Abstract
A library of oxygenated natural steroids, including physalins, withanolides, and perulactones, coupled with the synthetic cage-shaped right-side structure of type B physalins, was constructed. SAR studies for inhibition of NF-κB activation showed the importance of both the B-ring and the oxygenated right-side partial structure. The 5β,6β-epoxy derivatives of both physalins and withanolides showed similar profiles of inhibition of NF-κB activation and appeared to act on NF-κB signaling via inhibition of phosphorylation and degradation of IκBα. In contrast, type B physalins with C5–C6 olefin functionality inhibited nuclear translocation and DNA binding of RelA/p50 protein dimer, which lie downstream of IκBα degradation, although withanolides having the same AB-ring functionality did not. These results indicated that the right-side partial structure of these steroids influences their mode of action.
Keywords: Physalis plants, highly oxygenated steroid, physalin, withanolide, synthetic analogues, SAR, NF-κB
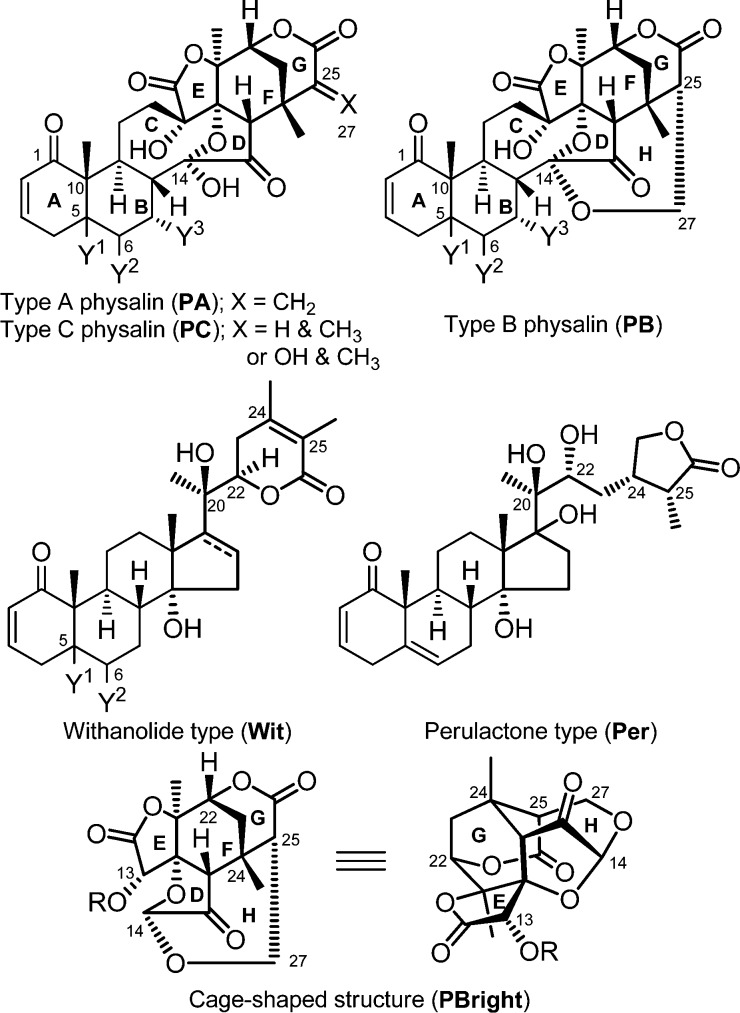
Oxygenated steroids are often of interest to synthetic chemists and medicinal chemists because of their complex structures and variety of biological activities. Among them, physalins, which were first isolated as bitter substances from Physalis plant, contain a particularly highly oxidized fused ring system with a 16,24-cyclo-13,14-secoergostane skeleton. More than 30 physalins have been identified, and most of them have been categorized into one of three classes, type A (PA), type B (PB), and type C (PC) (Figure 1). PBs, such as physalin B1 (PB-1, Table 1), have an additional internal acetal ring (H-ring), that forms a characteristic cage-shaped structure. Most physalins possess PB structure. PA and PB are structural isomers and were suggested to be in equilibrium in hot methanol.2
Figure 1.
Structures of type A (PA) and type B physalin (PB), withanolides (Wit), and perulactones (Per). (Y1, Y2 = double bond or epoxide; Y3 = H or OH).
