Abstract
Development of a safe and effective method for gene delivery to hepatocytes is a critical step toward gene therapy for liver diseases. Here, we assessed the parameters for gene delivery to the livers of large animals (pigs, 40–65 kg) using an image-guided hydrodynamics-based procedure that involves image-guided catheter insertion into the lobular hepatic vein and hydrodynamic injection of reporter plasmids using a computer-controlled injector. We demonstrated that injection parameters (relative position of the catheter in the hepatic vasculature, intravascular pressure upon injection, and injection volume) are directly related to the safety and efficiency of the procedure. By optimizing these parameters, we explored for the first time, the advantage of the procedure for sequential injections to multiple lobes in human-sized pigs. The optimized procedure resulted in sustained expression of the human α-1 antitrypsin gene in livers for more than 2 months after gene delivery. In addition, repeated hydrodynamic gene delivery was safely conducted and no adverse events were seen in the entire period of the study. Our results support the clinical applicability of the image-guided hydrodynamic gene delivery method for the treatment of liver diseases.
Keywords: gene therapy, human α-1 antitrypsin, hydrodynamic gene delivery, image-guided gene delivery, non-viral vector
Introduction
Development of a safe and effective procedure for liver-specific gene delivery is essential for gene therapy of many liver-related diseases.1 Among the methods developed,2 hydrodynamic gene delivery, originally developed for gene delivery to mouse liver by tail vein injection of plasmid DNA,3,4 has been explored for clinical applications [for recent reviews, see refs 2,5,6]. A few research groups have shown successful gene delivery in small pigs and rabbits.7,8,9,10 We have previously demonstrated high levels of gene expression in injected liver lobes11,12 using a computer-controlled injection device. Recently, the efficacy of hydrodynamic gene delivery has also been demonstrated ex vivo using human liver segments.13 The procedure of injecting a large volume of thrombopoietin-expressing plasmids directly into the hepatic vein has also been conducted in humans and resulted in a transient increase of platelet count.14
Despite these early successes demonstrating the simplicity, convenience, and safety of the procedure for hepatic gene delivery, most of the previous studies focused on proof of principle and feasibility, rather than on establishment of a clinically applicable procedure. For example, the pigs employed in these studies as an animal model were relatively small (body weight 20–25 kg), and the factors affecting gene delivery efficiency have not been identified and systematically assessed, resulting in poor reproducibility in animals9 and patients.14 In addition, the long-term effect of the hydrodynamic gene transfer with regard to persistence of transgene expression, tissue damage, and plasmid distribution has not been examined.
The objective of the current study was to fill the gaps between small animal studies and clinical applications. Using human-size pigs (40–60 kg) as the animal model, we demonstrated that optimal gene delivery to hepatocytes requires insertion of a balloon catheter at an appropriate section of the hepatic vein, an injection volume of 2.5× lobe volume, and sequential gene delivery to each of the liver lobes. Our results provide direct evidence in support of the clinical application of hydrodynamic gene delivery.
Results
Effect of the insertion site of a balloon catheter in the hepatic vein on intravascular pressure and gene delivery efficiency
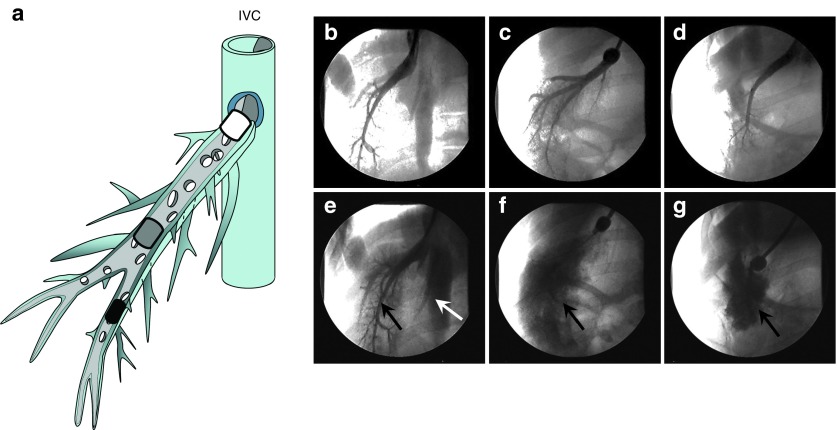
In image-guided hydrodynamic gene delivery to the liver, the balloon catheter should be lodged at a specific site in the hepatic vein. Anchoring the balloon catheter at the proximal site near the junction to the inferior vena cava (IVC) (white-rounded rectangle in Figure 1a) enables the DNA solution to reach the entire lobe. However, the pressure generated by the hydrodynamic injection will be lower with a fixed volume of DNA solution because it covers a larger area. In contrast, if one places the balloon catheter at a distant site from the junction of the hepatic vein to the IVC (black-rounded rectangle in Figure 1a), fewer hepatic cells in the lobe will be covered and the hydrodynamic pressure at the distal end is likely to be high because only a fraction of the lobe is included. Figure 1b–g show fluoroscopic images of the hepatic vein and the balloon catheter placed in the proximal (Figure 1b), intermediate (Figure 1c), or distal site (Figure 1d) of the hepatic vein in a lobe in 40–45 kg pigs relative to its position to the hepatic junction to the IVC. The areas covered by the contrast medium (Figure 1e) were wider when the catheter was placed at the proximal site (Figure 1b), but leakage of injected contrast medium to the IVC was evident (white arrow in Figure 1e). On the other hand, when performed from the distal end of the selected hepatic vein, the areas were narrower and no leakage was observed (Figure 1d,g). As expected, injection from the intermediate site (Figure 1c) resulted in a larger imaged area than the distal site, with no leakage of contrast medium to the IVC (Figure 1f).
Figure 1.
Influence of insertion site on vascular distribution of injected phase contrast medium. (a) Schematic presentation of the relative location for catheter insertion at proximal (white rounded rectangle), intermediate (gray rounded rectangle), and distal (black rounded rectangle) site in a hepatic vein. (b–d) Venography showing the blockade of blood vessel by balloon catheter located at (b) proximal, (c) intermediate, or (d) distal site of the right lateral vein. The ball-like structure represents the location of the catheter balloon. (e–g) Venography images showing distribution of contrast medium in the targeted liver lobes upon injection of 20 ml of contrast medium in 10 seconds from (e) proximal, (f) intermediate, or (g) distal site. The white arrow in (e) points to the IVC. Black arrows point the area where the injected contrast medium accumulates.
