Abstract
Was stone tool making a factor in the evolution of human hand morphology? Is it possible to find evidence in fossil hominin hands for this capability? These questions are being addressed with increasingly sophisticated studies that are testing two hypotheses; (i) that humans have unique patterns of grip and hand movement capabilities compatible with effective stone tool making and use of the tools and, if this is the case, (ii) that there exist unique patterns of morphology in human hands that are consistent with these capabilities. Comparative analyses of human stone tool behaviours and chimpanzee feeding behaviours have revealed a distinctive set of forceful pinch grips by humans that are effective in the control of stones by one hand during manufacture and use of the tools. Comparative dissections, kinematic analyses and biomechanical studies indicate that humans do have a unique pattern of muscle architecture and joint surface form and functions consistent with the derived capabilities. A major remaining challenge is to identify skeletal features that reflect the full morphological pattern, and therefore may serve as clues to fossil hominin manipulative capabilities. Hominin fossils are evaluated for evidence of patterns of derived human grip and stress-accommodation features.
Keywords: thumb, chimpanzees, muscle architecture, forceful precision grips, power (squeeze) grip
1. Introduction
Tools have been central to interpretations of fossil hominin hand anatomy, particularly since the discovery of the Homo habilis OH 7 hand bones at the same level as stone tools at Olduvai Gorge, Tanzania in 1960 [1–3]. Was H. habilis the tool maker? What might have been required for the hand to manipulate the tools? Should we look for evidence of hand proportions facilitating a precision grip between the thumb and index finger pad? Must there be evidence for a well-developed flexor pollicis longus muscle that could have secured a grip between the distal pads of the thumb and index finger? Did all species contemporary with stone tools make the tools? Perhaps of most concern, given the small number of fossil hand remains, might tool manipulation capabilities be inferred from just one or two ‘signals’ in the available bones? These questions persist as we review an array of hominin hand bones that has expanded substantially in recent decades. In spite of morphological evidence from virtually all regions of the hand in several fossil species, we are still uncertain about whether (and if so how) features in the fossils might reflect specific tool-making and tool-using capabilities.
Studies of human hand evolution have approached these questions through a synthesis of results from tests of two hypotheses, the first focusing primarily on behaviour and performance and the second mainly on form and function. The first hypothesis proposes that humans have unique patterns of grip and hand movement capabilities compatible with effective stone tool making and use of the tools. The second predicts that, if the first holds true, then there exist unique patterns of morphology in human hands that are consistent with these capabilities. Comparative studies of human tool making [4,5] and chimpanzee feeding behaviour [6] (which presents some challenges similar to stone tool making) have revealed distinctive forceful pinch grips used by humans that facilitate effective control of stones and minimization of injury to the fingers during tool behaviours. The comparative observations have also shown a distinctively human power (squeeze) grip of bones and sticks that enhances acceleration and impulse force by cylindrical tools [7]. Correlates to these human grip capabilities have been found in morphology that combines derived patterns of muscle architecture and functionally associated joint surface areas, orientations and curvatures, with elements shared with various non-human anthropoid species [8,9]. A critical review of evidence for tool manipulative capabilities and their morphological correlates follows, framed by the behavioural and morphological hypotheses described above.
2. Behaviour: hand grips and movements in tool making, tool use and feeding
(a). Humans: experiments in stone tool making and use of the tools
The most direct source of evidence for grips and hand movements that might have been required for effective and habitual prehistoric stone tool behaviours comes from archaeologists who have learned to replicate prehistoric stone tools. Experiments monitoring the kinematics of the tool behaviours, associated muscle recruitment and stresses on the hand bones and joints are beginning to illuminate hand functions likely to have become important as the behaviours increased in frequency and sophistication. The experiments are also generating predictions about hand morphology that might reflect the demands of the behaviours. Early experiments revealed two important capabilities that would have been advantageous to bimanual hard hammer percussion manufacture of stone tools. One capability is for controlling and stabilizing stones by each hand simultaneously, during application of strong percussive forces between the stones in removal of flakes from cores [4,5]. (Neural correlates of this bimanual challenge are the subject of particularly interesting research [10].) A second capability is for exposing the working surfaces of the stones during flake removal, minimizing the portion of the stone covered by the hand by using only the thumb and finger pads, thus avoiding crushing of the fingers [5]. A new category of grips emerged, forceful precision grips [5,6], including the three-jaw chuck ‘baseball grip’ and the cradle grip, which cup the stone by the thumb and finger pads and are able to keep the working edges of the tool exposed while at the same time withstanding large external forces (figure 1a–c). Muscles that are strongly recruited during these activities are the opponens pollicis and other intrinsic muscles of both the thumb and little finger, which facilitate cupping of the radial and ulnar sides of the hand in accommodation to the variable shapes of the stones and stabilize the thumb and fifth finger against displacement of stones from the hands [11]. Strong signals from the flexor pollicis longus muscle are elicited by wielding cylindrical sticks with the power (squeeze) grip ([11]; figure 1c). This muscle was strongly recruited during use of three- and four-jaw chuck grips in stone tool-making and tool using by subjects in another experiment [12] but not in the Marzke et al. [11] study. Differences in trial lengths may in part explain the different results. Fatigue tends to occur with repeated high-level contraction of the muscle [11] and leads to alternative thumb function.
Figure 1.
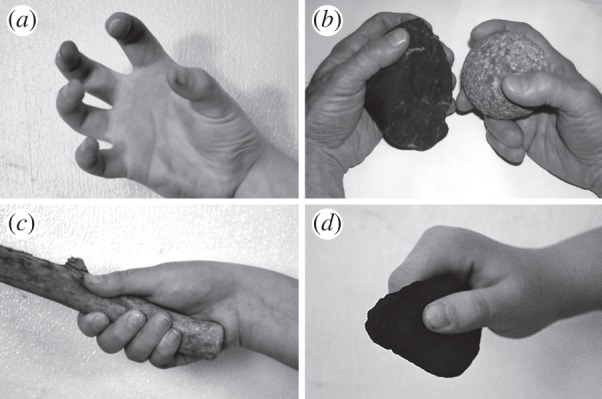
Human hand postures and grips. (a) Cupping of the hand accommodates tool shapes. (b) Cradle pinch grip of a core (left) and three-jaw chuck pinch grip of a hammerstone (right). (c) Power (squeeze) grip of a cylindrical wood tool. (d) Pad-to-side grip of a flake.