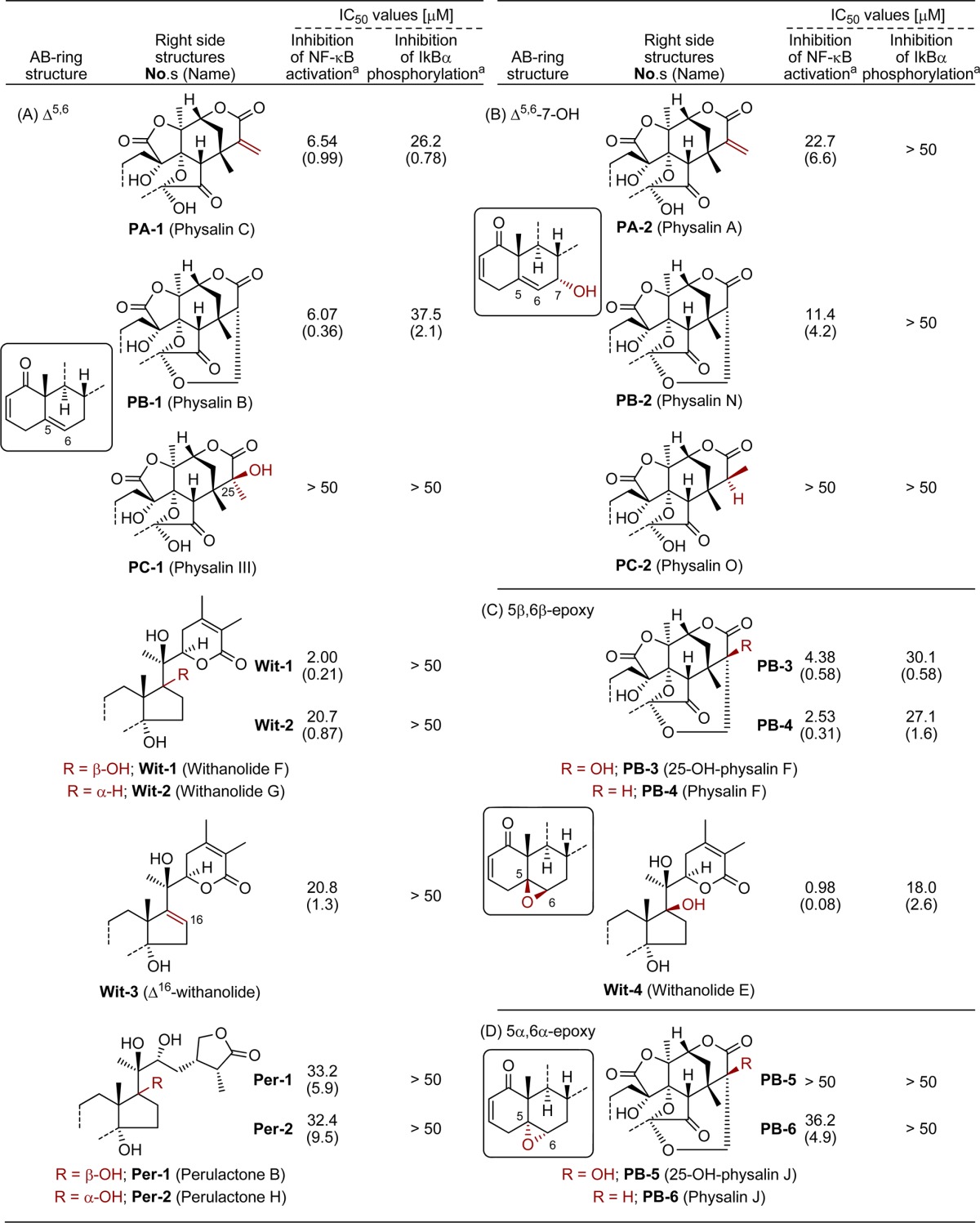
Table 1. Inhibition of NF-κB Activation and IκBα Phosphorylation by Oxygenated Natural Steroids.
IC50 values are means of three independent experiments. SD values are shown in parentheses.
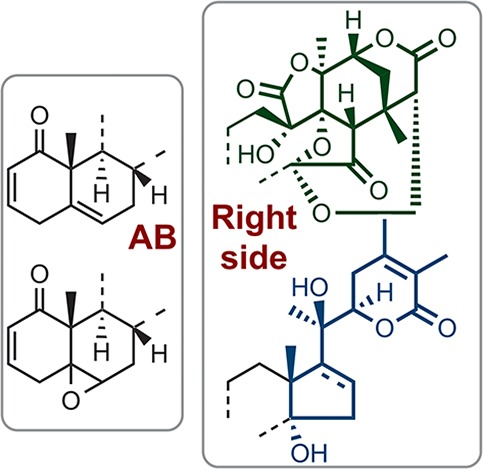
Physalins were found to exhibit cytotoxicity3,4 and anti-inflammatory activity.5 They also serve as regulators of intracellular signal transduction, showing inhibition of TNF-α- or PMA-induced NF-κB activation,6,7 inhibition of hedgehog/GLI-mediated transcription,8 and inhibition of the ubiquitin–proteasome pathway.9 A limited SAR study of the cytotoxicity of naturally occurring physalins and their derivatives to HeLa cells has been reported, focusing on structural differences in the AB-rings.10 However, SAR focusing on the unique oxygenated structure has not been reported, presumably due to the limited variability of the right-side structure of natural physalins. We were particularly attracted to the unique cage-shaped DEFGH-ring of PBs as a candidate for a new core structure of bioactive compounds, and we hypothesized that this structure contributes to the specific biological activities of PBs. We anticipated that SAR studies of a compound library consisting of steroidal natural products possessing different oxygenated structures in combination with synthetic DFGH11 or DEFGH-ring12PB-type derivatives would allow us to test this hypothesis, thus establishing the influence of the cage-shaped structure on the biological activities. Here, we report the construction of a library of oxygenated steroids and an examination of their SAR, focusing on the inhibitory activity toward the canonical NF-κB activation pathway.13 As a result, we concluded that right-side partial structure of physalins (PBright) and withanolides is indeed important for their activity.