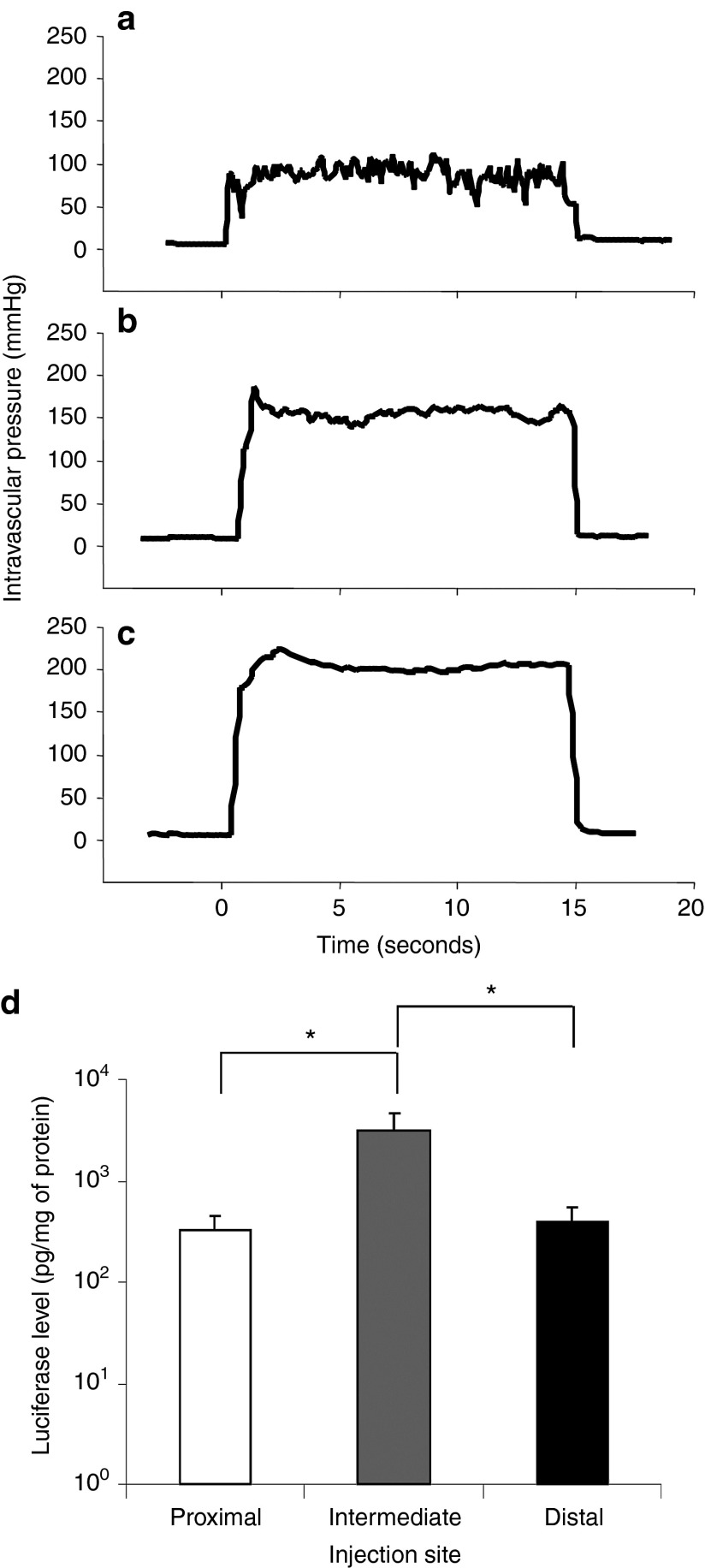
The insertion site of the catheter in the hepatic vein influences the vascular pressure. With an injection of 600 ml of pCMV-Luc plasmid in saline (100 µg/ml) in 15 seconds at 300 psi, the intravascular pressure quickly reached a peak level that was maintained until the injection was completed. When injected from the proximal site, the pressure was stabilized at 100 mmHg (Figure 2a). The injection at the intermediate site generated an intravascular pressure of approximately 150 mmHg (Figure 2b) compared with 200 mmHg (Figure 2c) at the distal site. The level of luciferase gene expression in the injected lobe was analyzed to examine the effect of the site of catheter insertion on gene delivery efficiency. Luciferase activity (Figure 2d) showed the highest level at 3,142 pg/mg of protein with the injection at the intermediate site being 10-fold higher than the level achieved with injections at the proximal or distal sites (P < 0.05). These results suggest that injecting from the intermediate site near the junction of the first branch of the hepatic vein is the best for placement of the balloon catheter. Two out of the five pigs injected at the distal site had identifiable tissue damage (rupture) to the injected lobes.
Figure 2.
Effect of injection site on intravascular pressure and level of reporter gene expression. Image-guided hydrodynamic injection of saline-containing pCMV-Luc plasmid (100 µg/ml) was performed from the injection sites shown in Figure 1 at an injection pressure of 300 psi. Intravascular pressure upon the injection from (a) proximal, (b) intermediate, and (c) distal site and average level of luciferase gene expression 24 hours after the hydrodynamic gene delivery in the targeted liver lobe (d). Two pigs were used for each measurement and the average luciferase activity was calculated from 15 liver samples collected from different parts of each targeted liver lobe (n = 30 for each injection site). The values represent mean ± SD. *P < 0.05, one-way ANOVA followed by Bonferroni's multiple comparison test.
Effect of injection volume on hydrodynamic gene delivery
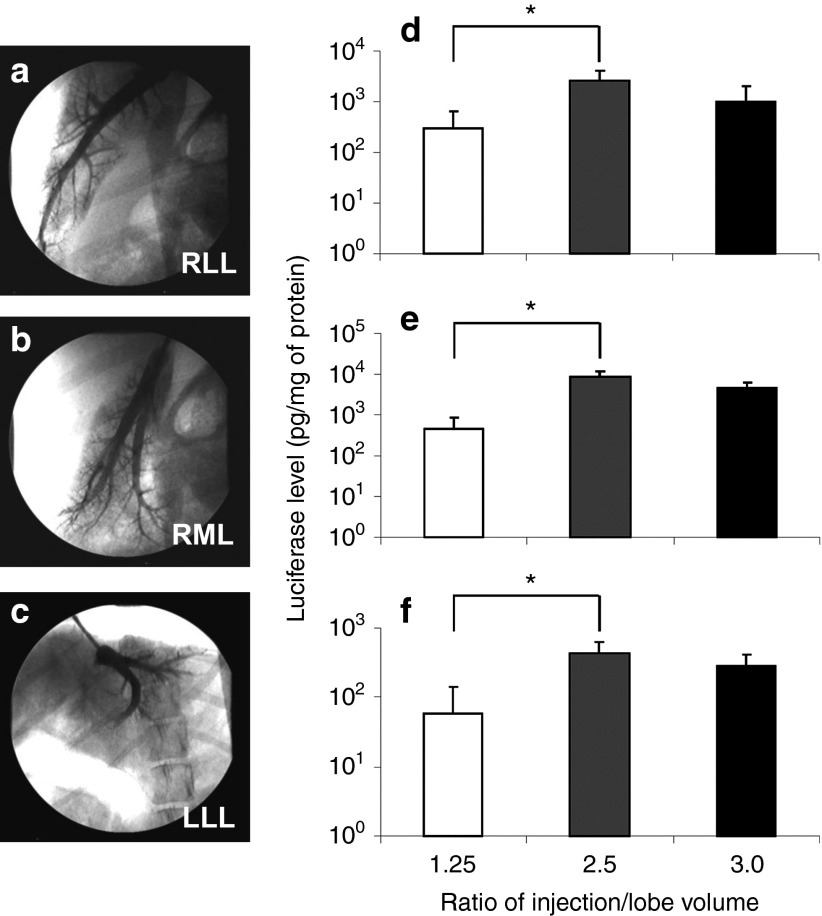
Next, the effect of injection volume on the efficiency of gene delivery was examined. On the basis of the maximum expansion rate of 2.5× the lobe volume that we previously reported,8 injection volumes at 1.25×, 2.5×, and 3.0× lobe volume were examined for gene delivery efficiency with injections at the intermediate site to assess the effect of injection volume on gene delivery. The injection was performed either to the right lateral lobe (RLL) from the right lateral hepatic vein, Figure 3a, to the right medial lobe (RML) from the right medial hepatic vein, Figure 3b, and to the left lateral lobe (LLL) from the left lateral hepatic vein, Figure 3c. Animals were sacrificed 24 hours after the final injection for the luciferase assay. The levels of luciferase gene expression in the RLL, RML, and LLL are shown in Figure 3d,e,f, respectively. Significantly higher levels of luciferase expression at 2,551, 8,469, and 440 pg/mg of protein in the RLL, RML, and LLL, respectively, were achieved with an injection volume of 2.5× lobe volume compared with that achieved with 1.25× lobe volume (P < 0.05). An increase in the injection volume to 3.0× lobe volume did not further increase luciferase gene expression. These results suggest that 2.5× lobe volume was the best injection volume among those tested.
