Abstract
The ρ1 GABAC receptor is a ligand-gated chloride ion channel that shows promise as a therapeutic target for myopia, sleep disorders, memory and learning facilitation, and anxiety-related disorders. As such, there is a need for molecular probes to understand the role GABAC receptors play in physiological and pathological processes. To date, no labeled (either radioactive or fluorescent) GABAC selective ligand has been developed that can act as a marker for GABAC receptor visualization and localization studies. Herein, we report a series of fluorescent ligands containing different-sized linkers and fluorophores based around (S)-4-ACPBPA [(4-aminocyclopenten-1-yl)-butylphosphinic acid], a selective GABAC antagonist. One of these conjugates, (S)-4-ACPBPA-C5-BODIPY (13), displayed moderate potency (IC50 = 58.61 μM) and selectivity (>100 times) for ρ1 over α1β2γ2L GABAA receptors. These conjugates are novel lead agents for the development of more potent and selective fluorescent probes for studying the localization and function of GABAC receptors in living cells.
Keywords: Human ρ1 GABAC receptors, CNS-related disorders, GABAC antagonists, fluorescent and biotinylated probes, homology modeling and docking
γ-Aminobutyric acid 1 [GABA (Figure 1)] is the most abundant inhibitory neurotransmitter in the mammalian central nervous system (CNS) and is essential for the overall balance between neuronal excitation and inhibition.1,2 GABA released from GABAergic axon terminals influences neurons via GABAA, GABAB, and GABAC (GABAρ) receptors. These receptors are grouped on the basis of their subunit composition, gating properties, and pharmacological profiles. GABAA and GABAC receptors are ligand-gated chloride ion channels that mediate fast synaptic inhibition when activated by GABA,1 whereas GABAB receptors are G-protein-coupled receptors that mediate slow, longer-lasting synaptic inhibition by increasing potassium and decreasing calcium conductances when activated by GABA.3
Figure 1.
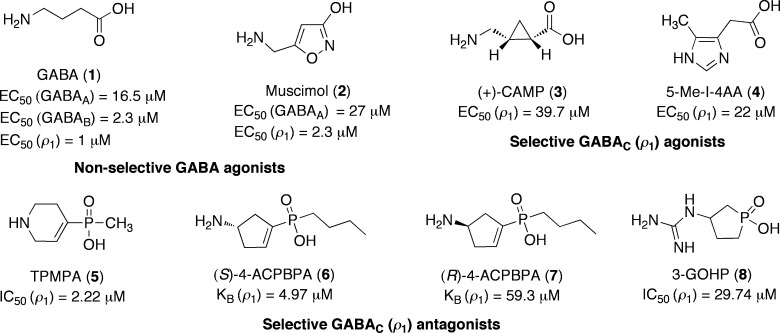
Structures of GABA (1), the GABAA agonist and GABAC partial agonist muscimol (2), the GABAC selective agonists (+)-CAMP (3) and 5-Me-I-4AA (4), and GABAC selective antagonists TPMPA (5), (S)-4-ACPBPA (6), (R)-4-ACPBPA (7), and 3-GOHP (8).
Ionotropic GABAA receptors are transmembrane protein complexes composed of five heteropentameric subunits. To date, 16 human GABAA receptor subunits have been identified and classified as α (α1–α6), β (β1–β3), γ (γ1–γ3), δ, ε, π, and θ.4 In contrast, ionotropic GABAC receptors have pharmacology, physiology, and subunit compositions distinct from those of GABAA.5,6 In mammals, GABAC receptors are composed of ρ subunits (ρ1–ρ3), forming homopentameric assemblies of five ρ subunits (ρ1, ρ2, or ρ3 subunits) or pseudoheteropentameric complexes comprising different ρ subunits (a combination of ρ1 and ρ2 or ρ2 and ρ3 subunits).5,7,8 GABAC receptors are insensitive to bicuculline and (−)-baclofen, selectively activated by (+)-CAMP 3 [(+)-cis-2-(aminomethyl)cyclopropanecarboxylic acid (Figure 1)] and 5-Me-I-4AA 4 (5-methyl-1H-imidazole-4-acetic acid), and inhibited by TPMPA 5 (1,2,5,6-tetrahydropyridine-4-yl-methyl-phosphinic acid), (S)-4-ACPBPA 6 {[(S)-4-aminocyclopenten-1-yl]-butylphosphinic acid}9 and 3-GOHP 8 [3-(guanido)-1-oxo-1-hydroxy-phospholane].10 This clearly indicates that the GABA binding sites of GABAC and GABAA receptors are not identical.
