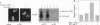Abstract
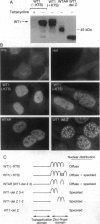
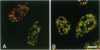
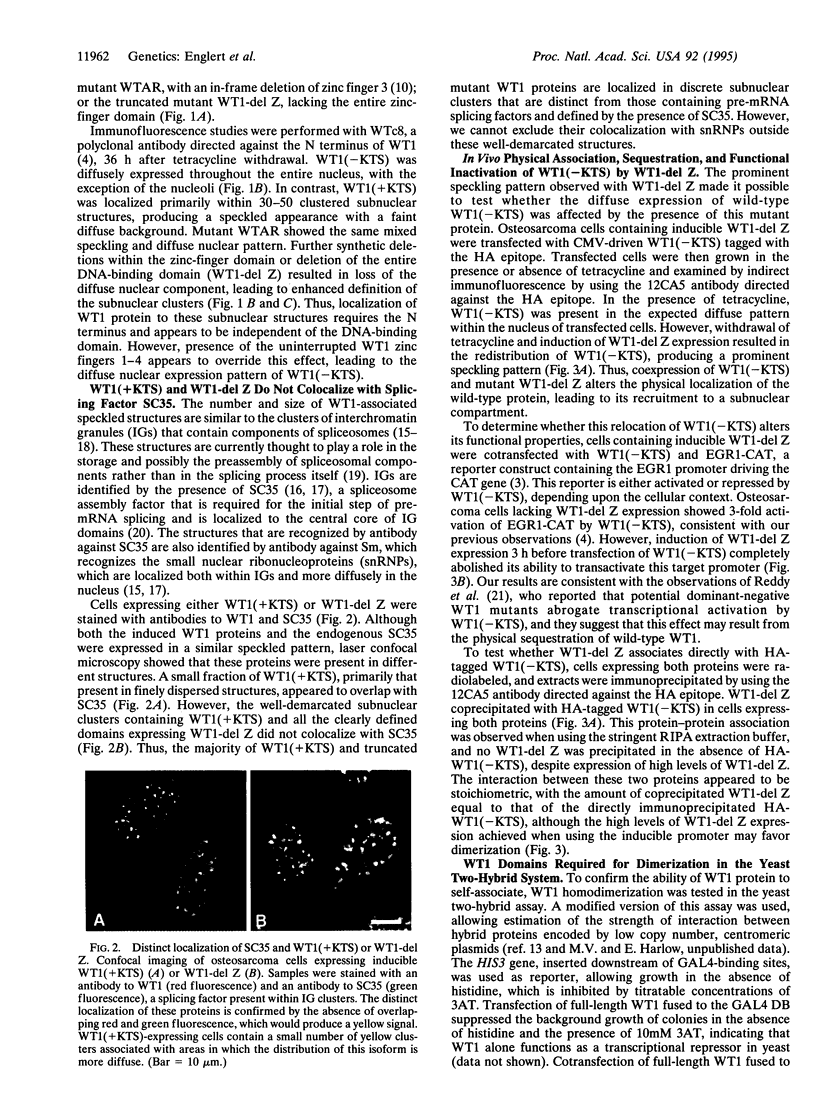
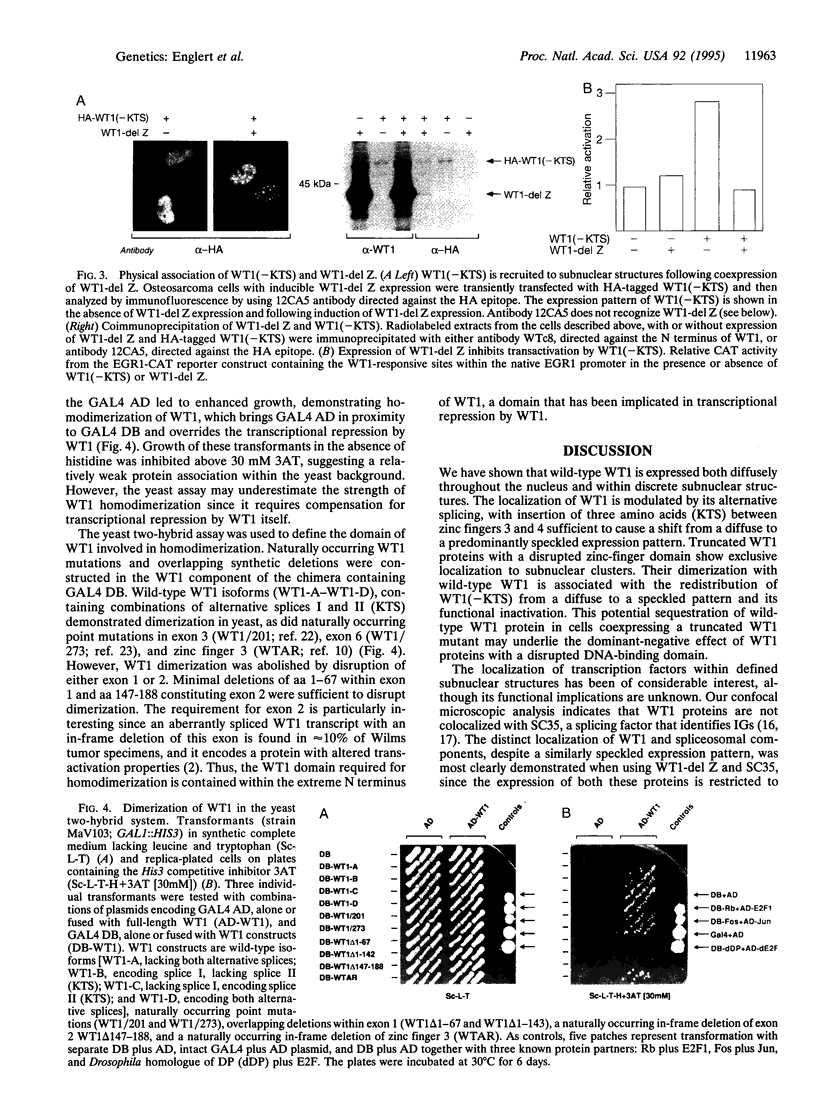
WT1 encodes a zinc-finger protein, expressed as distinct isoforms, that is inactivated in a subset of Wilms tumors. Both constitutional and somatic mutations disrupting the DNA-binding domain of WT1 result in a potentially dominant-negative phenotype. In generating inducible cell lines expressing wild-type isoforms of WT1 and WT1 mutants, we observed dramatic differences in the subnuclear localization of the induced proteins. The WT1 isoform that binds with high affinity to a defined DNA target, WT1(-KTS), was diffusely localized throughout the nucleus. In contrast, expression of an alternative splicing variant with reduced DNA binding affinity, WT1 (+KTS), or WT1 mutants with a disrupted zinc-finger domain resulted in a speckled pattern of expression within the nucleus. Although similar in appearance, the localization of WT1 variants to subnuclear clusters was clearly distinct from that of the essential splicing factor SC35, suggesting that WT1 is not directly involved in pre-mRNA splicing. Localization to subnuclear clusters required the N terminus of WT1, and coexpression of a truncated WT1 mutant and wild-type WT1(-KTS) resulted in their physical association, the redistribution of WT1(-KTS) from a diffuse to a speckled pattern, and the inhibition of its transactivational activity. These observations suggest that different WT1 isoforms and WT1 mutants have distinct subnuclear compartments. Dominant-negative WT1 proteins physically associate with wild-type WT1 in vivo and may result in its sequestration within subnuclear structures.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baird P. N., Santos A., Groves N., Jadresic L., Cowell J. K. Constitutional mutations in the WT1 gene in patients with Denys-Drash syndrome. Hum Mol Genet. 1992 Aug;1(5):301–305. doi: 10.1093/hmg/1.5.301. [DOI] [PubMed] [Google Scholar]