Figure 3.
Effect of injection volume on hydrodynamic gene delivery efficiency. (a) The balloon catheter was inserted into the right lateral hepatic vein (RLHV), (b) right medial hepatic vein (RMHV), or (c) left lateral hepatic vein (LLHV) followed by the venography. Each lobe was injected from the intermediate site with the volume of 1.25×, 2.5×, or 3.0× lobe volume. (d) Level of luciferase gene expression in right lateral lobe (RLL), (e) right medial lobe (RML), (f) and left lateral lobe (LLL). Two lobes from two different animals were used for each volume and 15 liver samples from different parts of each targeted lobe were used for luciferase assay (n = 30 for each data point). The values represent mean ± SD. *P < 0.05, one-way ANOVA followed by Bonferroni's multiple comparison test.
Effect of sequential injections to multiple liver lobes
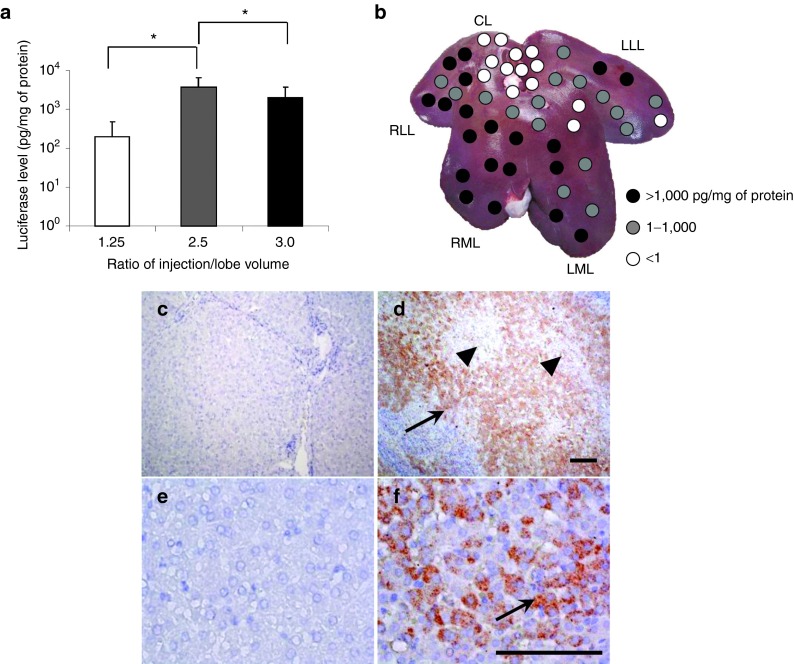
Sequential hydrodynamic injections of pCMV-Luc to the RLL, RML, and LLL were conducted in three pigs with injection volumes of 1.25×, 2.5×, and 3.0×, respectively, of the lobe volume at the intermediate site of the hepatic vein. To minimize the accumulated volume effect in systemic dynamics, each injection was completed at an interval of approximately 30 minutes. The highest luciferase expression was 3,699 pg/mg of protein (Figure 4a) with a 2.5× lobe volume, which was 18-fold higher than that of 1.25x lobe volume and twofold higher than that of 3.0× lobe volume (P < 0.05). Site-specific luciferase gene expression with sequential injection at 2.5× lobe volume of the RLL, RML, and LLL showed relatively homogeneous gene expression in those injected lobes and a heterogeneous pattern in noninjected left medial lobe (LML) (Figure 4b). Of note, LML showed a significant level of gene expression (Figure 4b), which was most likely due to the reported vascular connection between the RML and LML.15 Gene expression in noninjected LML was high when the RML was injected with 2.5× lobe volume, which was 2,000-fold higher than that of 1.25× (P < 0.05); no further increase was observed with 3.0× volume of RML (Supplementary Figure S1a online).
Figure 4.
Effect of sequential injections to multiple liver lobes. A sequential injection of pCMV-Luc plasmids to each of the three hepatic veins in RLL, RML, and LLL in three pigs was performed. (a) Average luciferase activity for the entire liver after sequential injection to three lobes of each animal using injection volumes of 1.25×, 2.5×, or 3.0× lobe volume of the targeted lobe. Ten samples were collected from RLL, RML, LML, LLL, and the caudate lobe (CL) (n = 50 for each injection volume). The values represent mean ± SD. *P < 0.05, One-way ANOVA followed by Bonferroni's multiple comparison test. (b) Map of reporter gene expression level in the liver. Ten liver samples were collected from different parts of each liver lobe. (c–f) Immunohistochemical staining of liver sections was performed using an anti-luciferase antibody. Three samples were collected from each lobe and representative images from noninjected CL (c, e) and injected RML (d, f) are shown. Scale bars represent 100 µm. Black arrows indicate hepatocytes stained positive with anti-luciferase antibody. Black arrowheads indicate the central vein in the acinus.