First, known two PAs (PA-1–2), three PBs (PB-1–2 and 4) and one PC (PC-2) were isolated from Physalis alkekengi var. franchetii, cultivated at RIKEN. Further phytochemical investigation resulted in the discovery of a new constituent with physalin structure, but not classifiable as PA or PB. Its molecular formula was determined as C28H32O10 by HRMS, and its planar structure was deduced from detailed analysis of 1H, 13C, and 2D NMR measurements (1H–1H COSY, HSQC, HMBC, and NOESY; Figure S1, Supporting Information). Although this compound superficially resembles PA-1 and PB-1, it contains an additional methyl group and lacks the C25–C27 functionality of PAs (exo-methylene) and PBs (CH–CH2O), suggesting that the new compound is a hydrated derivative of PA-1. We named this new compound physalin III (PC-1, Table 1A), though the same compound has already been described as a derivative of a natural product.10 The S-configuration of the hydroxyl group at C-25 was determined from NOESY correlations, as shown in Figure S1, Supporting Information.
Our oxygenated steroidal library was further expanded by phytochemical investigation of Physalis peruviana, from which natural products have only recently been investigated.15 We independently examined natural products of this plant cultivated at RIKEN and identified five withanolides (Wit-1–4 and Wit-S1(14)) and two perulactones (Per-1–2) as major components. Finally, synthesis of a cage-shaped DFGH-ring compound (PBright-1),11 three DEFGH-ring compounds (PBright-2–4),12 and a DEFG-ring compound (PAright-1) along with five PBs (PB-3, 5–6, and S1–S2), which were isolated previously,10,14 afforded a library of 24 oxygenated steroids.
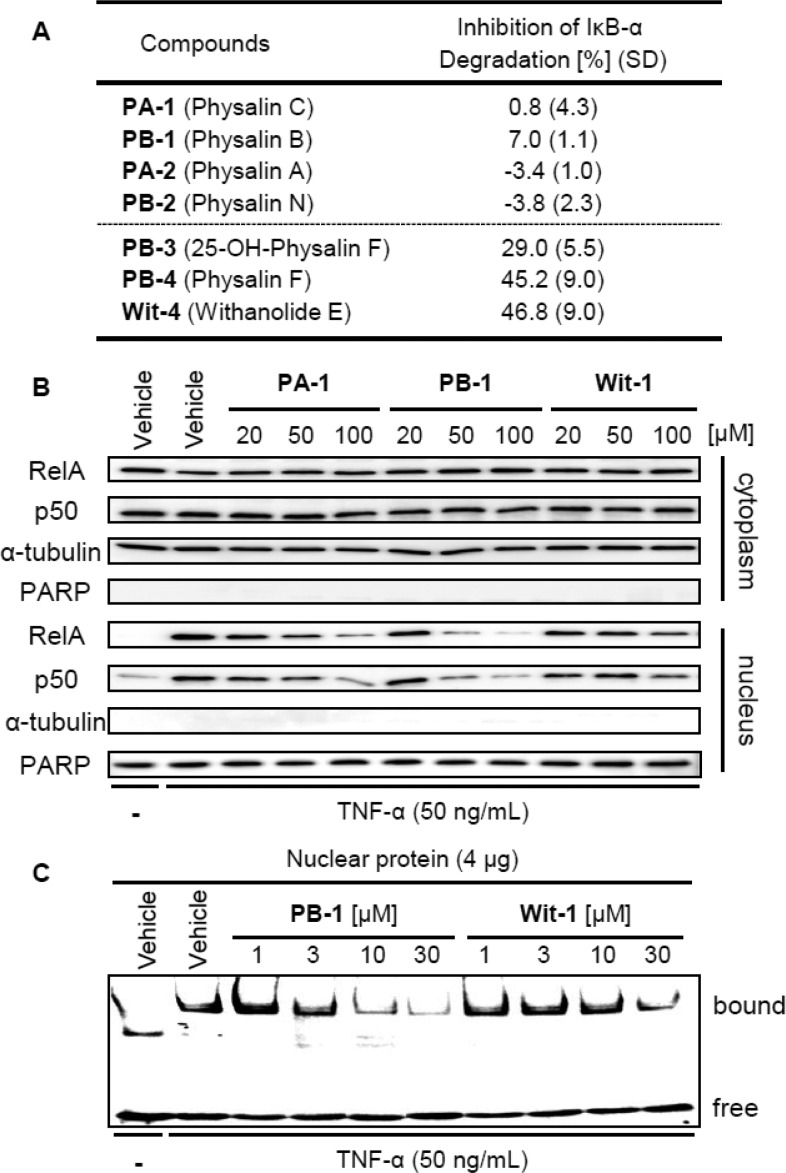
The inhibitory effects of natural products in our library on NF-κB activation stimulated by TNF-α were first evaluated (Table 1). NF-κB signaling is well-known to be involved in various diseases, including inflammation and cancers, and thus has attracted attention as a drug target.13 Heinrich and co-workers reported that physalin B (PB-1) and physalin F (PB-4), but not physalin D (PB-S1),14 inhibited canonical NF-κB activation, while PB-4 also showed inhibition of noncanonical NF-κB activation.6 We tested all the compounds in our library utilizing HeLa cells stably transfected with a NF-κB-driven luciferase reporter gene. The IC50 values are shown in Table 1. In agreement with the previous report, physalin B (PB-1) showed a modest inhibitory activity (IC50: 6.07 μM). The effect of the oxygenated right-side partial structure could be assessed by comparing the inhibitory activities of compounds having the same AB-ring structure. We found that C5–C6 olefin derivatives such as physalin B (PB-1), physalin C (PA-1, IC50 = 6.54 μM), and withanolide F (Wit-1, IC50 = 2.00 μM) were more potent inhibitors than other natural products. PC-1 was almost inactive (Table 1A). These results indicate that the oxygenated right-side structures of PA, PBs, and withanolide F (Wit-1) have an important role in the inhibition of NF-κB activation. In a series of compounds having an additional hydroxyl group on C7, similar SAR was observed, though the presence of this hydroxyl group decreased the inhibitory activity. Namely, physalin A (PA-2, IC50 = 22.7 μM) and physalin N (PB-2, IC50 = 11.4 μM) showed slightly lower inhibitory activity than physalin C (PA-1) and physalin B (PB-1), respectively, while physalin O was almost inactive (PC-2, IC50 = >50 μM) (Table 1B).
