlation between tool use (or niche complexity), social learning, innovation, brain size, slow life-history pace and general (or cultural) intelligence, whereas the different traits by themselves cannot account for the diversity of tool use across taxa [30,68,71,120]. Finally, it is consistent with previously proposed evolutionary factors for tool use in that: (i) predispositions and/or intelligence stimulate the occurrence of tool innovations; (ii) ecological factors provide opportunities for practice and determine the usefulness of tool innovations; (iii) intelligence stimulates a more flexible integration of such tool innovations in the behavioural repertoire, that are subsequently more likely to be socially transmitted and thus to be retained within the population's repertoire; especially (iv) in socially scaffolded learning environments [108,121,122].
In summary, evidence from observational and experimental studies indicate that using tools seems to be more cognitively demanding than performing the same behaviour by beak or hand, and flexible use seems to coincide with plasticity during development. Whereas the first conclusion applies to tool use, the latter may apply to ‘tool-free’ behaviours as well and hence certain ‘tool-free’ behaviours may thus very well be more cognitively demanding than certain behaviours involving tool use. Although the term ‘habitual tool use’ is often used to imply socially learned and flexible tool use, this inference could be incorrect if based on geographical variation alone. Social learning is unlikely to be limited to cases of habitual tool use [5,6], and socially acquired tool use may still be rather inflexible in it is expression (i.e. no adjusting of the behaviour to a slightly modified task, such as the use of rocks by sea otters). Flexible tool use may still be based on cognitive strategies that do not require social input (e.g. woodpecker finches) and relying on one snapshot of a population is unrealistic and misses the individual variation. Finally, the variation within and among routine rather than habitual tool users across foraging and non-foraging contexts requires further study. After all, human tool use is also of a customary rather than habitual nature. To study social and cognitive influences on tool use, developmental approaches and fitness outcomes, thus, may be more fruitful than the focus on geographical differences. Only then we can begin to unravel how the ability for tool use evolved and relates to cognitive evolution and cumulative technology and culture (see also [95]).
(a). Considerations for future work
Investigation on the ontogeny and adaptive function of tool use in animals with slow life histories, including humans, is a considerable enterprise. While field studies reveal much about the social and ecological circumstances that favour tool use, laboratory experiments (for some species) are ideal for understanding intrinsic mechanisms and manipulating specific extrinsic factors (e.g. social exposure to a task). Implementing new technological advances in both lines of work may provide us with further insights. Filming with remote cameras can reveal more details about object play, foraging efficiency and tool-use acquisition (including errors) that are either rarely seen by field observers or difficult to quantify in situ. Field experiments can help elucidate the importance of ecological factors and learning opportunities in more natural settings (e.g. presence of raw material, or terrestrial versus arboreal settings). Experiments in captive settings are needed to account for social and ecological influences (presence of discarded tools, food, conspecifics or material), and for controlled investigation of, for example, the ontogeny of manipulative ability and cognitive–perceptual skills using object, tool, or non-object-mediated tasks (e.g. looking-time experiments). Such experimental work may help uncover the link between tool use and specific cognitive processes (e.g. executive control). Future work aimed at pinpointing the cognitive skills required for tool use could moreover help identify candidates for adaptive change in habitual tool users. Potential fitness effects of tool use, as well as how these may be influenced by personality traits (e.g. boldness, neophilia and sociability) [123], are currently still largely unexplored study themes. Examination of how cognitive traits and foraging strategies are manifested differently among individuals, according to varying conditions, how they change during development and affect fitness are essential for understanding the adaptive significance of such traits [95]. A broader comparative approach including routine but non-habitual and/or non-subsistence tool use and ‘tool-free’ skills, is furthermore needed to relate the adaptive function of tool use to other foraging strategies and other behavioural contexts. Although we have a long way to go in determining what factors, including cognitive, shaped inter- and intraspecific variation in tool use, ontogenetic research is a most promising approach [18,21].
Acknowledgements
We thank Dora Biro and all participants of the Royal Society international scientific seminar on ‘Tool-use as adaption’ for fruitful discussions on the evolution of tool use, which has inspired us in our writing. We also acknowledge the Anthropological Institute of the University of Zürich, in particular Carel van Schaik, Karin Isler and Caroline Schuppli for stimulating discussions and data. Finally, we thank Michael Haslam and the anonymous reviewers for their suggestions in improving this manuscript.
Footnotes
Funding statement
J.M. would also like to acknowledge the following financial sources: NSF 0941487, 0918308 and ONR 10230702. E.M. would like to acknowledge PanEco (Switzerland) and the A. H. Schultz Foundation for their financial support.