Lobe-specific luciferase gene expression was verified via the immunohistochemical method using an anti-luciferase antibody (Figure 4c,d,e,f). More hepatocytes were stained positive in the RLL, RML (Figure 4d,f), and LLL than in the noninjected CL (Figure 4c,e). The positively stained cells in the RML were distributed around the central vein (black arrowhead in Figure 4d), which is connected to the hepatic veins in those injected lobes. The positive cells in the LML were distributed more heterogeneously at the edge of the Glisson's sheath (Supplementary Figure S1b,c online). Gene expression was not observed in the CL, kidney, lung, heart, spleen, brain, or muscle (data not shown). No change in physiological parameters was observed during or after the injection, including electrocardiogram, heart rate, respiration rate, blood pressure, and oxygen saturation (Supplementary Figure S2 online). These results provided direct evidence to support the notion that sequential injection of 2.5× volume of the RLL, RML, and LLL to the intermediate site of the hepatic veins is safe and that gene delivery to hepatocytes is efficient.
Effect of repeat injection and persistency of gene expression
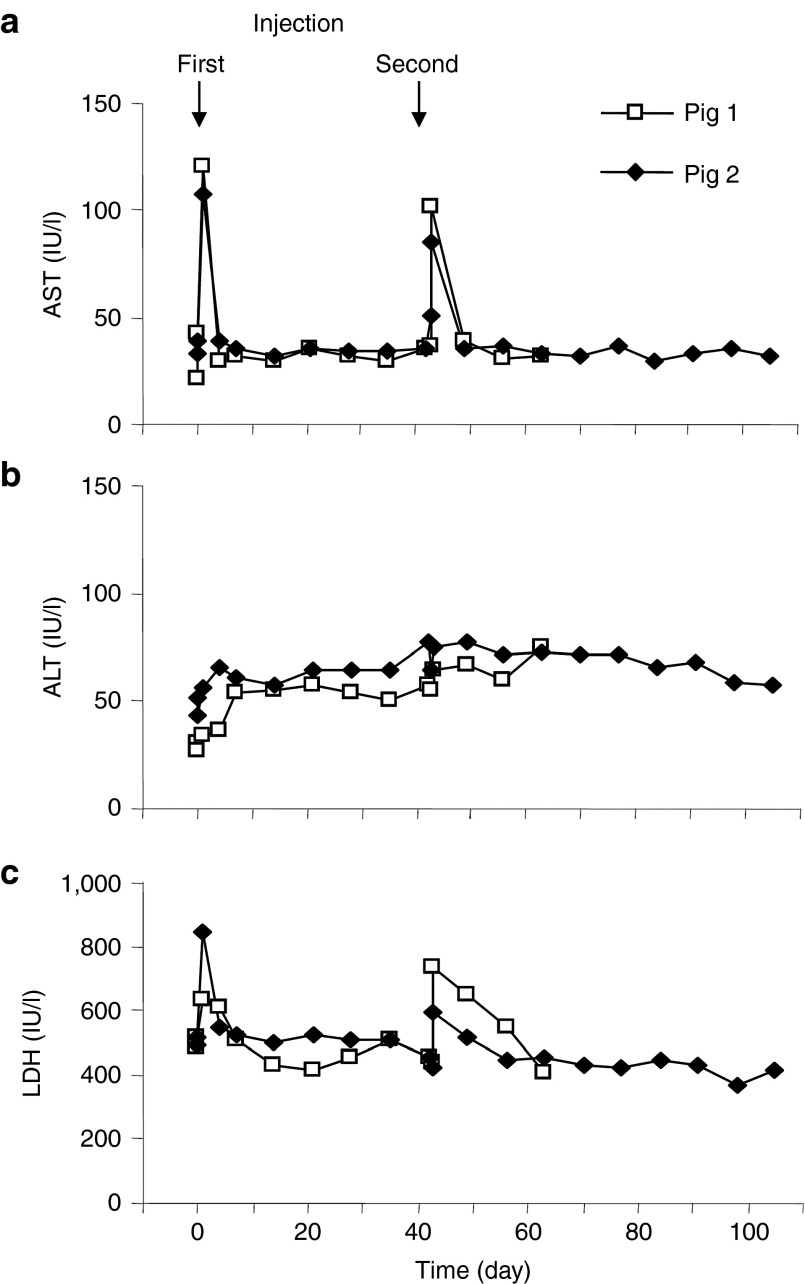
To examine the gene delivery efficiency and the safety of repeated hydrodynamic gene delivery in large animals, a long-term gene expression study was undertaken with optimum injection parameters obtained from the experiments mentioned above. The human α-1 antitrypsin (hAAT) gene encoding a serum protein was employed as a reporter. The pCAG-hAAT plasmid DNA (100 µg/ml in saline) was hydrodynamically injected into the RLL, RML, and LLL in two 40-kg pigs (pigs 1 and 2) at an injection volume of 2.5× lobe volume from the intermediate injection site. The total volume injected was approximately 4% of the body weight. The same procedure was carried out using the same animals (second injection) 6 weeks later, when the pigs weighed approximately 60 kg. The physiological parameters, blood biochemistry, and histology of the injected lobes were examined for safety assessment. No change in physiological parameters including heart rate, blood pressure, and oxygen saturation was observed (Supplementary Figure S2 online). Blood samples were collected from both pigs before (time = 0), 2 hours, and 1, 4, 7, 14, 21, 28, 35, 42 (the day when the second injection was administered), 43, 49, 56, and 63 days after the first hydrodynamic injections, and were collected 70, 77, 84, 91, 98, and 105 days from pig 2. Figure 5 shows the serum concentrations of three liver-related enzymes over time. A fourfold increase in aspartate aminotransferase (Figure 5a) and a twofold increase in alanine aminotransferase (Figure 5b) and lactate dehydrogenase (Figure 5c) were observed 24 hours after each injection. Levels of these enzymes returned to normal range 4 days after the injection. The values of other serum components (total protein, albumin, globulin, γ-glutamic aminotransferase, alkaline phosphatase, total bilirubin, blood urea nitrogen, creatinine, glucose, sodium ion, potassium ion, and chloride ion) were also obtained, and no change was observed (data not shown). A standard ELISA was carried out with a kit (ab108799, Abcam, Cambridge, MA) for detection of serum hAAT on all serum samples collected. Unfortunately, hAAT was not detectable in these serum samples.
Figure 5.
Effect of hydrodynamic gene delivery on serum concentrations of marker enzymes. Blood samples were collected from the ear vein or peripheral vein in the limb of animals before (time = 0), 2 hours, 1, 4, 7, 14, 21, 28, 35, 42 (the day when the second injection was administered), 43, 49, 56, and 63 days after the first injection from pig 1 and 2 and as well as 70, 77, 84, 91, 98, and 105 days after the first injection from pig 2. Concentrations of (a) aspartate aminotransferase (AST), (b) alanine aminotransferase (ALT), (c) and lactate dehydrogenase (LDH). Arrows indicate first and second injection on day 0 and 42. The white square and black diamond-shape represent results of pig 1 and pig 2, respectively.