GABAA receptors are widely distributed in the CNS,1 whereas GABAC receptors are mainly expressed in the superior colliculus,11 cerebellum,12 lateral amyglada,13 hippocampus (strong ρ2 subunit expression),14 and, most prominently, the retina (strong ρ1 subunit expression).7 Various studies show that GABAC receptors are potential therapeutic targets for myopia, sleep disorders, managing memory-related disorders, and peripheral antinociception.5,13 However, understanding the role of GABAC receptors and information about the processes triggered by ligand–receptor interactions in living cells are still limited because of the lack of pharmacological tools.
Fluorescent ligands have proven to be useful tools offering a wealth of information about the mapping or identification of ligand binding sites,15,16 the mechanism of ligand binding,17 the physical nature of the binding pocket, the movement and internalization of receptors in living cells,18 and the localization as well as visualization of the labeled receptors.19 Biotinylated probes are useful for the isolation and purification of target proteins of the receptor fragment for crystallization and X-ray studies of the particular receptor-binding site,20 the localization and visualization of the receptor by using antibodies,21 and the study of ligand–protein interactions in living cells.22
In addition, fluorescent and biotinylated probes represent a faster, safer, and less expensive alternative to radioligands in receptor studies, circumventing several drawbacks associated with radioligand studies such as health and safety concerns and the need for a large number of cells with strong receptor expression.23 Thus, the identification of fluorescent or biotinylated ligands for GABAC receptors is of great interest. The development of these tagged ligands usually starts with the selection of a potent and selective pharmacophore, which is then conjugated with the desired tag through an appropriate linker.
To date, only a few fluorescent and biotinylated GABAB receptor antagonist ligands have been reported,24 and N-acylation of the amino moiety of muscimol (2), a widely used GABAA agonist that it is also a potent partial agonist at human ρ1 GABAC receptors with fluorophores, has been shown to result in ligands that bind to the GABAA receptors.25 Vu et al. presented evidence that muscimol linked through a 6-aminohexanoyl chain to biotin (muscimol-biotin; EC50 = 20 μM) and BODIPY (muscimol-BODIPY) retained substantial agonist activity compared to muscimol (2; EC50 = 2 μM) at GABAC receptors,26 indicating a steric tolerance in the region around the ligand’s amine binding site of the GABAC receptor. To the best of our knowledge, no selective fluorescent ligand for GABAC receptors has been described in the literature. Therefore, we envisioned that incorporating fluorophores or the versatile biotin moiety into selective GABAC ligands could lead to selective probes. Herein, we report the design, synthesis, and pharmacological evaluation of the selective fluorescent and biotinylated probes for ρ1 GABAC receptors.
We selected two of our previously reported selective and potent GABAC antagonists, (S)-4-ACPBPA 6 and (R)-4-ACPBPA 7 (Figure 1), for the design of the fluorescent GABAC antagonists.9 Both were synthesized according to our previously published protocol.9 The length of the spacer chain is known to significantly impact the affinity of ligand–fluorophore conjugates for the receptors; therefore, we chose a linker size that varied from 0 to 10 carbons to introduce molecular flexibility into the conjugated fluorescent ligand. We incorporated fluorescent groups with relatively small to large molecular volumes to study the effect of perturbation on receptor affinity. We used the fluorophores NMA (N-methylanthranilic acid), BODIPY (4,4-difluoro-4-bora-3a,4a-diaza-s-indacene), and NBD (7-nitrobenz-2-oxa-1,3-diazol-4-yl) chloride, which are known to have different excitation wavelengths and distinctive properties, to diversify fluorescence experiments. BODIPY has been extensively utilized for the development of fluorescent probes because of its distinctive and useful features, including hydrophobicity, photochemical stability, insensitivity to changes in experimental conditions, efficient uptake into cell membranes, and high extinction coefficient and fluorescence quantum yield.27 Initially, we synthesized fluorescent probes using the potent and selective GABAC antagonist (S)-4-ACPBPA 6 by linking the small fluorophore NMA through linkers of various sizes.