References
- 1.Shumaker RW, Walkup KR, Beck BB. 2011. Animal tool behavior: the use and manufacture of tools by animals. Baltimore, MD: Johns Hopkins University Press. [Google Scholar]
- 2.McGrew WC. 1992. Chimpanzee material culture. Implications for human evolution. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 3.Whiten A, Goodall J, McGrew WC, Nishida T, Reynoldsk V, Sugiyama Y, Tutin CEG, Wrangham RW, Boesch C. 1999. Cultures in chimpanzees. Nature 399, 682–685. ( 10.1038/21415) [DOI] [PubMed] [Google Scholar]
- 4.van Schaik CP, Ancrenaz M, Borgen G, Galdikas B, Knott CD, Singleton I, Suzuki A, Utami SS, Merrill M. 2003. Orangutan cultures and the evolution of material culture. Science 299, 102–105. ( 10.1126/science.1078004) [DOI] [PubMed] [Google Scholar]
- 5.Laland KN, Janik VM. 2006. The animal cultures debate. Trends Ecol. Evol. 21, 542–547. ( 10.1016/j.tree.2006.06.005) [DOI] [PubMed] [Google Scholar]
- 6.Langergraber KE, et al. 2011. Genetic and ‘cultural’ similarity in wild chimpanzees. Proc. R. Soc. B 278, 408–416. ( 10.1098/rspb.2010.1112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Riedman ML, Estes JA. 1990. The sea otter (Enhydra lutris): behavior, ecology, and natural history. Washington, DC: US Fish and Wildlife Service. [Google Scholar]
- 8.Kenward B, Rutz C, Weir AAS, Kacelnik A. 2006. Development of tool use in New Caledonian crows: inherited action patterns and social influences. Anim. Behav. 72, 1329–1343. ( 10.1016/j.anbehav.2006.04.007) [DOI] [Google Scholar]
- 9.Holzhaider JC, Hunt GR, Gray RD. 2010. The development of Pandanus tool manufacture in wild New Caledonian crows. Behaviour 147, 553–586. ( 10.1163/000579510X12629536366284) [DOI] [Google Scholar]
- 10.Tebbich S, Taborsky M, Fessl B, Blomqvist D. 2001. Do woodpecker finches acquire tool-use by social learning? Proc. R. Soc. Lond. B 268, 2189–2193. ( 10.1098/rspb.2001.1738) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tebbich S, Sterelny K, Teschke I. 2010. The tale of the finch: adaptive radiation and behavioural flexibility. Phil. Trans. R. Soc. B 365, 1099–1109. ( 10.1098/rstb.2009.0291) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sargeant BL, Mann J. 2009. Developmental evidence for foraging traditions in wild bottlenose dolphins. Anim. Behav. 78, 715–721. ( 10.1016/j.anbehav.2009.05.037) [DOI] [Google Scholar]
- 13.Sanz CM, Morgan DB. 2007. Chimpanzee tool technology in the Goualougo Triangle, Republic of Congo. J. Hum. Evol. 52, 420–433. ( 10.1016/j.jhevol.2006.11.001) [DOI] [PubMed] [Google Scholar]
- 14.McGrew WC. 2013. Is primate tool use special? Chimpanzee and New Caledonian crow compared. Phil. Trans. R. Soc. B 368, 20120422 ( 10.1098/rstb.2012.0422) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sanz CM, Morgan DB. 2013. Ecological and social correlates of chimpanzee tool use. Phil. Trans. R. Soc. B 368, 20120416 ( 10.1098/rstb.2012.0416) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ottoni EB, Izar P. 2008. Capuchin monkey tool use: overview and implications. Evol. Anthropol. 17, 171–178. ( 10.1002/evan.20185) [DOI] [Google Scholar]
- 17.Fragaszy DM, Biro D, Eshchar Y, Humle T, Izar P, Resende B, Visalberghi E. 2013. The fourth dimension of tool use: temporally enduring artefacts aid primates learning to use tools. Phil. Trans. R. Soc. B 368, 20120410 ( 10.1098/rstb.2012.0410) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gumert MD, Hoong LK, Malaivijitnond S. 2011. Sex differences in the stone tool-use behavior of a wild population of Burmese long-tailed macaques (Macaca fascicularis aurea). Am. J. Primatol. 73, 1239–1249. ( 10.1002/ajp.20996) [DOI] [PubMed] [Google Scholar]
