| Title: | 2′-Substituted Carba-Nucleoside Analogues for Antiviral Treatment | ||
| Patent Application Number: | WO 2013/138236 A1 | Publication date: | 19 September 2013 |
| Priority Application: | US 61/610,411 | Priority date: | 13 March 2012 |
| Inventors: | Clarke, M. O. H. | ||
| Assignee Company: | Gilead Sciences, Inc.; 333 Lakeside Drive, Foster City, CA 94404, USA | ||
| Disease Area: | Influenza (Flu) | Biological Target: | Influenza RNA-dependent RNA polymerase (RdRp) |
| Summary: | The invention in this patent application relates to 2′-substituted carba-nucleoside analogues represented generally by formula (I). These compounds are inhibitors of RNA-dependent RNA polymerase (RdRp) of the Orthomyxoviridae family of viruses that include influenza A and B viruses and may potentially provide a treatment for influenza infections. | ||
| The influenza virus is a single-strand, negative-sense, segmented RNA virus of the Orthomyxovirus family that uses RNA-dependent RNA polymerase (RdRp) to synthesize the viral RNAs needed for replication. Some new anti-influenza agents such as the experimental drug favipiravir and the compounds of formula (I) described in this patent application introduce a promising novel mechanism of action for the treatment of influenza infections. These compounds act by inhibiting the action of influenza RdRp and target the virus replication process to stop or slow down its replication. This may potentially provide better alternative treatment for influenza virus infections with low potential for emergence of drug resistance. | |||
| Important Compound Classes: | 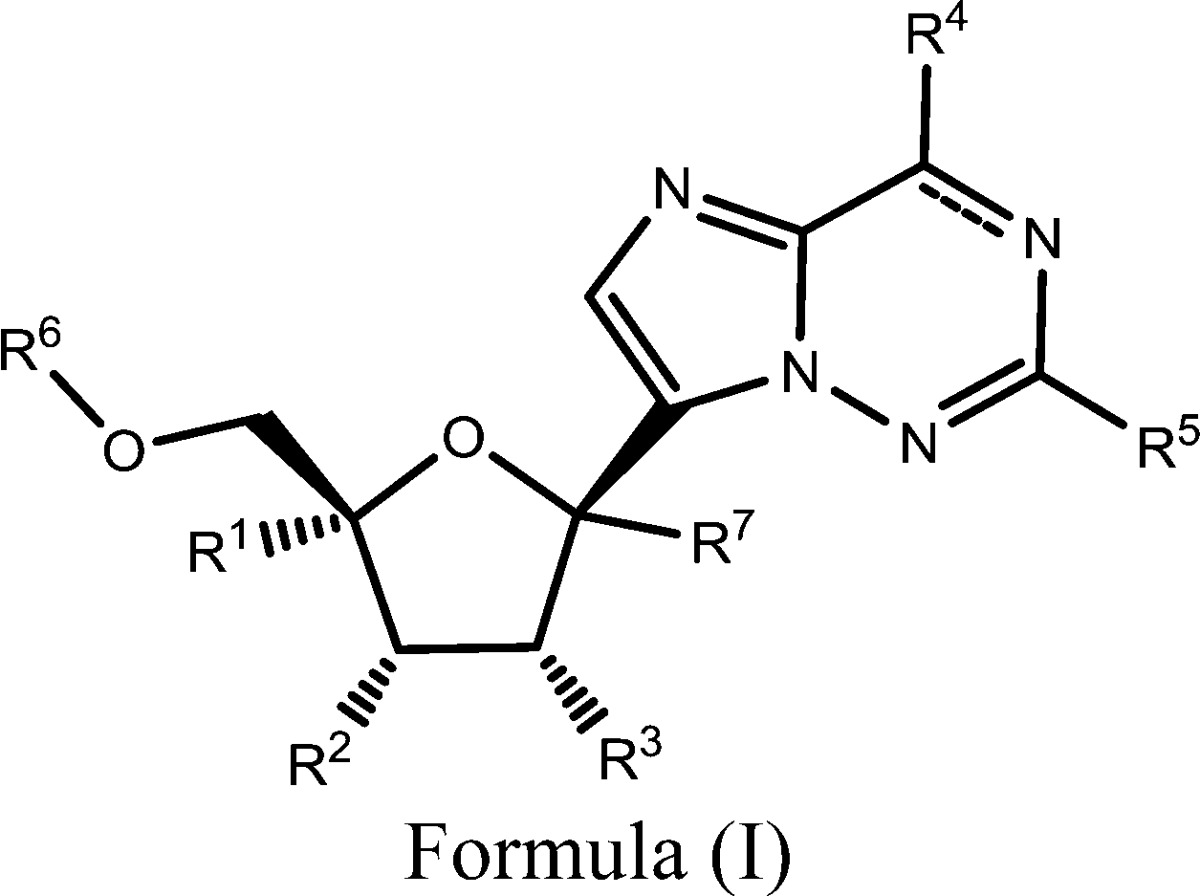 |
||
| Key Structures: | Representative examples of formula (I) compounds: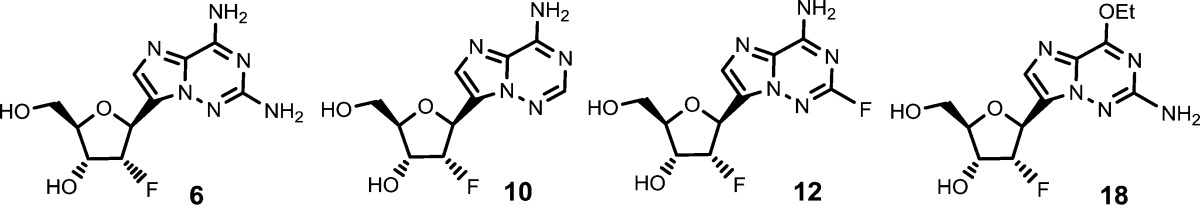
|
||
| Biological Assay: |
|
||
| Biological Data: | Data from normal human bronchial/tracheal epithelial cell influenza infection assay for compounds 6, 10, 12, and 18 (structures above)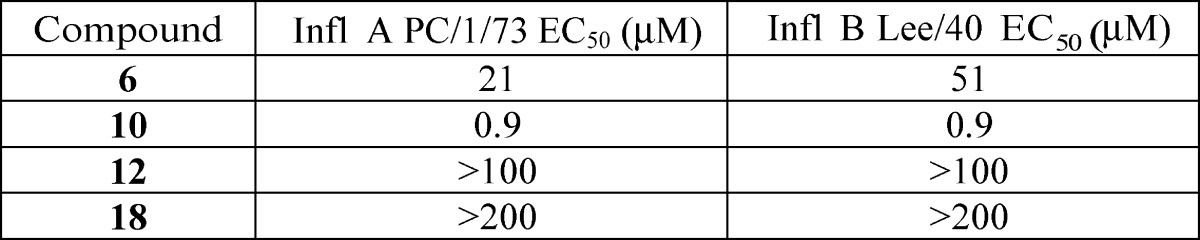
|
||