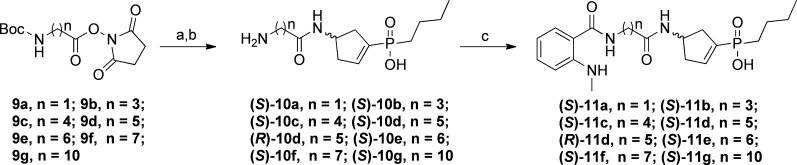
The synthesis of (S)-4-ACPBPA-NMA- and (R)-4-ACPBPA-C5-NMA-conjugated target compounds (S)-11a–g and (R)-11d is depicted in Scheme 1. N-Succinimidyl esters 9a–g were prepared by protection of the amine function of the amino acids with a tert-butyloxycarbonyl group (Boc), followed by activation of the acid function of the amino acids with an N-hydroxysuccinimide (NHS) (see the Supporting Information for the experimental details). The activated N-succinimidyl esters 9a–g were further reacted with the GABAC antagonist (S)-4-ACPBPA 6 in an aqueous NaHCO3 solution at room temperature, and subsequent removal of the Boc group using TFA in CH2Cl2 to afford compounds 10a–g in moderate to good yields. Target compounds (S)-11a–g were obtained by amide coupling of compounds (S)-10a–g with NHS-activated fluorophore NMA (NMA N-succinimidyl ester) in an aqueous NaHCO3 solution at room temperature. (R)-10d was obtained from 9d as described above for the preparation of (S)-10d. (R)-11d was synthesized by reaction of (R)-4-ACPBPA 7 with the previously prepared ester (R)-10d under basic conditions at room temperature.
Scheme 1. Synthesis of Fluorescent ρ1 GABAC Antagonists (S)-11a–g and (R)-11d.
Reagents and conditions: (a) (S)-4-ACPBPA 6 or (R)-4-ACPBPA 7 [for (R)-10d], NaHCO3, water/DME/THF (1:1:0.3), room temperature for 16 h, 81–89%; (b) TFA/DCM (1:1), room temperature for 1–2 h, 92–98%; (c) NMA N-succinimidyl ester, NaHCO3, water/DME/THF (1:1:0.3), room temperature for 16 h, 75–84%.
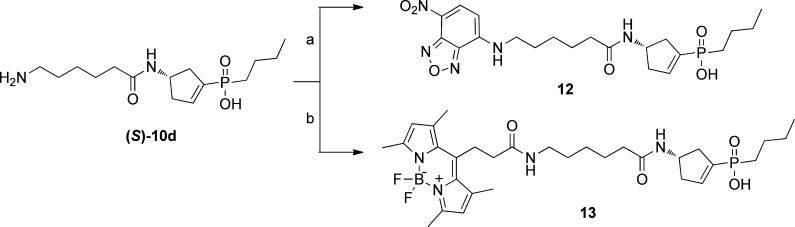
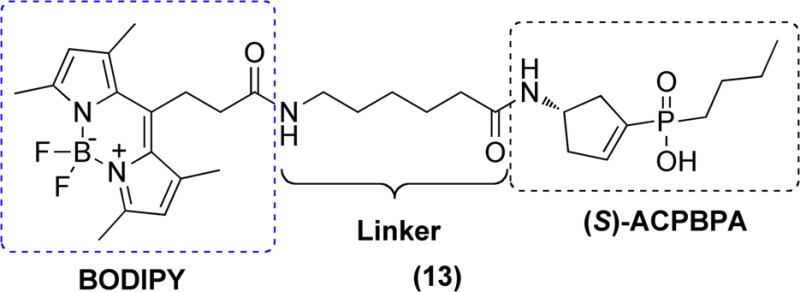
Fluorescent compounds 12 and 13 containing the NBD and BODIPY moieties were prepared as depicted in Scheme 2. Condensation of 7-nitrobenz-2-oxa-1,3-diazol-4-yl chloride (NBD chloride) with (S)-10d in a K2CO3/DMF mixture at room temperature afforded target compound 12 in moderate yield. The synthesis of BODIPY N-succinimidyl ester (see the Supporting Information for the experimental details) was achieved in two steps according to a previously described method.28 Compound 13 was obtained in moderate yield by reaction of BODIPY succinimidyl ester and the primary amine of (S)-10d in a DIPEA/DMF mixture at room temperature.
Scheme 2. Synthesis of Fluorescent ρ1 GABAC Antagonists 12 and 13.
Reagents and conditions: (a) 7-nitrobenz-2-oxa-1,3-diazol-4-yl chloride (NBD-Cl), K2CO3, DMF, room temperature for 24 h, 76%; (b) BODIPY N-succinimidyl ester, DIPEA, DMF, room temperature for 24 h, 72%.
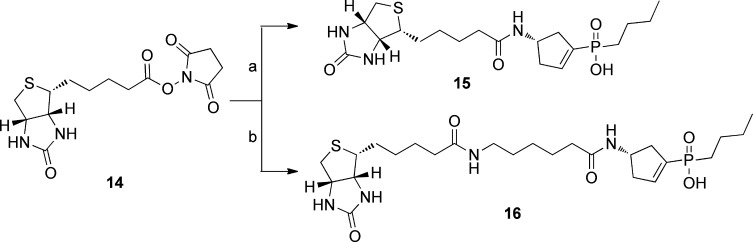
Biotinylated conjugates 15 and 16 were prepared according to Scheme 3. Formation of an amide bond between biotin N-succinimidyl ester 14 and the corresponding primary amine of (S)-4-ACPBPA 6 and compound (S)-10d in a DIPEA/DMF mixture at room temperature afforded biotinylated conjugates 15 and 16, respectively.