- 19.Gumert MD, Malaivijitnond S. 2013. Long-tailed macaques select mass of stone tools according to food type. Phil. Trans. R. Soc. B 368, 20120413 ( 10.1098/rstb.2012.0413) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.St Clair JJH, Rutz C. 2013. New Caledonian crows attend to multiple functional properties of complex tools. Phil. Trans. R. Soc. B 368, 20120415 ( 10.1098/rstb.2012.0415) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruxton GD, Hansell MH. 2011. Fishing with a bait or lure: a brief review of the cognitive issues. Ethology 117, 1–9. ( 10.1111/j.1439-0310.2010.01848.x) [DOI] [Google Scholar]
- 22.Payne SF, Jameson RJ. 1984. Early behavioral development of the sea otter, Enhydra lutris. J. Mammal. 65, 527–531. ( 10.2307/1381114) [DOI] [Google Scholar]
- 23.Tinker TM, Guimaraes PR, Jr, Novak M, Marquitti FM, Bodkin JL, Staedler M, Bentall G, Estes JA. 2012. Structure and mechanism of diet specialisation: testing models of individual variation in resource use with sea otters. Ecol. Lett. 15, 475–483. ( 10.1111/j.1461-0248.2012.01760.x) [DOI] [PubMed] [Google Scholar]
- 24.Bluff LA, Troscianko J, Weir AAS, Kacelnik A, Rutz C. 2010. Tool use by wild New Caledonian crows Corvus moneduloides at natural foraging sites. Proc. R. Soc. B 277, 1377–1385. ( 10.1098/rspb.2009.1953) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hunt GR, Holzhaider JC, Gray RD. 2012. Prolonged parental feeding in tool-using New Caledonian crows. Ethology 118, 423–430. ( 10.1111/j.1439-0310.2012.02027.x) [DOI] [Google Scholar]
- 26.Holzhaider JC, Sibley MD, Taylor AH, Singh PJ, Gray RD, Hunt GR. 2011. The social structure of New Caledonian crows. Anim. Behav. 81, 83–92. ( 10.1016/j.anbehav.2010.09.015) [DOI] [Google Scholar]
- 27.Holzhaider JC, Hunt GR, Gray RD. 2010. Social learning in New Caledonian crows. Learn. Behav. 38, 206–219. ( 10.3758/LB.38.3.206) [DOI] [PubMed] [Google Scholar]
- 28.Hunt GR, Gray RD. 2004. The crafting of hook tools by wild New Caledonian crows. Proc. R. Soc. Lond. B 271, S88–S90. ( 10.1098/rsbl.2003.0085) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mannu M, Ottoni EB. 2009. The enhanced tool-kit of two groups of wild bearded capuchin monkeys in the Caatinga: tool making, associative use, and secondary tools. Am. J. Primatol. 71, 242–251. ( 10.1002/ajp.20642) [DOI] [PubMed] [Google Scholar]
- 30.MacKinnon K. 2013. Ontogeny of social behavior in the genus Cebus and the application of an integrative framework for examining plasticity and complexity in evolution. In Building babies (eds Clancy K, Hinde K, Rutherford J.), pp. 387–408. New York, NY: Springer. [Google Scholar]
- 31.Fragaszy DM, Visalberghi E, Fedigan LM. 2004. Development. In The complete capuchin: the biology of the genus Cebus (eds Fragaszy DM, Visalberghi E, Fedigan LM.), pp. 106–125. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 32.Moura ACdeA, Lee PC. 2010. Wild capuchins show male-biased feeding tool use. Int. J. Primatol. 31, 457–470. ( 10.1007/s10764-010-9406-6) [DOI] [Google Scholar]
- 33.Perry S. 2011. Social traditions and social learning in capuchin monkeys (Cebus). Phil. Trans. R. Soc. B 366, 988–996. ( 10.1098/rstb.2010.0317) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Souto A, Bione CBC, Bastos M, Bezerra BM, Fragaszy D, Schiel N. 2011. Critically endangered blonde capuchins fish for termites and use new techniques to accomplish the task. Biol. Lett. 7, 532–535. ( 10.1098/rsbl.2011.0034) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Humle T, Snowdon CT, Matsuzawa T. 2009. Social influences on ant-dipping acquisition in the wild chimpanzees (Pan troglodytes verus) of Bossou, Guinea, West Africa. Anim. Cogn. 12, S37–S48. ( 10.1007/s10071-009-0272-6) [DOI] [PubMed] [Google Scholar]
- 36.Byrne RW, Byrne JME. 1993. Complex leaf-gathering skills of mountain gorillas (Gorilla gorilla beringei): variability and standardization. Am. J. Primatol. 31, 241–261. ( 10.1002/ajp.1350310402) [DOI] [PubMed] [Google Scholar]
- 37.Kaplan H, Hill K, Lancaster J, Hurtado AM. 2000. A theory of human life history evolution: diet, intelligence, and longevity. Evol. Anthropol. 9, 156–185. () [DOI] [Google Scholar]