Rolian et al. [13] tested the hypothesis that derived human features associated with forceful precision grips are required for effective stone tool behaviour. In a biomechanical analysis, they measured external forces on the thumb generated by production and use of stone flakes and determined how these forces affect internal muscle and joint mechanics. They found that variability in the amount of force required to stabilize the digits varies with digit length and that relative thumb robusticity is a factor in the size of joint contact stresses. Pressure on the thumb was found to be greater during manufacture of the tools than in use of resulting flakes [13].
An experiment by Williams et al. [14] measured forces and pressures sustained by the thumb relative to those on the index and third fingers during Oldowan stone tool making. Loads on the fingers were found to be relatively higher than that on the thumb, a finding that appears to contradict the results of the Rolian et al. 's experiment. However, as they note, differences between the studies in force measurement techniques and the range of grips and hand movements monitored are likely to be some of the factors that contributed to the different findings.
A kinematic analysis of upper limb movements during stone tool knapping revealed that the wrist flick from extension to flexion significantly increases mechanical work and force of the hammerstone strike on cores in flake removal [15].
(b). Chimpanzees: observations of hand grips and movements in feeding and tool use
Forceful precision grips of objects by a single hand do not appear to occur among captive or wild chimpanzees [6]. A study looking directly for single-handed forceful precision grips has not revealed them [16] and they are not specifically described or illustrated in reports of chimpanzee tool use [17] or feeding [18,19]. (Lack of agreement on precision grip terminology has hampered comparative studies, generating clarification and inclusion of forceful precision grips in a recent brief communication [20].) Chimpanzee feeding often presents the same demands as human tool behaviours for strong grasping of an object (in this case food) together with exposure of a good portion of the food for biting, where the teeth pull against the hand on the exposed portion of food. However, in these cases, a second hand is brought into play to resist displacement of the food, and in some cases, a grasping foot as well [6]. In fact, watching the seamless interaction of hands and feet during chimpanzee feeding reminds one that while bipedality freed human hands for tool manipulation, it also deprived us of half of our manipulative grasping apparatus. The lack of forceful precision grip capability is put into relief by descriptions and videotapes of Kanzi, a bonobo attempting to remove flakes from a core using the hard hammer percussion technique taught him by Toth [21,22]. The core rests in the upturned fingers and palm and the hammerstone is held generally within the radial side of the thumb and proximal index finger with the hand pronated. Many relatively weak strikes are required to remove a flake. One of several possible explanations offered for limitations in the effectiveness of this activity is biomechanical limitation to control of the stones by the hand and the difficulty in avoiding smashing of the fingers [23].
(c). Hamadryas baboons: observations of hand grips during feeding
In systematic studies of manipulative behaviour, the hand of captive Hamadryas baboons was observed to retrieve foods and deliver them to the mouth with precision grips between the thumb and index finger pads and between the thumb and side of the index finger [24,25]. However, the precision grips were not forceful enough to hold foods against resistance, for example in breaking hard biscuits with two hands [24].
(d). Long-tailed macaques: observations of hand grips in processing shelled foods
A recent field study of grips used by long-tailed macaques to process shellfish with stone tools revealed a grip of a stone axe described as a form of precision pinching by the thumb and finger pads [26]. This is the first suggestion of a possible non-human primate forceful precision grip that until now has been considered distinctive of humans. Future study of the behaviour and of hand morphology in these macaques is in progress [26].
(e). Capuchin monkeys: observations of stone tool use in pounding nuts
Capuchins use large stones bimanually with bipedal posture to pound nuts [27]. Laboratory experiments have shown that Cebus apella monkeys have a variety of power and precision grips for smaller stones used to throw, crack nuts and cut through acetate to obtain food [28]. The precision grips are reported to be weak [29].
3. Morphology: human bone, joint and muscle structure and functions consistent with grips, movements and stresses associated with modern human replication of stone tools
Comparative dissections, analyses of muscle architecture, three-dimensional shape and biomechanical analyses of joint morphology and measurements of hand segment ratios are revealing a distinctive modern human pattern of features. This pattern enables functions that facilitate the forceful precision grips and movements associated with hard hammer percussion and stone tool use. It also includes the structure that accommodates the hand to stresses associated with these activities. These features are described below with references and are summarized in table 1.
Table 1.
Morphological features consistent with hard hammer percussion manufacture of stone tools and with use of the tools. (PCSA, physiological cross-sectional area.)
| 1. features compatible with forming and securing forceful precision grips |
|---|
| (a) short fingers relative to thumb length [29] |
| (b) broad distal phalangeal tuberosity (‘apical tuft’) [9] |
| (c) extrinsic deep flexor muscle to thumb, reflected by gabled ridge on distal phalanx [9] |
| (d) mobile proximal pollical ungual pulp, reflected by ungual fossa and spines [9] |
| (e) flexor pollicis longus asymmetric attachment mark possibly reflecting phalanx pronationa [9] |
| (f) proximo-distal orientation of joint between capitate and metacarpal 2a [30] |
| (g) proximo-distal orientation of joint between trapezium and metacarpal 2a [30] |
| (h) long first dorsal interosseous muscle metacarpal 1 belly, reflected by attachment marka [31] |
| (i) proportionately large opponens pollicis PCSA and moment armsb [32] |
| (j) proportionately large oblique adductor pollicis muscle PCSA and flexor moment armb [32] |
| 2. features advantageous for distributing and accommodating the associated large forces |
|---|
| (a) proportionately large trapezial joint surface area for metacarpal 1a [30] |
| (b) proportionately large trapezial joint surface area for scaphoida [30] |
| (c) moderate (not marked, not flat) trapeziometacarpal joint curvaturea [33] |
| (d) broad boot-shaped trapezoida [30] |
| (e) proportionately large capitate/trapezoid joint surfacesa [30] |
| (f) metacarpal 3 styloid processa [34] |
| (g) radiocarpal capability for wrist flick (extension/flexion) [35] |
aDerived in humans.
bDerived in humans; associated skeletal features not yet investigated by dissection or quantified.