Scheme 3. Synthesis of Biotinylated ρ1 GABAC Antagonists 15 and 16.
Reagents and conditions: (a) (S)-4-ACPBPA 6, DIPEA, DMF, room temperature for 24 h, 75%; (b) (S)-10d, DIPEA, DMF, room temperature for 24 h, 81%.
We examined the functional characterization of fluorescent and biotinylated probes on human recombinant GABA receptors expressed in Xenopus oocytes using the two-electrode voltage clamp method (Table 1).9,10 All 12 probes [(S)-11a–g, (R)-11d, 12, 13, 15, and 16] were evaluated for activity alone and in the presence of GABA on GABAA (α1β2γ2L), and ρ1 GABAC receptors to determine whether they behave as agonists, antagonists, or modulators.
Table 1. Pharmacological Evaluation of Fluorescent and Biotinylated Probesa.
| human ρ1 GABAC receptor
% inhibition and IC50 μM (95% confidence interval) |
||||
|---|---|---|---|---|
| compound | spacer | 300 μM | 600 μMd | human α1β2γ2L GABAA receptor % inhibition at 300 μM |
| (S)-4-ACPBPA (6) | 97 ± 3%b | inactiveg | ||
| IC50 = 9.76, KB = 4.97 μMc | ||||
| (R)-4-ACPBPA (7) | 92 ± 7%b | inactiveg | ||
| KB = 59.3 μMc | ||||
| (S)-4-ACPBPA-C1-NMA [(S)-11a] | 1 | 8 ± 2% | ||
| (S)-4-ACPBPA-C3-NMA [(S)-11b] | 3 | 23 ± 4% | ||
| (S)-4-ACPBPA-C4-NMA [(S)-11c] | 4 | 31 ± 2% | ||
| (S)-4-ACPBPA-C5-NMA [(S)-11d] | 5 | 62 ± 7% | inactiveg | |
| (R)-4-ACPBPA-C5-NMA [(R)-11d] | 5 | 56 ± 9% | inactiveg | |
| (S)-4-ACPBPA-C6-NMA [(S)-11e] | 6 | 55 ± 3% | ||
| (S)-4-ACPBPA-C7-NMA [(S)-11f] | 7 | 24 ± 8% | ||
| (S)-4-ACPBPA-C10-NMA [(S)-11g] | 10 | 8 ± 3% | ||
| (S)-4-ACPBPA-C5-NBD (12) | 5 | IC50 = 281.42 (274.96–332.11)e | inactiveg | |
| IC50 = 193.27 (181.67–224.81)f | ||||
| (S)-4-ACPBPA-C5-BODIPY (13) | 5 | IC50 = 103.14 (95.36–114.87)e | inactiveg | |
| IC50 = 58.61 (45.37–71.63)f | ||||
| (S)-4-ACPBPA-C0-biotin (15) | 0 | inactiveb | ||
| (S)-4-ACPBPA-C5-biotin (16) | 5 | IC50 = 147.92 (139.45–174.13)e | 9 ± 7%g | |
| IC50 = 76.54 (68.33–102.96)f | ||||
Determined electrophysiologically in Xenopus laevis oocytes expressing the human ρ1 GABAC receptor or human α1β2γ2L GABAA receptors as previously described.9,10
Activity or percent inhibition by 300 μM compound of the current produced by a submaximal concentration of GABA (1 μM, EC50).
Data from ref (9).
Percent inhibition by 600 μM compound of the current produced by a submaximal concentration of GABA (1 μM, EC50), with a 5 min preincubation.
IC50 values for compounds without preincubation.
IC50 values for compounds with a 5 min preincubation.
Activity or percent inhibition by 300 μM compound of the current produced by a submaximal concentration of GABA (30 μM, EC50) without and with a 5 min preincubation. All data are means ± the standard error of the mean (n = 3 oocytes).