- 38.van Noordwijk MA, van Schaik CP. 2005. Development of ecological competence in Sumatran orangutans. Am. J. Phys. Anthropol. 127, 79–94. ( 10.1002/ajpa.10426) [DOI] [PubMed] [Google Scholar]
- 39.Harvey PH, Clutton-Brock TH. 1985. Life history variation in primates. Evolution 39, 559–581. ( 10.2307/2408653) [DOI] [PubMed] [Google Scholar]
- 40.Nishida T. 2012. Chimpanzees of the lakeshore: natural history and culture at Mahale. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 41.Nishie H. 2011. Natural history of Camponotus ant-fishing by the M group chimpanzees at the Mahale Mountains National Park, Tanzania. Primates 52, 329–342. ( 10.1007/s10329-011-0270-6) [DOI] [PubMed] [Google Scholar]
- 42.Lonsdorf EV. 2006. What is the role of mothers in the acquisition of termite-fishing behaviors in wild chimpanzees (Pan troglodytes schweinfurthii)? Anim. Cogn. 9, 36–46. ( 10.1007/s10071-005-0002-7) [DOI] [PubMed] [Google Scholar]
- 43.Sousa C, Biro D, Matsuzawa T. 2009. Leaf-tool use for drinking water by wild chimpanzees (Pan troglodytes): acquisition patterns and handedness. Anim. Cogn. 12, S115–S125. ( 10.1007/s10071-009-0278-0) [DOI] [PubMed] [Google Scholar]
- 44.Biro D, Sousa C, Matsuzawa T. 2006. Ontogeny and cultural propagation of tool use by wild chimpanzees at Bossou, Guinea: case studies in nut cracking and leaf folding. In Cognitive development in chimpanzees (eds Matsuzawa T, Tomonaga M, Tanaka M.), pp. 476–508. Tokyo, Japan: Springer. [Google Scholar]
- 45.Hayashi M, Inoue-Nakamura N. 2011. From handling stones and nuts to tool-use. In Chimpanzees of Bossou and Nimba (eds Matsuzawa T, Humle T, Sugiyama Y.), pp. 175–182. Osaka, Japan: Springer Japan. [Google Scholar]
- 46.Jaeggi AV, Dunkel LP, Van Noordwijk MA, Wich SA, Sura AAL, van Schaik CP. 2010. Social learning of diet and foraging skills by wild immature Bornean orangutans: implications for culture. Am. J. Primatol. 72, 62–71. ( 10.1002/ajp.20752) [DOI] [PubMed] [Google Scholar]
- 47.Jaeggi AV, van Noordwijk MA, van Schaik CP. 2008. Begging for information: mother-offspring food sharing among wild Bornean orangutans. Am. J. Primatol. 70, 533–541. ( 10.1002/ajp.20525) [DOI] [PubMed] [Google Scholar]
- 48.Mann J, Sargeant BL, Watson-Capps JJ, Gibson QA, Heithaus MR, Connor RC, Patterson E. 2008. Why do dolphins carry sponges? PLoS ONE 3, e3868 ( 10.1371/journal.pone.0003868) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sargeant BL, Mann J, Berggren P, Krutzen M. 2005. Specialization and development of beach hunting, a rare foraging behavior, by wild bottlenose dolphins (Tursiops sp.). Can. J. Zool. 83, 1400–1410. ( 10.1139/z05-136) [DOI] [Google Scholar]
- 50.Mann J, Smuts B. 1999. Behavioral development in wild bottlenose dolphin newborns (Tursiops sp.). Behaviour 136, 529–566. ( 10.1163/156853999501469) [DOI] [Google Scholar]
- 51.Mann J, Connor RC, Barre LM, Heithaus MR. 2000. Female reproductive success in bottlenose dolphins (Tursiops sp.): life history, habitat, provisioning, and group-size effects. Behav. Ecol. 11, 210–219. ( 10.1093/beheco/11.2.210) [DOI] [Google Scholar]
- 52.Rat-Fischer L, O'Regan JK, Fagard J. 2012. The emergence of tool use during the second year of life. J. Exp. Child Psychol. 113, 440–446. ( 10.1016/j.jecp.2012.06.001) [DOI] [PubMed] [Google Scholar]
- 53.Konner M. 2005. the kung and others. In Hunter–gatherer childhoods: evolutionary, developmental and cultural perspectives (eds Hewlett BS, Lamb ME.), pp. 19–64. New Brunsswick, NJ: Aldine Transaction. [Google Scholar]
- 54.Piaget J. 1977. The essential Piaget. New York, NY: Basic Books. [Google Scholar]
- 55.Schuppli C, Isler K, van Schaik CP. 2012. How to explain the unusually late age at skill competence among humans. J. Hum. Evol. 63, 1–8. ( 10.1016/j.jhevol.2012.08.009) [DOI] [PubMed] [Google Scholar]