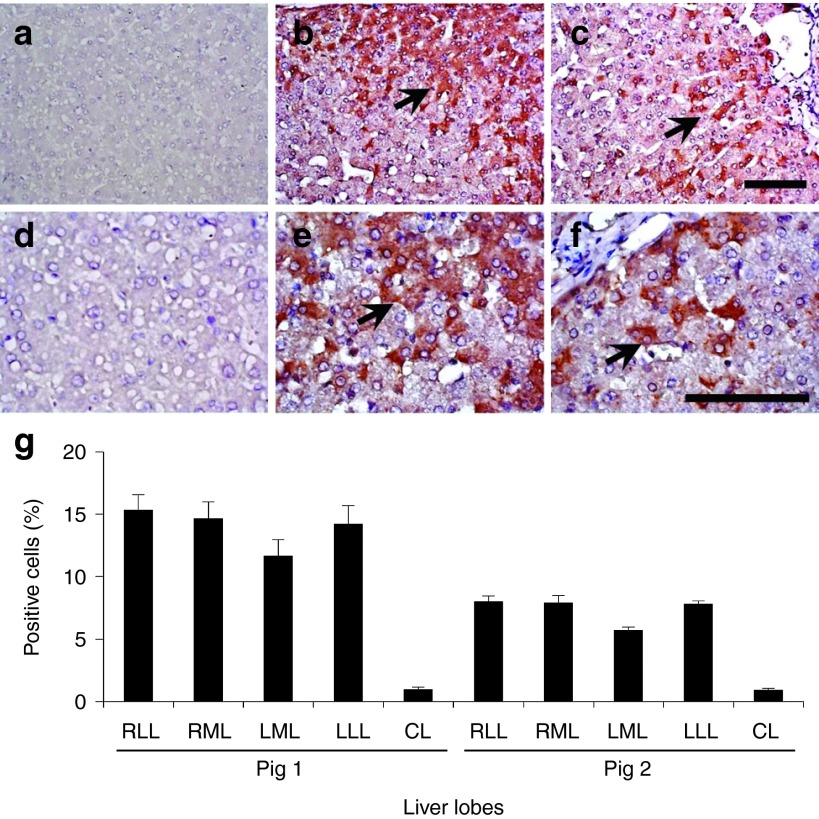
To examine the gene expression in hepatocytes, pig 1 was sacrificed on day 63 (3 weeks after the second set of injections) and pig 2 on day 105 (9 weeks after the second set of injections). Immunohistochemical staining was performed on 10 samples collected from liver tissue in pigs 1 and 2 with anti–hAAT antibody (Figure 6a–f), and positively stained cells were analyzed using Image J software.16 The gene-injected liver lobes showed 15.3, 14.6, 11.6, and 14.2% positively stained cells in the injected RLL (Figure 6b,e), RML, LLL, and noninjected LML, respectively, and less than 1% positive cells were found in the noninjected CL (Figure 6a,d) 3 weeks after injection (Figure 6g). The number of hAAT-expressing hepatocytes slowly decreased to 8.0, 7.9, 5.6, and 7.8% in the RLL (Figure 6c, f), RML, LML, and LLL, respectively, during the following 9 weeks after the second gene delivery (Figure 6g). These results suggest that hAAT expression in injected hepatocytes lasted 2 months after hydrodynamic gene delivery, and that the procedure could be carried out repeatedly with reasonable gene delivery efficiency.
Figure 6.
Persistent expression of human α-1 antitrypsin in the hepatocytes. Immunohistochemical staining of human α-1 antitrypsin was performed in the liver after the hydrodynamic gene delivery of pCAG-hAAT plasmid. (a, d) Noninjected CL in pig 1; (b, e) injected RML in pig 1; (c, f) injected RML in pig 2. Scale bar represents 100 µm (a, b, c, 200× d, e, f, 400×). Black arrows represent positively stained hepatocytes. (g) Quantitative analysis of positively stained cells. Ten liver tissue samples collected from each lobe (total of 50 samples in a liver) in pigs 1 and 2 and a quantitative analysis was performed on three fields (total of 150 fields in a liver) from each section. The values represent mean ± SD (n = 30 for each lobe). P < 0.05 between all injected lobes and CL. One-way ANOVA followed by Bonferroni's multiple comparison test.
Plasmid distribution
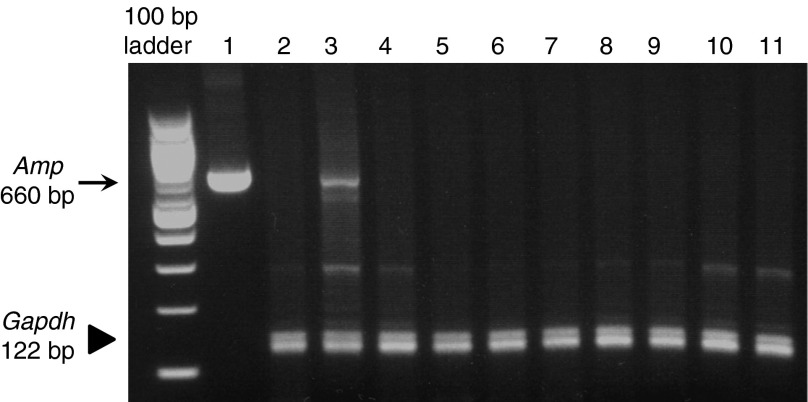
PCR analysis of DNA isolated from multiple organs from pigs 1 and 2 were conducted to determine whether the hAAT plasmid was delivered to organs other than the liver. A pair of ampicillin-resistant (Amp) gene-specific primers was utilized to detect the hAAT-expressing plasmid DNA using the glyceraldehyde 3-phosphate dehydrogenase (Gadph) gene as an internal control (Figure 7). The PCR product of Amp is a 660-nucleotide fragment that was detected in the injected liver lobe in pigs 1 and 2 (Figure 7, lane 3), but was not present in the liver of pigs who were not injected with the plasmid (Figure 7, lane 2). The spleens, kidneys, brains, hearts, stomachs, colons, ovaries, lungs, muscles, and small intestines of injected pigs showed no presence of the Amp gene. These results demonstrate that the procedure carried out in the liver did not result in gene transfer to cells in other organs, confirming the target specificity of image-guided gene delivery to the liver.
Figure 7.
Tissue distribution of plasmid DNA. Multiplex PCR analysis was performed on genomic DNA collected from pigs by 35 cycles of PCR amplification using primers for ampicillin resistance (Amp) and glyceraldehyde 3-phosphate dehydrogenase (Gapdh) genes, respectively. Lanes: 1, plasmid DNA; 2, normal liver without plasmid injection; 3, injected lobe in pig 2; 4, spleen; 5, kidney; 6, brain; 7, heart; 8, stomach; 9, colon; 10, ovary; 11, lung. The arrow represents the 660 bp Amp fragment. The arrowhead represents the 122 bp Gapdh fragment.