During the initial pharmacological screening, we found that stable and reproducible levels of inhibition of GABA (1 μM) at ρ1 GABAC receptors were reached only after a 5 min preincubation with compounds (S)-11a–g and (R)-11d (see the Supporting Information for details). The preincubation needed for some of the compounds for reaching stable and reproducible levels of inhibition of GABA may be due to a molecule’s size and lipophilicity needing additional time to diffuse into the binding site. In the (S)-4-ACPBPA-NMA series where the NMA group was attached to the primary amine of the ligand through an aminoalkanoyl chain, we observed that compounds with a chain length of fewer than five carbons [compounds (S)-11a–c] exhibited weak antagonist activity, compared to compound (S)-11d with a five-carbon chain. The activity for compounds (S)-11e–g decreased with an increasing chain length. These compounds had a chain length of 6–10 carbons, indicating that a linker of five carbons is optimal. The decrease in activity for compounds (S)-11a and (S)-11b (spacer size of fewer than four carbons) could be explained by the relative rigidity caused by the amido-containing chain with the ligand moiety at the binding site. With spacer sizes of more than six carbons, the fluorescent tag may occupy a less favorable area, resulting in a decrease in inhibitory activity.
The optimal chain length of the linker is important for the introduction of molecular flexibility for more convenient positioning of the ligand into the receptor. After optimization of the chain length for fluorescent probes, we linked (R)-4-ACPBPA through a 6-aminohexanoyl chain to NMA [(R)-11d] and evaluated its activity on ρ1 GABAC receptors. (R)-4-ACPBPA 7 is 12-fold less potent than (S)-4-ACPBPA 6 at the ρ1 GABAC receptor (Figure 1).9 However, (R)-4-ACPBPA-C5-NMA (R)-11d exhibited activity almost equal to that of (S)-4-ACPBPA-C5-NMA (S)-11d, indicating N-acylation of the amine removed the differential activity of enantiomers.
We also evaluated NBD and BODIPY (12 and 13, respectively) containing probes on both ρ1 GABAC and α1β2γ2L GABAA receptors with and without preincubation (Table 1). (S)-4-ACPBPA-C5-NBD 12 exhibited poor potency (IC50 = 281.42 μM) when tested without preincubation, but its activity was slightly improved (IC50 = 193.27 μM) with a 5 min preincubation at ρ1 GABAC receptors. (S)-4-ACPBPA-C5-BODIPY 13 displayed moderate and improved potency (IC50 = 58.61 μM with preincubation, and IC50 = 103.14 μM without preincubation) at ρ1 GABAC receptors. The increase in activity for (S)-4-ACPBPA-C5-BODIPY 13 but not for (S)-4-ACPBPA-C5-NMA (S)-11d and (S)-4-ACPBPA-C5-NBD 12 indicates that bulkier fluorophores linked by a 6-aminohexanoyl chain are more favorable for activity. These results confirmed the prediction of molecular modeling studies that the large hydrophobic region of the receptor is located adjacent to the binding site of the basic amino group of (S)-4-ACPBPA. As expected, probes (S)-11d, (R)-11d, 12, and 13 were found to be inactive (at 300 μM) at α1β2γ2L GABAA receptors.
We also evaluated biotinylated probes of (S)-4-ACPBPA (compounds 15 and 16) on the ρ1 GABAC receptor. Biotin-coupled probes are suitable for subcellular localization of receptors because biotin forms a tight complex with strept(avidin),21 which can be visualized by confocal microscopy using antibodies. Direct attachment of the biotin group to (S)-4-ACPBPA was deleterious for activity, (compound 15 was inactive at 300 μM). However, a linker length of five carbons (compound 16) is sufficient to restore moderate activity (IC50 = 76.54 μM with preincubation, and IC50 = 147.92 μM without preincubation) at ρ1 GABAC receptors. These findings represent the first demonstration of electrophysiological activity by N-acyl derivatives of selective antagonists (S)-4-ACPBPA 6 and (R)-4-ACPBPA 7.
To identify structural determinants for the observed activity for the ρ1 GABAC receptor, we flexibly docked the structures of (S)-4-ACPBPA 6 and fluorescent and biotinylated probes (S)-4-ACPBPA-C5-NMA (S)-11d, (S)-4-ACPBPA-C5-NBD 12, (S)-4-ACPBPA-C5-BODIPY 13, and (S)-4-ACPBPA-C5-biotin 16 into the ligand-binding site of a ρ1 GABAC homology model.29 Figure 2B shows predicted binding modes for (S)-4-ACPBPA-C5-NMA (S)-11d, (S)-4-ACPBPA-C5-NBD 12, (S)-4-ACPBPA-C5-BODIPY 13, and (S)-4-ACPBPA-C5-biotin 16, which show modes of binding for probe-linked (S)-4-ACPBPA similar to that of parent (S)-4-ACPBPA 6 (Figure 2A). The binding affinity is largely ascribed to various electrostatic interactions,29−31 including (i) a salt bridge interaction between the phosphinic acid and Arg104 and Arg158,29,30 (ii) hydrogen bond contacts of the amine of parent ligand or amide of probes with Glu196,9 and (iii) hydrogen bond contacts with Thr244.31 The docked model of compounds 6, (S)-11d, 12, 13, and 16 (Figure 3) orients the (S)-4-ACPBPA moiety in the active site cavity of the ρ1 GABAC receptor (shown with wire mesh) and a flexible spacer between the (S)-4-ACPBPA core and probe where the probe is pointing out of or residing in the noninteractive binding site of the receptors, which cannot be ruled out at this stage. The fluorophores or biotin tags are shown by molecular modeling studies to reside in a pocket away from the orthosteric site or extend into the extracellular space.