- 56.de Resende BD, Ottoni EB, Fragaszy DM. 2008. Ontogeny of manipulative behavior and nut-cracking in young tufted capuchin monkeys (Cebus apella): a perception–action perspective. Dev. Sci. 11, 828–840. ( 10.1111/j.1467-7687.2008.00731.x) [DOI] [PubMed] [Google Scholar]
- 57.Huffman MA, Quiatt D. 1986. Stone handling by Japanese macaques (Macaca fuscate): implications for tool use of stone. Primates 27, 413–423. ( 10.1007/BF02381887) [DOI] [Google Scholar]
- 58.Mann J, Patterson EM. 2013. Tool use by aquatic animals. Phil. Trans. R. Soc. B 368, 20120424 ( 10.1098/rstb.2012.0424) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sanz CM, Morgan DB. 2011. Elemental variation in the termite fishing of wild chimpanzees (Pan troglodytes). Biol. Lett. 7, 634–637. ( 10.1098/rsbl.2011.0088) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Patterson EM. 2012. Ecological and life history factors influence habitat and tool use in wild bottlenose dolphins (Tursiops sp.). PhD thesis, pp. 170, Georgetown University; , Washington, DC, USA. [Google Scholar]
- 61.Mann J, Stanton MA, Patterson EM, Bienenstock EJ, Singh LO. 2012. Social networks reveal cultural behaviour in tool-using using dolphins. Nat. Commun. 3, 980 ( 10.1038/ncomms1983) [DOI] [PubMed] [Google Scholar]
- 62.Michener GR. 2004. Hunting techniques and tool use by North American badgers preying on Richardson's ground squirrels. J. Mammal. 85, 1019–1027. ( 10.1644/BNS-102) [DOI] [Google Scholar]
- 63.Kenyon KW. 1969. The sea otter in the eastern Pacific Ocean. North American Fauna 68, 109–113. ( 10.3996/nafa.68.0001) [DOI] [Google Scholar]
- 64.Aversi-Ferreira TA, Diogo R, Potau JM, Bello G, Pastor JF, Aziz MA. 2010. Comparative anatomical study of the forearm extensor muscles of Cebus libidinosus (Rylands et al., 2000; Primates, Cebidae), modern humans, and other primates, with comments on primate evolution, phylogeny, and manipulatory behavior. Anat. Rec. 293, 2056–2070. ( 10.1002/ar.21275) [DOI] [PubMed] [Google Scholar]
- 65.Marzke MW. 2013. Tool making, hand morphology and fossil hominins. Phil. Trans. R. Soc. B 368, 20120414 ( 10.1098/rstb.2012.0414) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hirata S, Morimura N, Houki C. 2009. How to crack nuts: acquisition process in captive chimpanzees (Pan troglodytes) observing a model. Anim. Cogn. 12, S87–S101. ( 10.1007/s10071-009-0275-3) [DOI] [PubMed] [Google Scholar]
- 67.Sabbatini G, Truppa V, Hribar A, Gambetta B, Call J, Visalberghi E. 2012. Understanding the functional properties of tools: chimpanzees (Pan troglodytes) and capuchin monkeys (Cebus apella) attend to tool features differently. Anim. Cogn. 15, 1–14. ( 10.1007/s10071-012-0486-x) [DOI] [PubMed] [Google Scholar]
- 68.van Schaik CP, Isler K, Burkart JM. 2012. Explaining brain size variation: from social to cultural brain. Trends Cogn. Sci. 16, 277–284. ( 10.1016/j.tics.2012.04.004) [DOI] [PubMed] [Google Scholar]
- 69.Barrickman NL, Bastian ML, Isler K, van Schaik CP. 2008. Life history costs and benefits of encephalization: a comparative test using data from long-term studies of primates in the wild. J. Hum. Evol. 54, 568–590. ( 10.1016/j.jhevol.2007.08.012) [DOI] [PubMed] [Google Scholar]
- 70.Lefebvre L, Reader SM, Sol D. 2004. Brains, innovations and evolution in birds and primates. Brain Behav. Evol. 63, 233–246. ( 10.1159/000076784) [DOI] [PubMed] [Google Scholar]
- 71.Reader SM, Hager Y, Laland KN. 2011. The evolution of primate general and cultural intelligence. Phil. Trans. R. Soc. B 366, 1017–1027. ( 10.1098/rstb.2010.0342) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chappell J, Cutting N, Apperly IA, Beck SR. 2013. The development of tool manufacture in humans: what helps young children make innovative tools? Phil. Trans. R. Soc. B 368, 20120409 ( 10.1098/rstb.2012.0409) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Call J. 2010. Trapping the minds of apes: causal knowledge and inferential reasoning about object-object interactions. In The mind of the chimpanzee: ecological and experimental perspectives (eds Lonsdorf ER, Ross SR, Matsuzawa T.), pp. 464 Chicago, IL: University of Chicago Press. [Google Scholar]