- Carter K. C., Bowman D., Carrington W., Fogarty K., McNeil J. A., Fay F. S., Lawrence J. B. A three-dimensional view of precursor messenger RNA metabolism within the mammalian nucleus. Science. 1993 Feb 26;259(5099):1330–1335. doi: 10.1126/science.8446902. [DOI] [PubMed] [Google Scholar]
- Englert C., Hou X., Maheswaran S., Bennett P., Ngwu C., Re G. G., Garvin A. J., Rosner M. R., Haber D. A. WT1 suppresses synthesis of the epidermal growth factor receptor and induces apoptosis. EMBO J. 1995 Oct 2;14(19):4662–4675. doi: 10.1002/j.1460-2075.1995.tb00148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X. D., Maniatis T. Factor required for mammalian spliceosome assembly is localized to discrete regions in the nucleus. Nature. 1990 Feb 1;343(6257):437–441. doi: 10.1038/343437a0. [DOI] [PubMed] [Google Scholar]
- Haber D. A., Buckler A. J., Glaser T., Call K. M., Pelletier J., Sohn R. L., Douglass E. C., Housman D. E. An internal deletion within an 11p13 zinc finger gene contributes to the development of Wilms' tumor. Cell. 1990 Jun 29;61(7):1257–1269. doi: 10.1016/0092-8674(90)90690-g. [DOI] [PubMed] [Google Scholar]
- Haber D. A., Housman D. E. The genetics of Wilms' tumor. Adv Cancer Res. 1992;59:41–68. doi: 10.1016/s0065-230x(08)60302-4. [DOI] [PubMed] [Google Scholar]
- Haber D. A., Park S., Maheswaran S., Englert C., Re G. G., Hazen-Martin D. J., Sens D. A., Garvin A. J. WT1-mediated growth suppression of Wilms tumor cells expressing a WT1 splicing variant. Science. 1993 Dec 24;262(5142):2057–2059. doi: 10.1126/science.8266105. [DOI] [PubMed] [Google Scholar]