Structural impact of the lobe-specific gene transfer
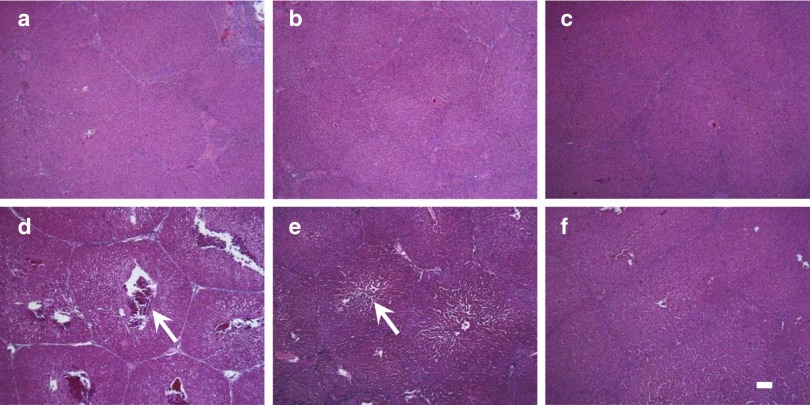
Hematoxylin & eosin staining was utilized to evaluate the impact of hydrodynamic injection on hepatocytes and the surrounding tissue at appropriate time points after the injection (Figure 8a–f). Significant enlargement of the central vein and sinusoid was observed immediately after the injection in the injected lobe (Figure 8d) with same setting as in the previous section; this enlargement started to subside after 4 hours (Figure 8e) and completely subsided after 24 hours (Figure 8f). Tissue damage was not observed in the noninjected CL (Figure 8a,b,c). In addition, chronic liver injury or accumulation of lymphocytes was not observed in pigs 1 and 2 in the long-term study (data not shown). These results suggest that our image-guided hydrodynamic gene delivery is safe and that the procedure could be carried out repeatedly on the same animal.
Figure 8.
Assessment of tissue damage by histochemistry. Hematoxylin & eosin staining of liver samples from CL (a–c), and RML (d–f). Samples were collected immediately following the injection (a, d), 4 hours after the injection (b, e), and 24 hours after the injection (c, f). Scale bars represent 100 µm. White arrows represent central vein in the lobular structure.
Discussion
Various methods of gene delivery have been studied, and a few have been clinically used for the treatment of diseases such as hemophilia and cancers.17 Among the various established methods, hydrodynamic gene delivery is relatively new and was first reported in 1999 by Liu et al.3 and Zhang et al.4 The biggest challenge over the last few years has been in the identification of the factors and parameters that have important roles in determining the safety and efficiency of hydrodynamic gene delivery to the liver. Previously, we and others have reported that hydrodynamic gene delivery can be applied for gene delivery to the liver, muscle, and kidney in small pigs and rabbits.7,8,9,10,11,12,18 Of note, Herrero et al. have demonstrated in a more recent study that effective hydrodynamic gene delivery is also achievable ex vivo in the human liver.13 A primary reason that the small size animals were used in the earlier studies is that they are easier to handle. However, the drawback in using these animals is that they appear too small for being used for the development of parameters clinically applicable. For example, in 20 kg pigs, the only catheter placement in the hepatic veins is the “proximal” site and the blood vessels at the intermediate and distal sites cannot be effectively examined, because they are too small for balloon catheter. Therefore, we employed larger pigs (40–65 kg) in the current study and assessed the effect of various parameters on gene delivery efficiency and potential tissue damage. We demonstrate that optimal gene delivery can be achieved by sequential injection of individual lobes by lodging a balloon catheter in the intermediate site of the hepatic vein at an intravascular pressure of 150 mmHg and a total volume of approximately 4% body weight shown in Figures 2 and 3. We also demonstrate that the same procedure repeatedly applied in the same animals with identical plasmids results in long-term gene expression in hepatocytes for at least 2 months without noticeable adverse effects (Figure 6).
Our results indicate that insertion and placement of the catheter under fluoroscopic image are important in the impact of injection, the efficiency of gene delivery, and the safety of the procedure. Further optimization of the injection volume achieved sequential lobe-specific hydrodynamic gene delivery targeting the entire liver with no evident plasmid in other organs (Figure 7). Serum levels of aspartate aminotransferase, alanine aminotransferase, and lactate dehydrogenase peaked at 24 hours and returned to normal ranges 4 days after the injection, as previously reported.9,10 The level of the elevation was lower than that of hydrodynamic gene delivery targeting the entire liver with IVC-clumped,12 and that of pseudo-hydrodynamic injection of helper- dependent adenoviral vectors with IVC-occlusion.19 Therefore, our procedure of catheter-based, lobe-specific hydrodynamic gene delivery is more desirable for clinical application.
Our long-term study shows approximately 10% of the hAAT-positive hepatocytes 2 months after gene transfer. The level was the same as positive hepatocytes 4 months after the injection in mice reported by Alino et al. using a pTG7101 plasmid containing the full length of hAAT.20 If the same level of efficiency of gene delivery can be achieved in patients with α-1 antitrypsin deficiency, we expect that the amount of normal hAAT protein in hepatocytes will compete with the abnormal mutated gene product for transcription and translation. Hence, it will result in a decrease of the accumulation of abnormal hAAT protein in hepatocytes, thereby reverting liver injury in hAAT-deficiency patients.21 Also, our successful hepatocyte-specific gene transfer suggests the promising option of transferring the gene expressing the protein that blocks the polymerization of mutant hAAT protein.21
It is important to note that our ELISA system was unable to detect hAAT in pig serum, whereas Alino et al. reported a serum level of 0.2 µg/ml of hAAT in pigs.10 They also observed a sustained therapeutic level at 1,000 µg/ml in mice23 using pTG7101, whereas we observed 100-fold lower levels in our study in mice.22 Therefore, the hAAT protein level in serum in this study using our plasmid might be below detection limit of ELISA (<1 ng/ml). This is likely due to the difference of the promoter, as TG7101 contains natural promoter for hAAT gene whereas our plasmid was driven by chicken β-actin promoter with CMV enhancer. Alternatively, it is also possible that the hAAT expressed are not secreted effectively by swine hepatocytes. Evidently, as serum hAAT is essential for the treatment of lung disease in hAAT deficiency,23 our work falls short in demonstrating therapeutic levels of hAAT, and additional studies are needed.
In summary, we identified factors affecting image-guided hydrodynamic gene delivery in human-size animals, and developed a clinically applicable procedure for liver-specific hydrodynamic gene delivery. Development of an automatic injector that can adjust injection pressure precisely to reproduce intravascular pressure and gene delivery efficiently may help physicians put this protocol into practice. Assessment of the applicability of this procedure in nonhuman primates that have more human-like livers is the next step toward clinical applications.