- 74.Hood B, Carey S, Prasada S. 2000. Predicting the outcomes of physical events: two-year-olds fail to reveal knowledge of solidity and support. Child Dev. 71, 1540–1554. ( 10.1111/1467-8624.00247) [DOI] [PubMed] [Google Scholar]
- 75.Santos LR, Hauser MD. 2002. A non-human primate's understanding of solidity: dissociations between seeing and acting. Dev. Sci. 5, F1–F7. ( 10.1111/1467-7687.t01-1-00216) [DOI] [Google Scholar]
- 76.Cacchione T, Call J, Zingg R. 2009. Gravity and solidity in four great ape species (Gorilla gorilla, Pongo pygmaeus, Pan troglodytes, Pan paniscus): vertical and horizontal variations of the table task. J. Comp. Psychol. 123, 168–180. ( 10.1037/a0013580) [DOI] [PubMed] [Google Scholar]
- 77.Hauser MD. 1999. Perseveration, inhibition and the prefrontal cortex: a new look. Curr. Opin. Neurobiol. 9, 214–222. ( 10.1016/S0959-4388(99)80030-0) [DOI] [PubMed] [Google Scholar]
- 78.Hood BM. 2004. Is looking good enough or does it beggar belief? Dev. Sci. 7, 415–417. ( 10.1111/j.1467-7687.2004.00358.x) [DOI] [PubMed] [Google Scholar]
- 79.Keen R. 2003. Representation of objects and events: why do infants look so smart and toddlers look so dumb? Curr. Dir. Psychol. Sci. 12, 79–83. ( 10.1111/1467-8721.01234) [DOI] [Google Scholar]
- 80.Price IL. 2009. Visuospatial reasoning in toddlers: a correlational study of door task performance. PhD dissertation, pp. 115, University of Massachusetts, Amherst, MA, USA. [Google Scholar]
- 81.Amici F, Aureli F, Call J. 2008. Fission-fusion dynamics, behavioral flexibility, and inhibitory control in primates. Curr. Biol. 18, 1415–1419. ( 10.1016/j.cub.2008.08.020) [DOI] [PubMed] [Google Scholar]
- 82.Kenward BEN, Schloegl C, Rutz C, Weir AAS, Bugnyar T, Kacelnik A. 2011. On the evolutionary and ontogenetic origins of tool-oriented behaviour in New Caledonian crows (Corvus moneduloides). Biol. J. Linn. Soc. 102, 870–877. ( 10.1111/j.1095-8312.2011.01613.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Seed AM, Call J, Emery NJ, Clayton NS. 2009. Chimpanzees solve the trap problem when the confound of tool-use is removed. J. Exp. Psychol. Anim. Behav. Processes 35, 23–34. ( 10.1037/a0012925) [DOI] [PubMed] [Google Scholar]
- 84.Taylor AH, Knaebe B, Gray RD. 2012. An end to insight? New Caledonian crows can spontaneously solve problems without planning their actions. Proc. R. Soc. B 279, 4977–4981. ( 10.1098/rspb.2012.1998) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Völter C, Call J. 2012. Problem solving in great apes (Pan paniscus, Pan troglodytes, Gorilla gorilla, and Pongo abelii): the effect of visual feedback. Anim. Cogn. 15, 923–936. ( 10.1007/s10071-012-0519-5) [DOI] [PubMed] [Google Scholar]
- 86.Tennie C, Hedwig D. 2009. How latent solution experiments can help to study differences between human culture and primate traditions. In Primatology: theories, methods and research (eds Potocki E, Krasinski J.), pp. 95–112. New York, NY: Nova Publishers. [Google Scholar]
- 87.Martin-Ordas G, Jaek F, Call J. 2012. Barriers and traps: great apes’ performance in two functionally equivalent tasks. Anim. Cogn. 15, 1007–1013. ( 10.1007/s10071-012-0504-z) [DOI] [PubMed] [Google Scholar]
- 88.Taylor AH, Hunt GR, Holzhaider JC, Gray RD. 2007. Spontaneous metatool use by New Caledonian crows. Curr. Biol. 17, 1504–1507. ( 10.1016/j.cub.2007.07.057) [DOI] [PubMed] [Google Scholar]