(a). Thumb
Short fingers relative to thumb length are clearly important for human control of tools by forceful precision grips. The ratio is lower in modern humans than that in great apes but is quite similar to the ratio in baboons [29]. The proportionately long thumb in baboons is compatible with both their quadrupedal terrestrial locomotion and retrieval of small foods by the hands [29]. A broad distal phalangeal tuberosity (‘apical tuft’) relative to base width provides a large surface area in humans for controlling grips. This ratio also is shared with baboons [9]. (Although a correlation has been found between force generation capabilities and tuberosity breadth in humans [36], forceful precision gripping by the thumb and index finger has not been observed in baboons [24,25].)
Apart from these shared ratios, there are many uniquely derived morphological features in humans. The relative areas of both the metacarpal and scaphoid surfaces of the trapezium are significantly larger in humans than that in the great apes and baboons and are thus capable of accommodating the larger joint compressive stresses associated with forceful precision and squeeze gripping [30]. Mutual surface curvatures at the trapeziometacarpal joint are moderate in humans, significantly lower than the marked curvature in chimpanzees, providing greater surface area for accommodation of axial thumb loads on the joint [33]. Lower curvature of the metacarpal base also reduces the projection of the proximal volar beak, lessening the potential for damage when it rides up on the trapezial saddle surface at the extreme ranges of opposition [33]. However, curvature is sufficient to maintain reasonable resistance to subluxation of the metacarpal with forces that tend to cause dorsal and radial displacement of the base.
Many differences consistent with differences in strength and effectiveness of precision grips have been found between humans and chimpanzees in thumb muscle architecture. Potential torque is significantly larger for the human opponens pollicis muscle, which brings the thumb metacarpal with its attached phalanges into opposition to the pads of the four fingers and is strongly recruited in tool behaviours [11,32]. Contributing to this torque advantage are a significantly larger physiological cross-sectional area (PCSA) and significantly larger flexion and abduction moment arms of the muscle [32]. (The larger moment arm provides more torque for a given PCSA, thus providing better leverage and limiting fatigue from muscle contraction.) The oblique portion of the human adductor pollicis muscle, an important flexor of the trapeziometacarpal and metacarpophalangeal joints, also has significantly larger PCSA and torque potential [32].
Many possible skeletal predictors of the pollical intrinsic muscle sizes and moment arms have been suggested in the literature on fossil hands (see, for example, [37]), including bone shapes, robusticity, joint surface areas and muscle attachment scars. However, only two have been tested. Maki & Trinkaus [38] found greater radial protrusion of the opponens pollicis muscle attachment in Neandertals and early Upper Palaeolithic Homo sapiens compared with later humans, indicating a longer opponens pollicis muscle moment arm for abduction and rotation. They suggest that this reflects differences in thumb stresses associated with different tool technologies. The attachment marking for the first metacarpal belly of the first dorsal interosseous muscle is significantly longer in humans than that in apes [31]. The muscle is in a position to stabilize the base of the metacarpal while the extrinsic and intrinsic thumb muscles are active [39], an advantage in maintaining forceful precision grips of stones during hard hammer percussion production of tools.
A deep extrinsic flexor muscle to the distal phalanx of the thumb (a portion of the flexor digitorum profundus muscle or a separate flexor pollicis longus muscle) is present in most non-human catarrhines, marked by a gabled attachment scar in some [9]. The muscle is frequently absent in chimpanzees [9]. Presumably, the muscle functions in the grasp of branches during locomotion by the non-human species as well as in manipulation of foods involving flexion of the pollical distal phalanx. It has not been possible yet to predict the size of this muscle from the attachment markings. However, humans are distinctive in the asymmetric pattern of this marking, distal to the volar fossa, together with asymmetry in ulnar spines associated with the mobile portion of the ungual pulp, which lies in the ungual fossa. In combination, these asymmetries reflect an apparently derived modern human pattern of loading on the pollical distal phalanx in manipulative behaviour [9].
(b). Carpometacarpal region
Three-dimensional comparative analyses of hominid joints in the region of the thumb and index finger have revealed significantly different joint surface orientations in humans that facilitate cupping of the human hand in adaptation to varying shapes of manipulated objects such as stone cores and hammerstones. The joints between the second metacarpal and both distal capitate and trapezium have a proximo/distal orientation [30] that permits metacarpal pronation [5], allowing for some ‘give’ when the metacarpal is stressed by abduction and rotation of the index finger in cupping of objects [5]. The three-dimensional analyses also show a derived human configuration of bones along the distal carpal row that appears to accommodate radioulnar stresses associated with strong pressure from the thumb [30]. The trapezoid bone not only is broader (and boot shaped) in humans than in chimpanzees but the joint between the trapezoid and capitate is also proportionately larger and located in a volar position, although occasionally associated with a dorsal articulation as well [30]. A styloid process on the proximal radiodorsal aspect of the third metacarpal appears to stabilize the central part of the palm against external volar forces that accompany hard hammer percussion by hand-held stones [34].
(c). Radiocarpal region
Radiocarpal facility for wrist extension is greater in humans and some monkeys than that in apes [35]. This facility is compatible with the human wrist flick observed just prior to hammerstone strike of a core observed in a simulated tool-making experiment [15].