The C5 and C6 functionality influenced the inhibitory activities of PBs and Wits. Three 5β,6β-epoxy derivatives showed higher inhibition potency than 5,6-olefin compounds (Table 1C). Indeed, physalin F (PB-4, IC50 = 2.53 μM) showed greater activity than PB-1. Withanolide E (Wit-4, IC50 = 0.98 μM) was the most potent inhibitor among our library compounds. The hydroxyl group on C25 did not disturb the biological activity of PB-4 since 25-hydroxyphysalin F (PB-3, IC50 = 4.38 μM) showed similar inhibitory activity to PB-4. Introduction of the 25-hydroxyl group would stabilize the cage-shaped ring system by precluding ring-opening of the H-ring via β-elimination, so this result suggested that formation of the PA-type α,β-unsaturated lactone moiety (G-ring) from PBs is not essential for physalins to inhibit NF-κB activation, at least in the case of 5β,6β-epoxy derivatives. The configuration of epoxide affected the inhibitory activity since physalin J (PB-6, IC50 = 36.2 μM) and 25-hydroxyphysalin J (PB-5, IC50 = > 50 μM), having 5α,6α-epoxide, showed less potent inhibitory activities (Table 1D).
Five key nuclear transcription factor proteins, RelA (p65), RelB, c-Rel, p105/p50, and p100/p52, are involved in NF-κB signaling. Among them, we focused the protein dimer of RelA and p50 because this dimer is representative of the canonical NF-κB pathway. At the steady-state condition before stimulation, this dimer forms a complex with inhibitor protein IκBα in cytoplasm. Stimulation by TNF-α triggers the canonical NF-κB pathway and activates IKK complex. The resulting IKKβ phosphorylates two N-terminal serines, Ser32 and Ser36 on IκBα, and this phosphorylation induces ubiquitination and proteasomal degradation of IκBα, resulting in nuclear translocation of RelA/p50 dimer.16,17 After several posttranslational modifications,18 the dimers bind to specific DNA sequences, inducing transcriptional activity. To examine the mode of action of our compounds, we investigated their effects on three key events in NF-κB signaling, i.e., IκBα phosphorylation, and nuclear translocation and DNA binding of the RelA and p50 dimer.
First, the inhibitory effects of the library compounds on IκBα phosphorylation stimulated by TNF-α treatment were assessed. Phosphorylated IκBα proteins in HeLa cells after stimulation with TNF-α for 7.5 min were quantified by ELISA, in the presence of proteasome inhibitor MG-132 to block the degradation of phosphorylated IκBα (Table 1).14PA-1 and PB-1 having C5–C6 olefin weakly inhibited IκBα phosphorylation. However, PB-1 was less potent than PA-1, even though they showed similar inhibitory activities on NF-κB activation. Moreover, Wit-1 was less potent (Table 1A). However, in the case of 5β,6β-epoxy derivatives PB-3–4 and Wit-4, potency for the inhibition of NF-κB activation was well correlated with that for inhibition of IκBα phosphorylation, although the IC50 values for IκBα phosphorylation were almost 10 times larger than those for NF-κB activation (Table 1C). These results suggest that inhibition of phosphorylation and degradation of IκBα might be implicated as one of the inhibition mechanisms of the 5β,6β-epoxy derivatives (PB-3–4 and Wit-4). Indeed, ELISA-based quantification of total IκBα proteins in HeLa cells after TNF-α stimulation for 12.5 min showed remarkable inhibition of the degradation of IκBα proteins by these 5β,6β-epoxy derivatives (Figures 2A and S4, Supporting Information). In contrast, C5–C6 olefin derivatives were less potent inhibitors.