- 89.Wimpenny JH, Weir AAS, Clayton L, Rutz C, Kacelnik A. 2009. Cognitive processes associated with sequential tool use in New Caledonian crows. PLoS ONE 4, e6471 ( 10.1371/journal.pone.0006471) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Auersperg AMI, von Bayern AMP, Gajdon GK, Huber L, Kacelnik A. 2011. Flexibility in problem solving and tool use of Kea and New Caledonian crows in a multi-access box paradigm. PLoS ONE 6, e20231 ( 10.1371/journal.pone.0020231) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Manrique HM, Völter CJ, Call J. 2013. Repeated innovation in great apes. Anim. Behav. 85, 195–202. ( 10.1016/j.anbehav.2012.10.026) [DOI] [Google Scholar]
- 92.Seed AM, Call J. 2010. Physical problem solving in tool-using and non-tool-using animals. In Encyclopedia of animal behaviour (ed. Brred M, Moore J.), pp. 778–785. Oxford, UK: Academic Press. [Google Scholar]
- 93.Teschke I, Wascher CAF, Scriba MF, von Bayern AMP, Huml V, Siemers B, Tebbich S. 2013. Did tool-use evolve with enhanced physical cognitive abilities? Phil. Trans. R. Soc. B 368, 20120418 ( 10.1098/rstb.2012.0418) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bird CD, Emery NJ. 2009. Insightful problem solving and creative tool modification by captive nontool-using rooks. Proc. Natl Acad. Sci. USA 106, 10 370–10 375. ( 10.1073/pnas.0901008106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Thornton A, Lukas D. 2012. Individual variation in cognitive performance: developmental and evolutionary perspectives. Phil. Trans. R. Soc. B 367, 2773–2783. ( 10.1098/rstb.2012.0214) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Herrmann E, Call J. 2012. Are there geniuses among the apes? Phil. Trans. R. Soc. B 367, 2753–2761. ( 10.1098/rstb.2012.0191) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.van Schaik CP, Fox EA, Fechtman LT. 2003. Individual variation in the rate of use of tree-hole tools among wild orang-utans: implications for hominin evolution. J. Hum. Evol. 44, 11–23. ( 10.1016/S0047-2484(02)00164-1) [DOI] [PubMed] [Google Scholar]
- 98.Singleton I, van Schaik CP. 2001. Orangutan home range size and its determinants in a Sumatran swamp forest. Int. J. Primatol. 22, 877–911. ( 10.1023/A:1012033919441) [DOI] [Google Scholar]
- 99.Morgan D, Sanz C, Onononga J, Strindberg S. 2006. Ape abundance and habitat use in the Goualougo Triangle, Republic of Congo. Int. J. Primatol. 27, 147–179. ( 10.1007/s10764-005-9013-0) [DOI] [Google Scholar]
- 100.Brown GR, Almond REA, van Bergen Y. 2004. Begging, stealing, and offering: food transfer in nonhuman primates. In Advances in the study of behavior (eds Slater P, Rosenblatt J, Snowdon C, Roper T, Brockmann J, Naguib M.), pp. 265–295. San Diego, CA: Elsevier Academic Press Inc. [Google Scholar]
- 101.Boesch C. 2013. Ecology and cognition of tool use in chimpanzees. In Tool use in animals: cognition and ecology (eds Sanz CM, Call J, Boesch C.), pp. 22–47. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 102.Holzhaider JC, Hunt GR, Campbell VM, Gray RD. 2008. Do wild New Caledonian crows (Corvus moneduloides) attend to the functional properties of their tools? Anim. Cogn. 11, 243–254. ( 10.1007/s10071-007-0108-1) [DOI] [PubMed] [Google Scholar]
- 103.Sousa C, Biro D, Matsuzawa T. 2009. Leaf-tool use for drinking water by wild chimpanzees (Pan troglodytes): acquisition patterns and handedness. Anim. Cogn. 12, 115–125. ( 10.1007/s10071-009-0278-0) [DOI] [PubMed] [Google Scholar]
- 104.Matthews LJ, Paukner A, Suomi SJ. 2010. Can traditions emerge from the interaction of stimulus enhancement and reinforcement learning? An experimental model. Am. Anthropol. 112, 257–269. ( 10.1111/j.1548-1433.2010.01224.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hirata S, Morimura N. 2000. Naive chimpanzees’ (Pan troglodytes) observation of experienced conspecifics in a tool-using task. J. Comp. Psychol. 114, 291–296. ( 10.1037/0735-7036.114.3.291) [DOI] [PubMed] [Google Scholar]
- 106.Hirata S, Celli M. 2003. Role of mothers in the acquisition of tool-use behaviours by captive infant chimpanzees. Anim. Cogn. 6, 235–244. ( 10.1007/s10071-003-0187-6) [DOI] [PubMed] [Google Scholar]