Materials and methods
Materials. The pCMV-Luc plasmid containing firefly luciferase cDNA driven by a CMV promoter and the pCAG-hAAT plasmid containing human α-1 antitrypsin gene driven by a chicken β-actin promoter with CMV enhancer, were purified by the CsCl-ethidiumbromide density gradient ultracentrifugation and kept in Tris-EDTA buffer. Purity of the plasmids was verified by absorbency at 260 and 280 nm and 1% agarose gel electrophoresis. The luciferase assay kit was from Promega (Madison, WI). The introducer and the 12 Fr sheath for image-guided catheter insertion were from COOK (Bloomington, IN) and the guide wire (ZIP wire) was from Boston Scientific (Natick, MA). Injection balloon catheters (10.5 Fr) were purchased from the Clinical Supply Co. (Kakamikahara, Gifu, Japan). The MIKRO TIP catheter transducer was from Millar (Houston, TX). Contrast medium (OXILAN) was from Guerbet (Bloomington, IN). WavelineVet Vital Signs Monitor for monitoring physiological parameters on animals was from DRE Veterinary (Louisville, KY). Pigs (female, 40–45 kg) were from Wally Whippo (Enon Valley, PA).
Animal catheterization. All animal experiments were conducted in full compliance with regulation of and approved by the Institutional Animal Care and Use Committee at the University of Pittsburgh, Pittsburgh, Pennsylvania. Under general anesthesia, animals were placed on the table of the fluoroscopy machine YSF-100 from Shimadzu Corp. (Nakagyo-ku, Kyoto, Japan). A 12 Fr short sheath was inserted in the jugular vein through a skin incision on the pigs. Following an insertion of the injection catheter, the balloon was inflated by injecting 1.5 ml of phase contrast medium. Obstruction of blood flow was verified by injecting a small volume of phase contrast medium into the vasculature through the catheter.
Hydrodynamic injection. Injections of saline containing either pCMV-Luc or pCAG-hAAT plasmid DNA (100 µg/ml), or phase contrast medium via the catheter were given to total of 42 pig livers by the HydroJector driven by the pressure provided by a gas (CO2) cylinder with its pressure regulator set at 300 psi.7,8 The venography was recorded using a VCR recorder connected to a fluoroscope. Intravascular pressure was transmitted to the computer using a MIKRO TIP catheter transducer inserted through the catheter to the hepatic veins. At the end of injection, the balloon remained inflated for 20 minutes and then slowly deflated. The sequential injection to multiple hepatic veins was performed with a 30 minutes interval in between. Animals received a postoperative analgesia with ketoprofen for 24 hours and an antibiotic with amoxicillin for 5 days.
Measurement of liver lobe volume. The RLL, RML, LML, and LLL in the pig liver was macroscopically identified and divided using deep interlobular and umbilical fissures according to segmental anatomy.15,24 The CL was identified by the presence of the small fissure adjoining the RLL on the visceral surface according to previously reported method.15 Three livers not injected with DNA were used to measure the volume of the liver lobes using water replacement method.25 Average lobe volume of RLL, RML, and LLL were 0.54, 0.6, and 0.4% BW, respectively (liver density: ~1.00 kg/l).26 The volume of each liver lobe for all animals used in the study was measured upon the euthanasia and the results coincided with predicted liver/body weight ratio.
Analysis of luciferase gene expression. Animals were euthanized and the liver was collected 24 hours after the hydrodynamic gene delivery of the pCMV-Luc plasmid. The luciferase and protein assays were performed according to the previously established procedure.3 The level of reporter gene expression was obtained as the relative light units (RLU) converted to luciferase protein (ng) per mg of total proteins in tissue homogenate, using the equation derived from the standard curve in which luciferase protein (pg) = 7.98 × 10-5 × RLU + 0.093 (R2 = 0.9999).3 A standard immunohistochemical staining was performed with a goat anti-luciferase polyclonal antibody (G7451, 1:50 dilution; Promega, Madison, WI), Vecstatin Elite ABC Goat IgG Kit (PK–6105; Vector Laboratories, Burlingame, CA), and DAB chromogen tablet (Muto Pure Chemicals CO, Bunkyo-ku, Tokyo, Japan) for luciferase staining. Microscopic examination was performed and photo images were recorded using an imaging system (AxioImager A1, Carl Zeiss Microscopy GmbH, Jena, Germany).
Persistent transgene expression in hepatocytes. Pigs were euthanized on day 63 or 105 after the first hydrodynamic gene delivery of pCAG-hAAT, and liver samples were collected from various lobes for hAAT expression analysis. Ten liver tissue samples were collected from all lobes, and standard immunohistochemical staining was performed with a rabbit anti–hAAT antibody (ab9373, 1:20 dilution; Abcam, Cambridge, MA). Every three fields from each section were captured and a quantitative analysis of positively stained cells was performed using Image J software (version 1.6.0_20, National Institutes of Health) as previously reported.16
Genomic DNA isolation and PCR analysis. Sections of the livers, spleens, kidneys, brains, hearts, stomachs, colons, ovaries, lungs, muscles, and small intestines were collected and DNA was isolated using phenol–chloroform extraction. Multiplex PCR was carried out on the samples and as well as a vector DNA with Ampicillin resistance gene primers and glyceraldehyde 3-phosphate dehydrogenase (Gapdh) gene as a reference. 2 µl of sample were amplified in a 20 µl PCR reaction at the program for 10 minutes at 94 °C, and 35 cycles for 1 minute at 94 °C, 1 minute at 56 °C, 1 minute at 72 °C, followed by a 10-minute extension at 72 °C. The primers used in PCR analysis were: Gapdh (forward), AGGTCGGAGTGAACGGATTTG; Gapdh (reverse), TGTAGACCATGTAGTGGAGGTCA; Amp (forward), TACAGGCATCGTGGTGTCAC; and Amp (reverse), AAATGTGCGCGGAACCCCTA.
Assessment of tissue damage. Blood samples were collected from the ear or peripheral vein in the limb from pigs at appropriate time points. Automated concentration determination was performed by the Cleveland Office of Marshfield Labs. Hematoxylin & eosin staining was performed on the liver tissues in the pathology lab of the Department of Pathology, University of Pittsburgh.
Statistical analysis. The luciferase assays were statistically evaluated by the analysis of variance followed by Bonferronis multiple comparison test.
SUPPLEMENTARY MATERIAL Figure S1.Level of gene expression in left medial lobe. Figure S2.Effect of repeat hydrodynamic injection on physiological parameters.
Acknowledgments
The authors would like to thank Michael Maranowski, Christopher Janssen and all staff members in the Division of Laboratory Animal Resources at the University of Pittsburgh for the excellent assistance in animal care. The authors would also like to thank Miss Ryan Fugett for the critical reading of the manuscript and English language review. The authors declare that they have no conflict of interest. The research in the authors' laboratory has been supported in part by grant support from the National Institute of Health (EB002946 and HL075542) to LD, and from the Japanese Society for the Promotion of Sciences (22890064 and 23790595), Takeda foundation, and Uehara Foundation to KK.
Supplementary material
Level of gene expression in left medial lobe.
Effect of repeat hydrodynamic injection on physiological parameters.