| Additional Information: | The most severe influenza infections are caused by type A virus. Influenza A virus infects humans and other species such as birds and pigs; it has caused all known pandemics and most epidemics. Type A virus undergoes sudden genetic changes called antigenic shifts associated with changes in the hemagglutinin (H) and neuraminidase (N) proteins, and such changes introduce new strains of the virus. Scientists have identified 15 hemagglutinin (H1 to H15) and 9 neuraminidase (N1 to N9) subtypes on type A influenza virus. The type A virus is named according to the hemagglutinin and neuraminidase subtypes, e.g., H1N1, H1N2, H3N2, etc. | ||
| Influenza is a highly contagious acute respiratory infection that infects 10–20% of the population annually. It is associated with significant morbidity and mortality in high-risk patient populations and responsible for >200,000 hospitalizations and 20,000–35,000 deaths annually in the US alone. Globally it results in 250,000–500,000 deaths annually, and it causes major pandemics. The most devastating is the 1918–19 pandemic (Spanish Flu) caused by H1N1 virus and resulted in an estimated 50–100 million deaths. | |||
| Available influenza therapy | |||
| |||
| Some experimental influenza treatments | |||
| |||
| Recent Review Articles: | 1. Seo S.; Englund J. A.; Nguyen J. T.; Pukrittayakamee S.; Lindegardh N.; Tarning J.; Tambyah P. A.; Renaud C.; Went G. T.; de Jong M. D.; Boeckh M. J.. Antiviral Ther. 2013, 18 (3), 377–386. | ||
| 2. Hedlund M.; Larson J. L.; Fang F.. Viruses 2010, 2, 1766–1781. | |||
| 3. Reviriego C.Drug Future 2010, 35 (9), 713–717. | |||
| 4. Smee D. F.; Hurst B. L.; Wong M. H.; Bailey K. W.; Tarbet E. B.; Morrey J. D.; Furuta Y.. Antimicrob. Agents Chemother. 2010, 54, 126–133. | |||
| 5. Malakhov M. P.; Aschenbrenner L. M.; Smee D. F.; Wandersee M. K.; Sidwell R. W.; Gubareva L. V.; Mishin V. P.; Hayden F. G.; Kim D. H.; Ing A.; Campbell E. R.; Yu M.; Fang F.. Antimicrob. Agents Chemother. 2006, 50 (4), 1470–1479. | |||
The authors declare no competing financial interest.