- 107.Meulman EJM, van Schaik CP. 2013. Orangutan tool use and the evolution of technology. In Tool use in animals: cognition and ecology (eds Sanz CM, Call J, Boesch C.), pp. 176–202. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 108.Meulman EJM, Sanz CM, Visalberghi E, van Schaik CP. 2012. The role of terrestriality in promoting primate technology. Evol. Anthropol. Issues News Rev. 21, 58–68. ( 10.1002/evan.21304) [DOI] [PubMed] [Google Scholar]
- 109.Ottoni EB, de Resende BD, Izar P. 2005. Watching the best nutcrackers: what capuchin monkeys (Cebus apella) know about others’ tool-using skills. Anim. Cogn. 8, 215–219. ( 10.1007/s10071-004-0245-8) [DOI] [PubMed] [Google Scholar]
- 110.Lonsdorf EV. 2005. Sex differences in the development of termite-fishing skills in the wild chimpanzees, Pan troglodytes schweinfurthii, of Gombe National Park, Tanzania. Anim. Behav. 70, 673–683. ( 10.1016/j.anbehav.2004.12.014) [DOI] [Google Scholar]
- 111.Pradhan GR, Tennie C, van Schaik CP. 2012. Social organization and the evolution of cumulative technology in apes and hominins. J. Hum. Evol. 63, 180–190. ( 10.1016/j.jhevol.2012.04.008) [DOI] [PubMed] [Google Scholar]
- 112.Horner V, Whiten A. 2005. Causal knowledge and imitation/emulation switching in chimpanzees (Pan troglodytes) and children (Homo sapiens). Anim. Cogn. 8, 164–181. ( 10.1007/s10071-004-0239-6) [DOI] [PubMed] [Google Scholar]
- 113.Whiten A, McGuigan N, Marshall-Pescini S, Hopper LM. 2009. Emulation, imitation, over-imitation and the scope of culture for child and chimpanzee. Phil. Trans. R. Soc. B 364, 2417–2428. ( 10.1098/rstb.2009.0069) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Caldwell CA, Millen AE. 2009. Social learning mechanisms and cumulative cultural evolution: is imitation necessary? Psychol. Sci. 20, 1478–1483. ( 10.1111/j.1467-9280.2009.02469.x) [DOI] [PubMed] [Google Scholar]
- 115.Boesch C. 1991. Teaching among wild chimpanzees. Anim. Behav. 41, 530–532. ( 10.1016/S0003-3472(05)80857-7) [DOI] [Google Scholar]
- 116.Matsuzawa T, Biro D, Humle T, Inoue-Nakamura N, Tonooka R, Yamakoshi G. 2001. Emergence of culture in wild chimpanzees: education by master-apprenticeship. In Primate origins of human cognition and behavior (ed. Matsuzawa T.), pp. 557–574. Tokyo, Japan: Springer [Google Scholar]
- 117.Harley HE, Putman EA, Roitblat HL. 2003. Bottlenose dolphins perceive object features through echolocation. Nature 424, 667–669. ( 10.1038/nature01846) [DOI] [PubMed] [Google Scholar]
- 118.Herrmann E, Hernandez-Lloreda MV, Call J, Hare B, Tomasello M. 2010. The structure of individual differences in the cognitive abilities of children and chimpanzees. Psychol. Sci. 21, 102–110. ( 10.1177/0956797609356511) [DOI] [PubMed] [Google Scholar]
- 119.Call J. 2013. Three ingredients for becoming a creative tool user. In Tool use in animals: cognition and ecology (eds Sanz CM, Call J, Boesch C.), pp. 3–20. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 120.Overington SE, Morand-Ferron J, Boogert NJ, Lefebvre L. 2009. Technical innovations drive the relationship between innovativeness and residual brain size in birds. Anim. Behav. 78, 1001–1010. ( 10.1016/j.anbehav.2009.06.033) [DOI] [Google Scholar]
- 121.Parker ST, Gibson KR. 1977. Object manipulation, tool use and sensorimotor intelligence as feeding adaptations in Cebus monkeys and great apes. J. Hum. Evol. 6, 623–641. ( 10.1016/S0047-2484(77)80135-8) [DOI] [Google Scholar]
- 122.van Schaik CP, Deaner RO, Merrill MY. 1999. The conditions for tool use in primates: implications for the evolution of material culture. J. Hum. Evol. 36, 719–741. ( 10.1006/jhev.1999.0304) [DOI] [PubMed] [Google Scholar]
- 123.Carere C, Locurto C. 2011. Interaction between animal personality and animal cognition. Curr. Zool. 57, 491–498. [Google Scholar]