Figure 2.
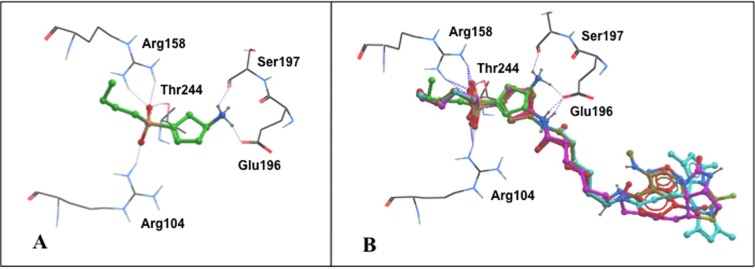
View of the ρ1 GABAC ligand-binding site with predicted binding modes. (A) Close-up of (S)-4-ACPBPA 6 (green carbons). (B) Close-up of (S)-4-ACPBPA-C5-NMA (S)-11d (brown carbons), (S)-4-ACPBPA-C5-NBD 12 (red carbons), (S)-4-ACPBPA-C5-BODIPY 13 (aqua blue carbons), and (S)-4-ACPBPA-C5-biotin 16 (magenta carbons) that are overlaid on (S)-4-ACPBPA 6 in the GABAC binding site, with the linker and fluorophores external to the orthosteric site.
Figure 3.
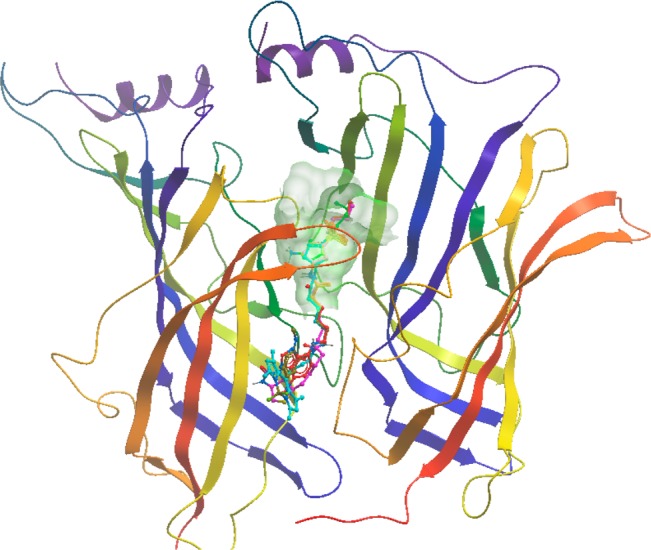
View of the (S)-4-ACPBPA moiety (wire mesh) in the active site cavity of the ρ1 GABAC receptor model and flexible spacer between the (S)-4-ACPBPA core and probes pointing away from the orthosteric site.
In conclusion, we have designed and synthesized for the first time fluorescent and biotinylated probes of selective antagonists for ρ1 GABAC receptors. Pharmacological studies show that 12, 13, and 16 exhibit activity similar to that of the previously reported muscimol-based probes,25,26 with the added advantage that they are selective GABAC receptors. These results offer new knowledge regarding the binding site and receptor flexibility of GABAC receptors. The described fluorescent and biotinylated probes will be useful tools for localizing, visualizing, and studying the physiopathological processes of GABAC receptors. Further optimization of probes at amino and phosphinic acid moieties of (S)-4-ACPBPA, in vitro pharmacological evaluation, and fluorescent spectroscopic characterizations of all the probes are underway.
Acknowledgments
We are thankful to Bruce Tattam and Dr. Keith Fischer for technical assistance with mass spectrometry. We are also thankful to Dr. Izumi Yamamoto, Dr. Nasiara Karim for injecting human ρ1, α1, β2, and γ2L mRNA into Xenopus oocytes for this study, and Dr. Heba Abdel-Halim for performing initial docking studies.
Supporting Information Available
Synthetic method, characterization of compounds, pharmacology, and molecular modeling. This material is available free of charge via the Internet at http://pubs.acs.org.