Figure 2.
(A) Inhibition of IκBα degradation. HeLa cells were stimulated with TNF-α (50 ng/mL) for 12.5 min after pretreatment either with test compound (50 μM) or DMSO for 30 min. Total IκBα proteins were quantified by ELISA. (B,C) Effect of PA-1, PB-1, and Wit-1 on translocation (B) and DNA binding (C) of RelA/p50 proteins. HeLa cells were stimulated with TNF-α (50 ng/mL) for 15 min (B) or 30 min (C) after pretreatment with various concentrations of compounds or with DMSO for 30 min. (B) Western blotting analysis of cytoplasmic or nuclear proteins by immunoblotting using anti-RelA, anti-p50, antitubulin (marker for cytoplasmic fraction), and anti-PARP (marker for nucleus fraction) antibodies. (C) Electrophoresis mobility shift assay (EMSA) using labeled DNA probe and nuclear fraction of HeLa cells.
Recently, Ma and co-workers reported that physalin A (PA-2) formed covalent bonds with all six cysteine residues of human recombinant IKKβ in vitro and proposed that IKKβ is a potential target for the anti-inflammatory activity of PA-2.19 Our results suggested that 5β,6β-epoxy derivatives of both PBs and Wits are also likely to act via the inhibition of IκBα phosphorylation and degradation, but C5–C6 olefin derivatives of PAs and PBs, including PA-2, are likely to have a different mode of action.
Withaferin A (Figure S3, Supporting Information), a structurally similar compound bearing a 5β,6β-epoxy group in a steroidal skeleton, is one of the most well-studied oxygenated steroids.20 Withaferin A shows multiple biological activities, including inhibition of NF-κB activation through IKKβ hyperphosphorylation.21,22 It has also been reported that annexin II is a potential target of withaferin A in connection with its actin microfilament-aggregating ability.23 On the basis of the experimental results and molecular modeling, it was proposed that the 5β,6β-epoxide in withaferin A forms a covalent bond with a cysteine residue of annexin II, so it is possible that modification of IKKβ or other proteins related to IκBα degradation by our 5β,6β-epoxy derivatives accounts for their potent inhibition of NF-κB activation (Figure 3). The stereochemistry of the epoxide group affected the inhibition of IκBα phosphorylation, further supporting the importance of the epoxide functionality. The 5β,6β-epoxy group favors high potency.
Figure 3.
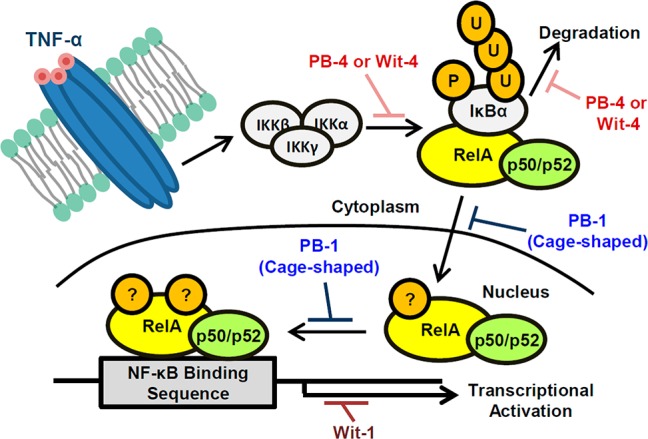
Schematic representation of NF-κB activation upon TNF-α stimulation and proposed points of action of oxygenated steroidal natural products. P, phosphorylation; U, ubiquitination; ?, some posttranslational modification.
We next focused on the C5–C6 olefin PAs, PBs, and Wits. As described above, nuclear translocation of RelA and p50 dimer occurs after IκBα degradation in the course NF-κB activation. Interestingly, Western blotting analysis of RelA and p50 in the cytosolic and nuclear fractions of HeLa cells revealed an apparent difference between physalins (PAs and PBs) and Wits (Figure 2B). Stimulation of HeLa cells by TNF-α resulted in nuclear accumulation of RelA and p50 proteins, as expected. In accordance with the observed inhibitory activity on NF-κB activation, both PA-1 and PB-1 dose-dependently inhibited the nuclear translocation of RelA and p50, though no significant change in the abundance of these proteins in the cytosolic fraction was observed.14 However, Wit-1 showed weaker inhibitory activity on nuclear translocation even at 100 μM, although it was a more potent inhibitor of NF-κB activation, as compared with PA-1 and PB-1. These results strongly indicate first that difference of the right-side partial structure affected the mode of action in the case of C5–C6 olefin derivatives, and second that PAs and PBs inhibited the nuclear translocation of RelA and p50 dimer, as well as NF-κB activation.