4. A new approach to interpreting fossil hominin hand morphology
The comparative analysis of catarrhine hand morphology has shown that there is indeed a derived pattern of features in the modern human hand whose functions are consistent with grips and stresses associated with habitual and effective manufacture and use of stone tools by the hard hammer percussion method simulated in experiments. One key to applying this finding to the functional analysis of fossil hominin hand morphology is to identify complementary capabilities, one for forceful gripping of stones and the other for accommodating stresses associated with bimanual percussion of the stones. To assist in this identification, features compatible with forceful precision grip formation and features advantageous to large force application are grouped in separate categories and listed in table 1. For example, features 1a,f,g would indicate the ability to cup stone cores and hammerstones in the hands, while only the presence of complementary features (2a–f) would be consistent with the possibility that the hands might have been used repeatedly for striking the stones together. Viewing the previously cited features in this way leads us to consider two questions together: (i) could the hands form the appropriate grips (category 1) and if so, (ii) were they built in a way that might have accommodated stresses generated by tool making and use of the tools (category 2)? Note that also within categories certain combinations of features may be particularly informative. For example, features a–c in category 1, shared with many non-human primates, are suggestive of tool making only if they are accompanied by evidence for well-developed pollical musculature (h–j). And within category 2, a broad trapezoid and large capitate/trapezoid joint surfaces (2d,e) might suggest radioulnar loading with tool making, but only if there were evidence in the thumb joints of large muscle forces that might have generated the radioulnar loads (2a–c).
5. Fossil hominin evidence for effective, habitual tool-making capabilities
The recent detailed, comprehensive and carefully reasoned analysis of the Australopithecus sediba hand fossils by Kivell et al. [37] addresses a wide range of features that have been proposed in the past as predictors of tool behaviours, and compares them morphologically and functionally with features available in all other hominin hand fossils. An extensive table summarizes their comparison and, together with their text, reviews the relevant literature.
The brief interpretation of fossil evidence outlined below applies a more limited approach to fossil hand bone analysis, considering only combinations of morphological features noted in §4 and table 1 that have been tested, either through dissections or quantitative analysis, as possible predictors of hand functions compatible with tool making. For most fossil species, this approach is impossible because of a paucity of hand bones associated with a single individual. Information about the features included here is drawn from Kivell et al. [37], except where additional references are provided.
The ratio of thumb length to finger length in Ardipithecus ramidus, measured by the ratio of first metacarpal to fifth metacarpal length, is most similar to that of Old World monkeys and Proconsul among Anthropoidea (table 1, 1a), and a marking on the pollical distal phalanx indicates that an extrinsic deep flexor muscle to the thumb (1c) was present [40]. These features are attributed to arboreal quadrupedal locomotion [40]. No elements of the pattern for cupping of the second and third digital rays or for accommodation of stresses transversely along the wrist are noted.
Considerable detail is available about Australopithecus afarensis hand bones and possible functions from descriptions of recent finds, reviews of previous functional analyses and new interpretations of the full range of features [37,41,42]. Several of the isolated hand bones of this species together exhibit a pattern of features that would have facilitated carpometacarpal cupping and control of objects by the thumb, second and third digital rays (table 1, 1a,f,g) but lack evidence for the accommodation of large loads either across the wrist or along the thumb (2a–e). This combination suggests a capability for tool behaviours such as throwing and pounding [4,43], using a three-jaw chuck by the thumb, index and third fingers, without generating the large or repeated internal loads on the thumb and wrist associated with later tool behaviours. Evidence for pounding of bones with stones for marrow extraction has been reported for A. afarensis at the Dikika site in Ethiopia dating to more than 3.39 Ma [44]. Cut and scraping marks on bones at the same site reflect flesh removal with sharp-edged stones [44]. Gripping of stone flakes between the thumb and side of the index finger (figure 1d) may have been enhanced by the facility for pronating the second metacarpal toward the thumb in A. afarensis (table 1, 1f,g) [5]. Of particular interest is the recent observation of a possibly asymmetrical attachment of the flexor pollicis longus muscle on the pollical distal phalanx [42] (described by the authors as asymmetry of the volar fossa but probably referring to the attachment marking distal to the fossa as they cite Shrewsbury et al. [9]). Debate continues about the finger/thumb length ratio (1a) [45,46] but does not indicate that the ratio would have been large enough to have precluded thumb/fingerpad manipulation of objects.
There appears to be an integrated pattern of features in hand bones from a single individual of A. sediba, dating to 1.977 Ma, that might have facilitated forceful precision grips and also tolerated stresses associated with habitual and effective bimanual tool making and tool use [37]. The proportionately short fingers relative to thumb length (table 1, 1a) and distal pollical phalangeal (1b,c) and carpometacarpal morphology (1f,g) would have facilitated forceful precision grips of stones, and bone and joint shapes and sizes should have accommodated stresses generated by forceful pinch and grasp by the thumb and fingers (2a–e). The hands are contemporary with stone tools that would have been manufactured with bimanual striking of hammerstones on cores. A very gracile pollical metacarpal seems inconsistent with the rest of the morphology [37]. Kivell et al. [37] point to other features of metacarpal 1 morphology that may reflect relatively large PCSA and moment arms of intrinsic pollical muscles that may offset the implications from metacarpal gracility. Thus, these features certainly should be tested as predictors of muscle architecture and internal bone and joint stresses.
Several features of Australopithecus africanus indicate a pattern that should have been compatible with forming and securing forceful precision grips (table 1, 1a–d,f,g) and accommodating external forces at the centre of the palm associated with tool making (2f). However, primitive scaphoid morphology [47] and apparently marked curvature of the joint at the base of the thumb [48] indicate that the hand may not have habitually sustained the large internal axial pollical loads and transverse carpal forces that can be accommodated by the modern human hand.