N.G. and H.-L.K. acknowledge support from an Endeavour International Postgraduate Research Scholarship (EIPRS) and a Faculty of Pharmacy Postgraduate Award, respectively. Both N.G. and H.-L.K. also acknowledge the John A. Lamberton Scholarship.
The authors declare no competing financial interest.
Supplementary Material
References
- Chebib M.; Johnston G. A. R. GABA-activated ligand gated ion channels: Medicinal chemistry and molecular biology. J. Med. Chem. 2000, 43, 1427–1447. [DOI] [PubMed] [Google Scholar]
- Minier F.; Sigel E.. Ligand-operated membrane channels: GABA. In Encyclopedia of Biological Chemistry; Lane W. L. M., Ed.; Elsevier: Oxford, U.K., 2004; Vol. 2. [Google Scholar]
- Marshall F. H.; Jones K. A.; Kaupmann K.; Bettler B. GABAB receptors: The first 7TM heterodimers. Trends Pharmacol. Sci. 1999, 20, 396–399. [DOI] [PubMed] [Google Scholar]
- Whiting P. J. GABA-A receptor subtypes in the brain: A paradigm for CNS drug discovery?. Drug Discovery Today 2003, 8, 445–450. [DOI] [PubMed] [Google Scholar]
- Ng C.; Kim H.-L.; Gavande N.; Yamamoto I.; Kumar R. J.; Mewett K. N.; Johnston G. A. R.; Hanrahan J. R.; Chebib M. GABAC Receptor Pharmacology and Implications for Myopia, Learning and Memory. Future Med. Chem. 2011, 3, 197–209. [DOI] [PubMed] [Google Scholar]
- Zhang J.; Xue F.; Chang Y. Structural determinants for antagonist pharmacology that distinguish the ρ1 GABAC receptor from GABAA receptors. Mol. Pharmacol. 2008, 74, 941–951. [DOI] [PubMed] [Google Scholar]
- Enz R.; Brandstaetter J. H.; Waessle H.; Bormann J. Immunocytochemical localization of the GABAC receptor ρ subunits in the mammalian retina. J. Neurosci. 1996, 16, 4479–4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogurusu T.; Yanagi K.; Watanabe M.; Fukaya M.; Shingai R. Localization of GABA receptor ρ2 and ρ3 subunits in rat brain and functional expression of homooligomeric ρ3 receptors and heterooligomeric ρ2ρ3 receptors. Recept. Channels 1999, 6, 463–475. [PubMed] [Google Scholar]
- Kumar R.; Chebib M.; Hibbs D.; Kim H.-L.; Johnston G.; Salam N.; Hanrahan J. Novel γ-Aminobutyric Acid ρ1 Receptor Antagonists; Synthesis, Pharmacological Activity and Structure-Activity Relationships. J. Med. Chem. 2008, 51, 3825–3840. [DOI] [PubMed] [Google Scholar]
- Gavande N.; Yamamoto I.; Salam N.; Ai T.-H.; Burden P.; Johnston G. A. R.; Hanrahan J. R.; Chebib M. Novel Cyclic Phosphinic acids as GABAC ρ Receptor Antagonists: Design, Synthesis and Pharmacology. ACS Med. Chem. Lett. 2011, 2, 11–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boue-Grabot E.; Roudbaraki M.; Bascles L.; Tramu G.; Bloch B.; Garret M. Expression of GABA receptor ρ subunits in rat brain. J. Neurochem. 1998, 70, 899–907. [DOI] [PubMed] [Google Scholar]
- Rozzo A.; Armellin M.; Franzot J.; Chiaruttini C.; Nistri A.; Tongiorgi E. Expression and dendritic mRNA localization of GABAC receptor ρ1 and ρ2 subunits in developing rat brain and spinal cord. Eur. J. Neurosci. 2002, 1747–1758. [DOI] [PubMed] [Google Scholar]
- Cunha C.; Monfils M.-H.; LeDoux J. E. GABAC receptors in the lateral amygdala: A possible novel target for the treatment of fear and anxiety disorders?. Front. Behav. Neurosci. 2010, 4, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alakuijala A. M.; Wegelius K.; Schmidt M.; Enz R.; Paulin L.; Saarma M.; Pasternack M. GABA receptor ρ subunit expression in the developing rat brain. Dev. Brain Res. 2005, 154, 15–23. [DOI] [PubMed] [Google Scholar]
- Turcatti G.; Nemeth K.; Edgerton M. D.; Knowles J.; Vogel H.; Chollet A. Fluorescent labeling of NK2 receptor at specific sites in vivo and fluorescence energy transfer analysis of NK2 ligand-receptor complexes. Recept. Channels 1997, 5, 201–207. [PubMed] [Google Scholar]