We further investigated whether these C5–C6 olefin derivatives inhibit DNA binding of RelA and p50 dimer by means of gel-shift assay (EMSA) using nuclear protein extracts of HeLa cells. TNF-α stimulation in the absence of test compounds induced binding of the protein dimer to the target DNA. Pretreatment with PB-1 resulted in significant, dose-dependent inhibition of the binding (Figure 2C). Wit-1 showed a similar effect but was less potent than PB-1. These compounds had no effect on DNA binding of the protein dimer itself in the absence of pretreatment with PB-1 (Figure S5, Supporting Information). Since blocking the nuclear translocation of protein dimer by PB-1 decreased the amount of dimer available for DNA binding, these results are consistent with the results in Figure 2B. The effect of PB-1 on DNA binding appeared to be more potent than that on nuclear translocation. Therefore, PB-1 might also play some role in the nucleus, such as interfering with posttranslational modification of RelA, to decrease DNA complex formation with RelA/p50 dimer (Figure 3).18 However, Wit-1 appears to mainly inhibit a subsequent process after DNA binding of RelA/p50 dimer.24
Finally, we evaluated the inhibitory activities of the synthetic right-side partial structure of PAs and PBs on NF-κB activation (Table 2). Pleasingly, we found that benzyl-protected PBright-4 (IC50 = 43.6 μM) showed relatively high inhibitory activity among the synthetic compounds. This result supports the idea that this partial structure of PBs exhibits biological activity. PAright-1 (IC50 = 85.8 μM), corresponding to the right half structure of PAs, was less potent than PBright-4. This tendency is consistent with that of natural PAs and PBs (Table 1). The benzyl group presumably acts as a hydrophobic substituent in place of the steroidal ABC-ring and was necessary for inhibitory activity because derivatives with an acetyl (PBright-3) or free hydroxyl group (PBright-2) did not show any inhibitory activity.25 Furthermore, PBright-4 also inhibited the nuclear translocation and DNA binding of RelA/p50 dimer, though at rather high concentration, whereas it showed only a weak inhibitory effect on IκBα phosphorylation and degradation (Figure S6, Supporting Information). These results support our hypothesis that the right-side partial structure of PBs and Wits has a key influence on the mode of action of the compounds.
Table 2. Inhibition of NF-κB Activation by Synthetic Right-Side Molecules of Physalin.
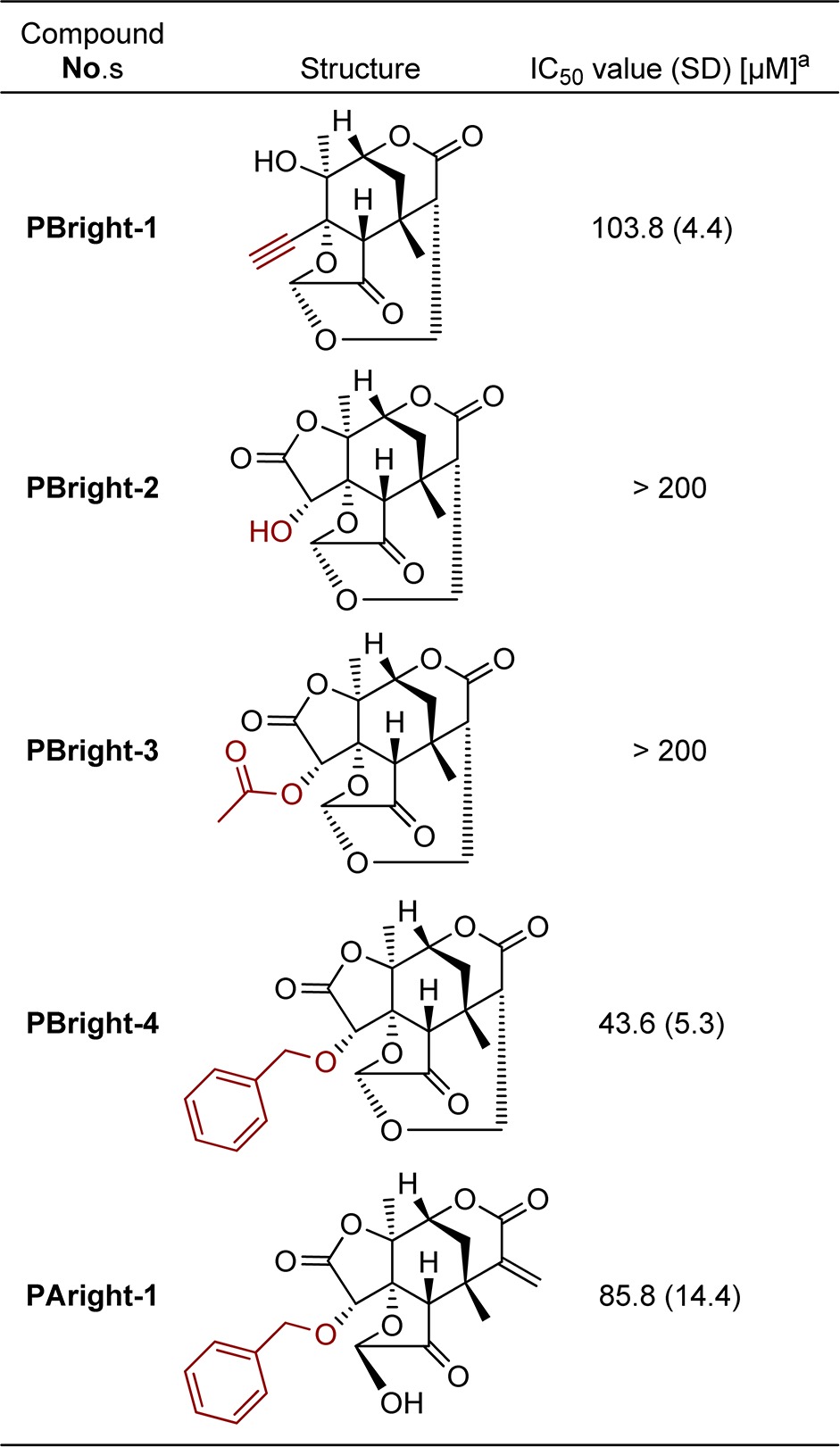
IC50 values are means of three independent experiments. Standard deviation is in parentheses.
In conclusion, we have shown that our approach of combining natural products and synthetic molecules to prepare a library of compounds for SAR study was effective both for finding potent inhibitors of NF-κB activation and for examining the structure-mode of action relationship. Further chemical–biology studies, including identification of target proteins and further evaluation of the biological activity of the cage-shaped DEFGH-ring are under way.
Acknowledgments
We thank the Molecular Characterization Team (RIKEN, Japan) for HRMS analysis and helpful discussions. M.M. is a Research Fellow of JSPS.