Data from the Swartkrans fossils for variables considered here are limited to the base and distal aspect of the thumb and are thus not sufficient for consideration of combinations of variables reflective of grip capabilities and possible tool-making stresses. The same is the case for the Olduvai H. habilis hand bones, which generated studies of tool-making morphology. Features considered here are available only for the OH 7 distal phalanx and the joint between the trapezium and second metacarpal. Evaluation of Homo antecessor and Homo erectus tool-making capabilities is similarly handicapped by the lack of a sufficient range of complementary informative features. Neandertal hand morphology reflects tool-making capabilities throughout [37,49]. However, there are interesting differences from modern humans in carpometacarpal joint orientations and shapes and in thumb phalangeal proportions that may reflect performance differences from modern humans [50,51].
The Late Pleistocene fossil hand bones of Homo floresiensis, associated with stone artefacts comparable to Oldowan tools [52], lack modern human morphology relating to both cupping the palm and accommodating stresses across the wrist [52–54]. The lack of stress-related features, as in australopiths, has been attributed to the likelihood that stresses across the wrist did not intensify until the later development of Acheulean tool making [30,31,52]. However, the absence of features that facilitate cupping of the human hand, and are present even in australopiths, is more puzzling, and puts into relief the twin problems of paucity of the fossil hand record and limited biomechanical models of hand functions relating to tool behaviours.
6. Future directions of research
Given the large geographical and time ranges of hominins, and current evidence for variable combinations of features in the fossil hominin hands, it should be expected that a variety of morphological patterns were compatible with tool behaviours. The need for additional data that would enhance our ability to identify morphological predictors of stone tool making has been noted throughout this brief review. Following are suggestions for studies that could substantially improve the functional interpretation of hand skeletal morphology in fossil hominins.
(a). Consideration of additional models for predicting grips and stresses associated with fossil hominin tool making and tool use
Gumert et al. ([26], p. 607) encourage research using a ‘tripartite non-human model of chimpanzees, capuchins and macaques’ to bring new perspectives on human tool use. New approaches are needed to analyse the morphology of hominin fossils in connection with their specific ecological and cultural contexts, rather than focusing exclusively on analysis based upon our current restricted human models. These approaches will dovetail with the developing interdisciplinary field of primate archaeology [55] in attempting to discern through comparative behaviours, morphology and material remains the earliest traces of specific tool behaviours. Detailed descriptions and illustrations of grips used by all these tool-using species, measurement of relative forcefulness of the grips and of stresses on the hand associated with tool using, and relevant hand morphology will be essential to this comparative approach.
(b). Testing of hypotheses that feeding behaviours are consistent with derived patterns of hand morphology
It has been hypothesized that a few derived morphological features found in some early hominin fossil hands may reflect repeated manipulative activities during food gathering and food processing [56] or processing of tough vegetation [8] rather than tool behaviours. Feeding hypotheses should be tested with simulations of feeding behaviours. Are the distinctively human grips recruited and do the behaviours generate forces that might explain derived features in the modern human thumb and wrist?
(c). Bone/muscle relationships
An assumption that relative metacarpal robusticity correlates with sizes of muscles that load the bones is implicit in most fossil hominin hand studies, but it should be tested directly and comparatively with dissections and quantitative analyses of catarrhine cadaver musculoskeletal specimens. Inferences of muscle size and loading patterns drawn from locations and shapes of skeletal features at sites of muscle attachments also need to be tested directly. Variation in metacarpal robusticity among individuals and between species may be affected by many factors including scaling relationships of joint dimensions and bone lengths [13], sexual dimorphism [37], sizes of each muscle that stresses the bone and also lengths of the muscle moment arms. Variability among fossils in metacarpal robusticity and joint surface sizes indicates that the two features do not necessarily reflect similar functional patterns [57].
(d). Fifth finger morphology
The fifth finger anchors the distinctive human power (squeeze) grip of cylindrical tools ([7]; figure 1c) and plays a critical role in stabilizing cores with grips by the thumb and four digital pads during hard hammer percussion manufacture of stone tools [11]. Thus, it is not surprising that humans appear to differ from other primates in several features of the finger and hypothenar region that should be examined quantitatively [7]. Data on this region would complement data already available for the thumb/index finger region in providing a much more comprehensive view of full hand capabilities.
(e). Metacarpal internal bony structure
Metacarpal head trabecular structure has been explored as a possible source of evidence for joint loading and posture [58–60]. Caveats to measurement and interpretation of this structure should be considered [60], and it is hoped that further studies will lead to possible new predictors of behaviours.
7. Did the human hand evolve in adaptation to stone tool making and use of the tools?
As Weiss notes [61], it cannot be directly determined that hominin hands evolved by natural selection in adaptation to tool making. Derived elements of the human pattern may have appeared at different times and have been compatible with other food-gathering and food-processing activities of early hominins. The proportionately long thumb, shared with some other living and fossil catarrhines, may be primitive for hominins [40,62,63] or have coevolved with the hallux/toes pattern [64]. However, it is clear from the prehistoric record that hands were exposed increasingly to large, repeated, prolonged stresses associated with tool making and use of the tools. These stresses are likely to have compromised functions of the hand that would have been crucial to a wide range of behaviours, even beyond those involving tools. (Vulnerability to similar stresses is clearly evident in many pathologies of the modern human hand [65].) Thus, the presence among fossil hominin species of morphological patterns with combinations of features consistent with the challenges of stone tool replication, and the persistence of one and probably more such patterns through many millennia, would be compelling evidence that tool making and use of the tools were significant factors in the evolution of hominin hand morphology. It is hoped that larger samples of fossil hands from early species of the genus Homo, as well as from australopiths will be found in order to trace the evolution of derived human hand morphology in more detail.
Acknowledgements
I thank Dora Biro for the opportunity to explore ‘Tool Use as Adaptation’ at a wonderfully broad and interactive level. Assistance of D. Hawkey with the figure is gratefully acknowledged.