- Valloton P.; Tairi A. P.; Wohland T.; Friedrich-Benet K.; Pick H.; Hovius R.; Vogel H. Mapping the Antagonist Binding Site of the Serotonin Type 3 Receptor by Fluorescence Resonance Energy Transfer. Biochemistry 2001, 40, 12237–12242. [DOI] [PubMed] [Google Scholar]
- Tairi A. P.; Hovius R.; Pick H.; Blasey H.; Bernard A.; Surprenant A.; Lunstrom K.; Vogel H. Ligand binding to the serotonin 5HT3 receptor studied with a novel fluorescent ligand. Biochemistry 1998, 37, 15850–15864. [DOI] [PubMed] [Google Scholar]
- Terrillon S.; Cheng L. L.; Stoev S.; Mouillac B.; Barberis C.; Manning M.; Durroux T. Synthesis and characterization of fluorescent antagonists and agonists for human oxytocin and vasopressin V1a receptors. J. Med. Chem. 2002, 45, 2579–2588. [DOI] [PubMed] [Google Scholar]
- Macchia M.; Salvetti F.; Bertini S.; Di Bussolo V.; Gattuso L.; et al. 7-Nitrobenzofurazan (NBD) derivatives of 5′-N-ethylcarboxamidoadenosine (NECA) as new fluorescent probes for human A3 adenosine receptors. Bioorg. Med. Chem. Lett. 2001, 11, 3023–3026. [DOI] [PubMed] [Google Scholar]
- Froestl W.; Bettler B.; Bittiger H.; Heid J.; Kaupmann K.; Mickel S. J.; Strub D. Ligands for the isolation of GABAB receptors. Neuropharmacology 1999, 38, 1641–1646. [DOI] [PubMed] [Google Scholar]
- Laitinen O. H.; Hytonen V. P.; Nordlund H. R.; Kulomaa M. S. Genetically engineered avidins and streptavidins. Cell. Mol. Life Sci. 2006, 63, 2992–3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavoff S. A.; Saghatelian A. Discovering ligand-receptor interactions. Nat. Biotechnol. 2012, 30, 959–961. [DOI] [PubMed] [Google Scholar]
- McGrath J. C.; Arribas S. M.; Daly C. J. Fluorescent ligands for the study of receptors. Trends Pharmacol. Sci. 1996, 17, 393–399. [DOI] [PubMed] [Google Scholar]
- Froestl W. Chemistry and Pharmacology of GABA-B receptor ligands. Adv. Pharmacol. 2010, 58, 19–62. [DOI] [PubMed] [Google Scholar]
- Meissner O.; Haberlein H. Lateral mobility and specific binding to GABAA receptors on hippocampal neurons monitored by fluorescence correlation spectroscopy. Biochemistry 2003, 42, 1667–1672. [DOI] [PubMed] [Google Scholar]
- Vu T. Q.; Chowdhury S.; Muni N. J.; Qian H.; Standaert R. F.; Pepperberg D. R. Activation of membrane receptors by a neurotransmitter conjugate designed for surface attachment. Biomaterials 2005, 26, 1895–1903. [DOI] [PubMed] [Google Scholar]
- Loudet A.; Burgess K. BODIPY dyes and their derivatives: Syntheses and spectroscopic properties. Chem. Rev. 2007, 107, 4891–4932. [DOI] [PubMed] [Google Scholar]
- Wang D.; Fan J.; Gao X.; Wang B.; Sun S.; Peng X. Carboxyl BODIPY dyes from bicarboxylic anhydrides: One-pot preparation, spectral properties, photostability and biolabeling. J. Org. Chem. 2009, 74, 7675–7683. [DOI] [PubMed] [Google Scholar]
- Abdel-Halim H.; Hanrahan J. R.; Hibbs D. E.; Johnston G. A. R.; Chebib M. A Molecular Basis for Agonist and Antagonist Actions at GABAC Receptors. Chem. Biol. Drug Des. 2008, 71, 306–327. [DOI] [PubMed] [Google Scholar]
- Adamian L.; Gussin H. A.; Tseng Y. Y.; Muni N.; Feng F.; Qian H.; Pepperberg D. R.; Liang J. Structural model of rho1 GABAC receptor based on evolutionary analysis: Testing of predicted protein-protein interactions involved in receptor assembly and function. Protein Sci. 2009, 18, 2371–2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto I.; Absalom N.; Carland J. E.; Doddareddy M.; Johnston G. A. R.; Hanrahan J. R.; Chebib M. Differentiating enantioselective actions of GABOB: A possible role for threonine 244 in the binding site of GABAC ρ1 receptors. ACS Chem. Neurosci. 2012, 3, 665–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.