Glossary
Abbreviations
- SAR
structure–activity relationship
- NF-κB
nuclear factor-kappa B
- IκB
inhibitor of kappa B
- TNF
tumor necrosis factor
- ELISA
enzyme-linked immunosorbent assay
- EMSA
electrophoretic mobility shift assay
Supporting Information Available
Isolation of compounds, experimental procedures, characterization of new compounds, 1H NMR, 13C NMR, and biological experiments. This material is available free of charge via the Internet at http://pubs.acs.org.
Author Contributions
All authors contributed to the research and writing of this manuscript.
This work was partially supported by Project Funding from RIKEN and Grant-in-Aid for Scientific Research (C) and Scientific Research on Innovative Area (Grant Numbers 22603014 and 24102534, respectively).
The authors declare no competing financial interest.
Supplementary Material
References
- Matsuura T.; Kawai M. Bitter principles of Physalis alkekengi var. francheti: structure of physalin B. Tetrahedron Lett. 1969, 10, 1765–1766. [Google Scholar]
- Makino B.; Kawai M.; Yamamura H.; Araki S.; Butsugan Y. Tautomerism between exomethylene type physalins and oxymethylene-bridged physalins. Pharmazie 2002, 57, 215–216. [PubMed] [Google Scholar]
- Antoun M. D.; Abramson D.; Tyson R. L.; Chang C. J.; McLaughlin J. L.; Peck G.; Cassady J. M. Potential antitumor agents. XVII. Physalin B and 25,26-epidihydrophysalin C from Witheringia coccoloboides. J. Nat. Prod. 1981, 44, 579–585. [DOI] [PubMed] [Google Scholar]
- Damu A. G.; Kuo P. C.; Su C. R.; Kuo T. H.; Chen T. H.; Bastow K. F.; Lee K. H.; Wu T. S. Isolation, structures, and structure–cytotoxic activity relationships of withanolides and physalins from Physalis angulata. J. Nat. Prod. 2007, 70, 1146–1152. [DOI] [PubMed] [Google Scholar]
- Vieira A. T.; Pinho V.; Lepsch L. B.; Scavone C.; Ribeiro I. M.; Tomassini T.; Ribeiro-dos-Santos R.; Soares M. B. P.; Teixeira M. M.; Souza D. G. Mechanism of the anti-inflammatory effects of the natural secosteroids physalins in a model of intestinal ischaemia and reperfusion injury. Br. J. Pharmacol. 2005, 146, 244–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobo-Herrera N. J.; Bremner P.; Márquez N.; Gupta M. P.; Gibbons S.; Muñoz E.; Heinrich M. Physalins from Witheringia solanacea as modulators of the NF-κB cascade. J. Nat. Prod. 2006, 69, 328–331. [DOI] [PubMed] [Google Scholar]
- Wu S. Y.; Leu Y. L.; Chang Y. L.; Wu T. S.; Kuo P. C.; Liao Y. R.; Teng C. M.; Pan S. L. Physalin F induces cell apoptosis in human renal carcinoma cells by targeting NF-kappaB and generating reactive oxygen species. PLoS One 2012, 7, e40727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoya T.; Arai M. A.; Koyano T.; Kowithayakorn T.; Ishibashi M. Naturally occurring small-molecule inhibitors of Hedgehog/GLI-mediated transcription. ChemBioChem 2008, 9, 1082–1092. [DOI] [PubMed] [Google Scholar]
- Vandenberghe I.; Créancier L.; Vispé S.; Annereau J. P.; Barret J. M.; Pouny I.; Samson A.; Aussagues Y.; Massiot G.; Ausseil F.; Bailly C.; Kruczynski A.; Physalin B. A novel inhibitor of the ubiquitin-proteasome pathway, triggers NOXA-associated apoptosis. Biochem. Pharmacol. 2008, 76, 453–462. [DOI] [PubMed] [Google Scholar]