References
- 1.Leakey LSB. 1960. Recent discoveries at Olduvai Gorge. Nature 188, 1050–1052. ( 10.1038/1881050a0) [DOI] [Google Scholar]
- 2.Napier JR. 1962. Fossil hand bones from the Olduvai Gorge. Nature 196, 409–411. ( 10.1038/196409a0) [DOI] [Google Scholar]
- 3.Susman RL, Creel N. 1979. Functional and morphological affinities of the subadult hand (O.H. 7) from Olduvai Gorge. Am. J. Phys. Anthropol. 51, 311–332. ( 10.1002/ajpa.1330510303) [DOI] [PubMed] [Google Scholar]
- 4.Marzke MW, Shackley MS. 1986. Hominid hand use in the Pliocene and Pleistocene: evidence from experimental archaeology and comparative morphology. J. Hum. Evol. 15, 439–460. ( 10.1016/S0047-2484(86)80027-6) [DOI] [Google Scholar]
- 5.Marzke MW. 1997. Precision grips, hand morphology, and tools. Am. J. Phys. Anthropol. 102, 91–110. () [DOI] [PubMed] [Google Scholar]
- 6.Marzke MW, Wullstein KL. 1996. Chimpanzee and human grips: a new classification with a focus on evolutionary morphology. Int. J. Primatol. 17, 117–139. (doi:10/1007BFO2696162) [Google Scholar]
- 7.Marzke MW, Wullstein KL, Viegas SF. 1992. Evolution of the power (‘squeeze’) grip and its morphological correlates in hominids. Am. J. Phys. Anthropol. 89, 283–298. (doi:10/1002/ajpa.1330890303) [DOI] [PubMed] [Google Scholar]
- 8.Marzke MW. 2006. Who made stone tools? In Stone knapping: the necessary conditions for a uniquely hominid behaviour (eds Roux V, Brill B.), pp. 243–255. Cambridge, UK: McDonald Institute Monograph Series. [Google Scholar]
- 9.Shrewsbury MM, Marzke MW, Linscheid RL, Reece SP. 2003. Comparative morphology of the pollical distal phalanx. Am. J. Phys. Anthropol. 121, 30–47. (doi:10/1002/ajpa.10192) [DOI] [PubMed] [Google Scholar]
- 10.Stout D, Toth N, Schick K, Chaminade T. 2008. Neural correlates of Early Stone Age toolmaking: technology, language and cognition in human evolution. Phil. Trans. R. Soc. B 363, 1939–1949. ( 10.1098/rstb.2008.0001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marzke MW, Toth N, Schick K, Reece S, Steinberg B, Hunt K, Linscheid RL. 1998. EMG study of hand muscle recruitment during hard hammer percussion manufacture of Oldowan tools. Am. J. Phys. Anthropol. 105, 315–332. () [DOI] [PubMed] [Google Scholar]
- 12.Hamrick MW, Churchill SE, Schmitt D, Hylander WL. 1998. EMG of the human flexor pollicis longus muscle: implications for the evolution of hominid tool use. J. Hum. Evol. 34, 123–136. ( 10.1006/jhev.1997.0177) [DOI] [PubMed] [Google Scholar]
- 13.Rolian C, Lieberman DE, Zermeno JP. 2011. Hand biomechanics during simulated stone tool use. J. Hum. Evol. 61, 26–41. ( 10.1016/j.jhevol.2011.01.008) [DOI] [PubMed] [Google Scholar]
- 14.Williams EM, Gordon AD, Richmond BG. 2012. Hand pressure distribution during Oldowan stone tool production. J. Hum. Evol. 62, 520–532. ( 10.1016/j.jhevol2012.02.005) [DOI] [PubMed] [Google Scholar]
- 15.Williams EM, Gordon AD, Richmond BG. 2010. Upper limb kinematics and the role of the wrist during stone tool production. Am. J. Phys. Anthropol. 141, 134–145. ( 10.1002/ajpa21302) [DOI] [PubMed] [Google Scholar]
- 16.Reece S, Marzke MW, Marchant LF, Mc Grew WC. 2000. Food object manipulation by chimpanzees in Mahale Mountains National Park. Am. J. Phys. Anthropol. 30(Suppl), 259–260. [DOI] [PubMed] [Google Scholar]
- 17.Boesch C, Boesch H. 1993. Different hand postures for pounding nuts with natural hammers by wild chimpanzees. In Hands of primates (eds Preuschoft H, Chivers DJ.), pp. 31–43. New York, NY: Springer. [Google Scholar]
- 18.Jones-Engel LE, Bard KA. 1996. Precision grip in young chimpanzees. Am. J. Primatol. 39, 1–15. () [DOI] [PubMed] [Google Scholar]
- 19.Pouydebat E, Laurin M, Gorce P, Bels V. 2008. Evolution of grasping among anthropoids. J. Evol. Biol. 21, 1732–1743. ( 10.1111/j.1420-9101.2008.01582.x) [DOI] [PubMed] [Google Scholar]
- 20.Marzke MW, Pouydebat E, Laurin M, Gorce P, Bels V. 2009. A clarification of Pouydebat et al. 2008, evolution of grasping among anthropoids. J. Evol. Biol. 22, 2554–2557. ( 10.1111/j.1420-9101.2009.01856x) [DOI] [PubMed] [Google Scholar]
- 21.Toth N, Schick KD, Savage-Rumbaugh ES, Sevcik RA, Rumbaugh DM. 1993. Pan the tool-maker: investigations into the stone tool-making and tool-using capabilities of a bonobo (Pan paniscus). J. Archaeol. Sci. 20, 81–91. ( 10.1006/jasc.1993.1006) [DOI] [Google Scholar]
- 22.Roffman I, Savage-Rumbaugh S, Rubert-Pugh E, Ronen A, Nevo E. 2012. Stone tool production and utilization by bonobo-chimpanzees (Pan paniscus). Proc. Natl Acad. Sci. USA 109, 14 500–14 503. ( 10.1073/pnas.1212855109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schick KD, Toth N, Garufi G. 1999. Continuing investigations into the stone tool-making and tool-using capabilities of a bonobo (Pan paniscus). J. Archaeol. Sci. 26, 821–832. ( 10.1006/jasc.1998.0350) [DOI] [Google Scholar]