References
- Kamimura K, Liu D. Physical approaches for nucleic acid delivery to liver. AAPS J. 2008;10:589–595. doi: 10.1208/s12248-008-9067-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamimura K, Suda T, Zhang G, Liu D. Advances in gene delivery systems. Pharmaceut Med. 2011;25:293–306. doi: 10.2165/11594020-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Song Y, Liu D. Hydrodynamics-based transfection in animals by systemic administration of plasmid DNA. Gene Ther. 1999;6:1258–1266. doi: 10.1038/sj.gt.3300947. [DOI] [PubMed] [Google Scholar]
- Zhang G, Budker V, Wolff JA. High levels of foreign gene expression in hepatocytes after tail vein injections of naked plasmid DNA. Hum Gene Ther. 1999;10:1735–1737. doi: 10.1089/10430349950017734. [DOI] [PubMed] [Google Scholar]
- Suda T, Liu D. Hydrodynamic gene delivery: its principles and applications. Mol Ther. 2007;15:2063–2069. doi: 10.1038/sj.mt.6300314. [DOI] [PubMed] [Google Scholar]
- Herweijer H, Wolff JA. Gene therapy progress and prospects: hydrodynamic gene delivery. Gene Ther. 2007;14:99–107. doi: 10.1038/sj.gt.3302891. [DOI] [PubMed] [Google Scholar]
- Suda T, Suda K, Liu D. Computer-assisted hydrodynamic gene delivery. Mol Ther. 2008;16:1098–1104. doi: 10.1038/mt.2008.66. [DOI] [PubMed] [Google Scholar]
- Kamimura K, Suda T, Xu W, Zhang G, Liu D. Image-guided, lobe-specific hydrodynamic gene delivery to swine liver. Mol Ther. 2009;17:491–499. doi: 10.1038/mt.2008.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshino H, Hashizume K, Kobayashi E. Naked plasmid DNA transfer to the porcine liver using rapid injection with large volume. Gene Ther. 2006;13:1696–1702. doi: 10.1038/sj.gt.3302833. [DOI] [PubMed] [Google Scholar]
- Aliño SF, Herrero MJ, Noguera I, Dasí F, Sánchez M. Pig liver gene therapy by noninvasive interventionist catheterism. Gene Ther. 2007;14:334–343. doi: 10.1038/sj.gt.3302873. [DOI] [PubMed] [Google Scholar]
- Eastman SJ, Baskin KM, Hodges BL, Chu Q, Gates A, Dreusicke R, et al. Development of catheter-based procedures for transducing the isolated rabbit liver with plasmid DNA. Hum Gene Ther. 2002;13:2065–2077. doi: 10.1089/10430340260395910. [DOI] [PubMed] [Google Scholar]
- Fabre JW, Grehan A, Whitehorne M, Sawyer GJ, Dong X, Salehi S, et al. Hydrodynamic gene delivery to the pig liver via an isolated segment of the inferior vena cava. Gene Ther. 2008;15:452–462. doi: 10.1038/sj.gt.3303079. [DOI] [PubMed] [Google Scholar]
- Herrero MJ, Sabater L, Guenechea G, Sendra L, Montilla AI, Abargues R, et al. DNA delivery to ‘ex vivo' human liver segments. Gene Ther. 2012;19:504–512. doi: 10.1038/gt.2011.144. [DOI] [PubMed] [Google Scholar]
- Khorsandi SE, Bachellier P, Weber JC, Greget M, Jaeck D, Zacharoulis D, et al. Minimally invasive and selective hydrodynamic gene therapy of liver segments in the pig and human. Cancer Gene Ther. 2008;15:225–230. doi: 10.1038/sj.cgt.7701119. [DOI] [PubMed] [Google Scholar]
- Court FG, Wemyss-Holden SA, Morrison CP, Teague BD, Laws PE, Kew J, et al. Segmental nature of the porcine liver and its potential as a model for experimental partial hepatectomy. Br J Surg. 2003;90:440–444. doi: 10.1002/bjs.4053. [DOI] [PubMed] [Google Scholar]
- Vrekoussis T, Chaniotis V, Navrozoglou I, Dousias V, Pavlakis K, Stathopoulos EN, et al. Image analysis of breast cancer immunohistochemistry-stained sections using ImageJ: an RGB-based model. Anticancer Res. 2009;29:4995–4998. [PubMed] [Google Scholar]
- The Journal of Gene Medicine. Gene therapy clinical trials worldwide. http://www.wiley.com/legacy/wileychi/genmed/clinical/ ( Accessed on 27 February 2013).
- Kamimura K, Zhang G, Liu D. Image-guided, intravascular hydrodynamic gene delivery to skeletal muscle in pigs. Mol Ther. 2010;18:93–100. doi: 10.1038/mt.2009.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunetti-Pierri N, Stapleton GE, Palmer DJ, Zuo Y, Mane VP, Finegold MJ, et al. Pseudo-hydrodynamic delivery of helper-dependent adenoviral vectors into non-human primates for liver-directed gene therapy. Mol Ther. 2007;15:732–740. doi: 10.1038/sj.mt.6300102. [DOI] [PubMed] [Google Scholar]
- Aliño SF, Crespo A, Dasí F. Long-term therapeutic levels of human alpha-1 antitrypsin in plasma after hydrodynamic injection of nonviral DNA. Gene Ther. 2003;10:1672–1679. doi: 10.1038/sj.gt.3302065. [DOI] [PubMed] [Google Scholar]
- Fairbanks KD, Tavill AS. Liver disease in alpha 1-antitrypsin deficiency: a review. Am J Gastroenterol. 2008;103:2136–41; quiz 2142. doi: 10.1111/j.1572-0241.2008.01955.x. [DOI] [PubMed] [Google Scholar]
- Zhang G, Song YK, Liu D. Long-term expression of human alpha1-antitrypsin gene in mouse liver achieved by intravenous administration of plasmid DNA using a hydrodynamics-based procedure. Gene Ther. 2000;7:1344–1349. doi: 10.1038/sj.gt.3301229. [DOI] [PubMed] [Google Scholar]
- Flotte TR, Mueller C. Gene therapy for alpha-1 antitrypsin deficiency. Hum Mol Genet. 2011;20 R1:R87–R92. doi: 10.1093/hmg/ddr156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couinaud C. Liver anatomy: portal (and suprahepatic) or biliary segmentation. Dig Surg. 1999;16:459–467. doi: 10.1159/000018770. [DOI] [PubMed] [Google Scholar]
- Heymsfield SB, Fulenwider T, Nordlinger B, Barlow R, Sones P, Kutner M. Accurate measurement of liver, kidney, and spleen volume and mass by computerized axial tomography. Ann Intern Med. 1979;90:185–187. doi: 10.7326/0003-4819-90-2-185. [DOI] [PubMed] [Google Scholar]
- Yuan D, Lu T, Wei YG, Li B, Yan LN, Zeng Y, et al. Estimation of standard liver volume for liver transplantation in the Chinese population. Transplant Proc. 2008;40:3536–3540. doi: 10.1016/j.transproceed.2008.07.135. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Level of gene expression in left medial lobe.
Effect of repeat hydrodynamic injection on physiological parameters.