- Kawai M.; Makino B.; Yamamura H.; Araki S.; Butsugan Y.; Ohya J. Cytotoxic activity of physalins and related compounds against HeLa cells. Pharmazie 2002, 57, 348–350. [PubMed] [Google Scholar]
- Ohkubo M.; Hirai G.; Sodeoka M. Synthesis of DFGH ring system of type B physalins: highly oxygenated, cage-shaped molecules. Angew. Chem., Int. Ed. 2009, 48, 3862–3866. [DOI] [PubMed] [Google Scholar]
- Morita M.; Hirai G.; Ohkubo M.; Koshino H.; Hashizume D.; Maruoka K.; Sodeoka M. Kinetically controlled one-pot formation of DEFGH-rings of type B physalins through domino-type transformations. Org. Lett. 2012, 14, 3434–3437. [DOI] [PubMed] [Google Scholar]
- Hoffmann A.; Baltimore D. Circuitry of nuclear factor κB signaling. Immunol. Rev. 2006, 210, 171–186. [DOI] [PubMed] [Google Scholar]
- See Supporting Information.
- Fang S. T.; Liu J. K.; Li B. Ten new withanolides from Physalis peruviana. Steroids 2012, 77, 36–44. [DOI] [PubMed] [Google Scholar]
- Hayden M. S.; Ghosh S. Shared principles in NF-κB signaling. Cell 2008, 132, 344–362. [DOI] [PubMed] [Google Scholar]
- Karin M.; Ben-Neriah Y. Phosphorylation meets ubiquitination: the control of NF-κB activity. Annu. Rev. Immunol. 2000, 18, 621–663. [DOI] [PubMed] [Google Scholar]
- Huang B.; Yang X. D.; Lamb A.; Chen L. F. Posttranslational modifications of NF-κB: another layer of regulation for NF-κB signaling pathway. Cell Signaling 2010, 22, 1282–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji L.; Yuan Y.; Luo L.; Chen Z.; Ma X.; Ma Z.; Cheng L. Physalins with anti-inflammatory activity are present in Physalis alkekengi var. franchetii and can function as Michael reaction acceptors. Steroids 2012, 77, 441–447. [DOI] [PubMed] [Google Scholar]
- Vanden Berghe W.; Sabbe L.; Kaileh M.; Haegeman G.; Heyninck K. Molecular insight in the multifunctional activities of Withaferin A. Biochem. Pharmacol. 2012, 84, 1282–1291. [DOI] [PubMed] [Google Scholar]
- Kaileh M.; Vanden Berghe W.; Heyerick A.; Horion J.; Piette J.; Libert C.; De Keukeleire D.; Essawi T.; Haegeman G. Withaferin A strongly elicits IκB kinase β hyperphosphorylation concomitant with potent inhibition of its kinase activity. J. Biol. Chem. 2007, 282, 4253–4264. [DOI] [PubMed] [Google Scholar]
- Grover A.; Shandilya A.; Punetha A.; Bisaria V. S.; Sundar D. Inhibition of the NEMO/IKKβ association complex formation, a novel mechanism associated with the NF-κB activation suppression by Withania somnifera’s key metabolite withaferin A. BMC Genomics 2010, 11, S25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falsey R. R.; Marron M. T.; Gunaherath G. M.; Shirahatti N.; Mahadevan D.; Gunatilaka A. A.; Whitesell L. Actin microfilament aggregation induced by withaferin A is mediated by annexin II. Nat. Chem. Biol. 2006, 2, 33–38. [DOI] [PubMed] [Google Scholar]
- Selectivity of Wit-1 for the NF-κB-promoted transcription was confirmed by control experiment; see Figure S7, Supporting Information.
- Synthesis of PBright-3 and PAright-1 is shown in Scheme S1, Supporting Information.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.