- 24.Guthrie EA. 1991. Variability of the primate trapeziometacarpal articulation: description and functional evolution significance. MA thesis, Arizona State University, Tempe, AZ, USA. [Google Scholar]
- 25.Jude J. 1993. Manipulative behaviour of Hamadryas baboons. Senior thesis, Arizona State University, Tempe, AZ, USA. [Google Scholar]
- 26.Gumert MD, Kluck M, Malaivijitnond S. 2009. The physical characteristics and usage patterns of stone axe and pounding hammers used by long-tailed macaques in the Andaman Sea region of Thailand. Am. J. Primatol. 71, 594–608. ( 10.1002/ajp20694) [DOI] [PubMed] [Google Scholar]
- 27.Liu Q, Simpson K, Izar P, Ottoni E, Visalberghi E, Fragaszy D. 2009. Kinematics and energetics of nut-cracking in wild capuchin monkeys (Cebus libidinosus) in Piauí, Brazil. Am. J. Phys. Anthropol. 138, 210–220. ( 10.1002/ajpa.20920) [DOI] [PubMed] [Google Scholar]
- 28.Westergaard GC, Suomi SJ. 1997. Capuchin monkey (Cebus apella) grips for the use of stone tools. Am. J. Phys. Anthropol. 103, 131–135. () [DOI] [PubMed] [Google Scholar]
- 29.Napier J. 1993. Hands (revised edn) Princeton, NJ: Princeton University Press. [Google Scholar]
- 30.Tocheri MW. 2007. Three-dimensional riddles of the radial wrist: derived carpal and carpometacarpal joint morphology in the genus Homo and the implications for understanding the evolution of stone tool-related behaviors in hominins. Dissertation, Arizona State University, Tempe, AZ, USA. [Google Scholar]
- 31.Tocheri MW, Orr CM, Jacofsky MC, Marzke MW. 2008. The evolutionary history of the hominin hand since the last common ancestor of Pan and Homo. J. Anat. 212, 544–562. ( 10.1111/j.1469-7580.2008.00865.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marzke MW, Marzke RF, Linscheid RL, Smutz P, Steinberg B, Reece S, An KN. 1999. Chimpanzee thumb muscle cross sections, moment arms and potential torques, and comparisons with humans. Am. J. Phys. Anthropol. 110, 163–178. () [DOI] [PubMed] [Google Scholar]
- 33.Marzke MW, Tocheri MW, Steinberg B, Femiani JD, Reece SP, Linscheid RL, Orr CM, Marzke RF. 2010. Comparative 3D quantitative analyses of trapeziometacarpal joint surface curvatures among living catarrhines and fossil hominins. Am. J. Phys. Anthropol. 141, 38–51. ( 10.1002/ajpa.21112) [DOI] [PubMed] [Google Scholar]
- 34.Marzke MW, Marzke RF. 1987. The third metacarpal styloid process in humans: origin and functions. Am. J. Phys. Anthropol. 73, 415–431. ( 10.1002/ajpa.1330730403) [DOI] [PubMed] [Google Scholar]
- 35.Orr CM. 2012. Kinematics and morphometrics of the radiocarpus in anthropoids with implications for reconstructing the evolution of hominin wrist mechanics. Am. J. Phys. Anthropol. 54(Suppl.), 229–230. [Google Scholar]
- 36.Bimson B, Ottevanger J, Roberts N, Macho G, Percy D, Whitehouse GH. 1997. Hominid thumb strength predicted by high resolution magnetic resonance imaging and force measurements in living subjects. Magn. Reson. Imaging 15, 899–908. ( 10.1016/S0730-725x(97)00007-6) [DOI] [PubMed] [Google Scholar]
- 37.Kivell TL, Kibii JB, Churchill SE, Schmid P, Berger LR. 2011. Australopithecus sediba hand demonstrates mosaic evolution of locomotor and manipulative abilities. Science 333, 1411–1417. ( 10.1126/science.1202625) [DOI] [PubMed] [Google Scholar]
- 38.Maki J, Trinkaus E. 2011. Opponens pollicis mechanical effectiveness in Neandertals and early modern humans. PaleoAnthropology 2011, 62–71. ( 10.4207/PA.2011.ART43) [DOI] [Google Scholar]
- 39.Hollister A, Buford W, Myers L. 1992. Axes of rotation of the thumb carpometacarpal joint. J. Orthop. Res. 10, 454–460. ( 10.1002/jor.1100100319) [DOI] [PubMed] [Google Scholar]
- 40.Lovejoy CO, Simpson SW, White TD, Asfaw B, Suwa G. 2009. Careful climbing in the Miocene: the forelimbs of Ardipithecus ramidus and humans are primitive. Science 326, 70e1–70e8. ( 10.1126/science.1175827) [DOI] [PubMed] [Google Scholar]
- 41.Drapeau MSM, Ward CV, Kimbel WH, Johanson DC, Rak Y. 2005. Associated cranial and forelimb remains attributed to Australopithecus afarensis from Hadar, Ethiopia. J. Hum. Evol. 48, 593–642. ( 10.1016/j.jhevol.2005.02.005) [DOI] [PubMed] [Google Scholar]
- 42.Ward CV, Kimbel WH, Harmon EH, Johanson DC. 2012. New postcranial fossils of Australopithecus afarensis from Hadar, Ethiopia (1990–2007). J. Hum. Evol. 63, 1–51. ( 10.1016/j.jhevol.2012.02.005) [DOI] [PubMed] [Google Scholar]
- 43.Marzke MW. 1983. Joint functions and grips of the Australopithecus afarensis hand, with special reference to the region of the capitate. J. Hum. Evol. 12, 197–211. (doi:10/1016/S0047-2484(83)80025-6) [Google Scholar]
- 44.McPherron SP, Alemseged Z, Marean CW, Wynn JG, Reed D, Geraads D, Bobe R, Béarat HA. 2010. Evidence for stone-tool-assisted consumption of animal tissues before 3.39 million years ago at Dikika, Ethiopia. Nature 466, 857–860. ( 10.1038/nature09248) [DOI] [PubMed] [Google Scholar]
- 45.Rolian C, Gordon AD, Hallgrimson B. 2011. Assessing manual proportions in Australopithecus afarensis using Monte Carlo resampling. Am. J. Phys. Anthropol. 52(Suppl.), 256. [Google Scholar]
- 46.Alba DM, Moyà-Solà S, Köhler M. 2003. Morphological affinities of the Australopithecus afarensis hand on the basis of manual proportions and relative thumb length. J. Hum. Evol. 44, 225–254. (doi:10/1016/S0047-2484(02)00207-5) [DOI] [PubMed] [Google Scholar]
- 47.Kibii JM, Clarke RJ, Tocheri MW. 2011. A hominin scaphoid from Sterkfontein, Member 4: morphological description and first comparative phenetic 3D analyses. J. Hum. Evol. 61, 510–517. ( 10.1016/j.jhevol.2011.06.001) [DOI] [PubMed] [Google Scholar]
- 48.Clarke RJ. 1999. Discovery of complete arm and hand of the 3.3 million-year-old Australopithecus skeleton from Sterkfontein. S. Afr. J. Sci. 95, 477–480. [Google Scholar]
- 49.Trinkaus E. 1983. The Shanidar Neandertals. New York, NY: Academic Press. [Google Scholar]
- 50.Niewoehner WA, Weaver A, Trinkaus E. 1997. Neandertal capitate-metacarpal articular morphology. Am. J. Phys. Anthropol. 103, 219–233. () [DOI] [PubMed] [Google Scholar]
- 51.Trinkaus E, Villemeur I. 1991. Mechanical advantages of the Neandertal thumb in flexion: a test of an hypothesis. Am. J. Phys. Anthropol. 84, 249–260. ( 10.1002/ajpa.1330840303) [DOI] [PubMed] [Google Scholar]
- 52.Tocheri MW, et al. 2007. The primitive wrist of Homo floresiensis and its implications for hominin evolution. Science 317, 1743–1745. ( 10.1126/science.1147143) [DOI] [PubMed] [Google Scholar]
- 53.Larson SG, Jungers WL, Tocheri MW, Orr CM, Morwood MJ, Sutikna T, Rokhus DA, Djubiantono T. 2009. Descriptions of the upper limb skeleton of Homo floresiensis. J. Hum. Evol. 57, 555–570. ( 10.1016/j.jhevol.2008.06.007) [DOI] [PubMed] [Google Scholar]
- 54.Orr CM, et al. 2013. New wrist bones of Homo floresiensis from Liang Bua (Flores, Indonesia). J. Hum. Evol. 64, 109–129. (doi:10-0.1016/j.jhevol.2012.10.003) [DOI] [PubMed] [Google Scholar]
- 55.Haslam M, et al. 2009. Primate archaeology. Nature 460, 339–344. ( 10.1038/nature08188) [DOI] [PubMed] [Google Scholar]
- 56.Moyà-Solà A, Köhler M, Alba DM, Almécija S. 2008. Taxonomic attribution of the Olduvai Hominid 7 manual remains and the functional interpretation of hand morphology in robust australopithecines. Fol. Primatol. 79, 215–250. ( 10.1159/000113458) [DOI] [PubMed] [Google Scholar]
- 57.Richmond BG, Green DJ, Braun DR, Mbua E, Griffin NL, Chirchir H, Harris JWK. 2011. New fossils from Ileret, Kenya, and the evolution of hominin hand function. Am. J. Phys. Anthropol. 52(Suppl.), 253. [Google Scholar]
- 58.Zeininger A, Richmond BG, Hartman G. 2011. Metacarpal head biomechanics: a comparative backscattered electron image analysis of trabecular bone mineral density in Pan troglodytes, Pongo pygmaeus, and Homo sapiens. J. Hum. Evol. 60, 703–710. ( 10.1016/j.jhevol.2011.01.002) [DOI] [PubMed] [Google Scholar]
- 59.Lazenby RA, Skinner MM, Hublin J-J, Boesch C. 2011. Metacarpal trabecular architecture variation in the chimpanzee (Pan troglodytes): evidence for locomotion and tool-use? Am. J. Phys. Anthropol. 144, 215–225. ( 10.1002/ajpa.21390) [DOI] [PubMed] [Google Scholar]
- 60.Kivell TL, Skinner MM, Lazenby R, Hublin J-J. 2011. Methodological considerations for analyzing trabecular architecture: an example from the primate hand. J. Anat. 218, 209–225. () [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weiss KM. 2012. Agnotology. Evol. Anthropol. 21, 96–100. (doi:10/1002evan21303) [DOI] [PubMed] [Google Scholar]
- 62.Almécija S, Moyà-Solà S, Alba DM. 2010. Early origin for human-like precision grasping: a comparative study of pollical distal phalanges in fossil hominins. PLoS ONE 5, e11727 ( 10.1371/journal.pone.0011727) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Almécija S, Alba DM, Moyà-Solà S. 2012. The thumb of Miocene apes: new insights from Castell de Barberà (Catalonia, Spain). Am. J. Phys. Anthropol. 148, 436–450. ( 10.1002/ajpa.22071) [DOI] [PubMed] [Google Scholar]
- 64.Rolian C, Lieberman DE, Hallgrimsson B. 2010. The coevolution of human hands and feet. Evolution 64, 1558–1568. ( 10.1111/j.1558-5646.2010.00944.x) [DOI] [PubMed] [Google Scholar]
- 65.Cooney WP., III (ed.) 2010. The wrist. Diagnosis and operative treatment. Philadelphia, Pennsylvania: Wolters Kluwer/Lippincott. [Google Scholar]