- Haber D. A., Sohn R. L., Buckler A. J., Pelletier J., Call K. M., Housman D. E. Alternative splicing and genomic structure of the Wilms tumor gene WT1. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9618–9622. doi: 10.1073/pnas.88.21.9618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber D. A., Timmers H. T., Pelletier J., Sharp P. A., Housman D. E. A dominant mutation in the Wilms tumor gene WT1 cooperates with the viral oncogene E1A in transformation of primary kidney cells. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):6010–6014. doi: 10.1073/pnas.89.13.6010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreidberg J. A., Sariola H., Loring J. M., Maeda M., Pelletier J., Housman D., Jaenisch R. WT-1 is required for early kidney development. Cell. 1993 Aug 27;74(4):679–691. doi: 10.1016/0092-8674(93)90515-r. [DOI] [PubMed] [Google Scholar]
- Larsson S. H., Charlieu J. P., Miyagawa K., Engelkamp D., Rassoulzadegan M., Ross A., Cuzin F., van Heyningen V., Hastie N. D. Subnuclear localization of WT1 in splicing or transcription factor domains is regulated by alternative splicing. Cell. 1995 May 5;81(3):391–401. doi: 10.1016/0092-8674(95)90392-5. [DOI] [PubMed] [Google Scholar]
- Little M. H., Prosser J., Condie A., Smith P. J., Van Heyningen V., Hastie N. D. Zinc finger point mutations within the WT1 gene in Wilms tumor patients. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):4791–4795. doi: 10.1073/pnas.89.11.4791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez J., Georgoff I., Martinez J., Levine A. J. Cellular localization and cell cycle regulation by a temperature-sensitive p53 protein. Genes Dev. 1991 Feb;5(2):151–159. doi: 10.1101/gad.5.2.151. [DOI] [PubMed] [Google Scholar]
- Mattaj I. W. RNA processing. Splicing in space. Nature. 1994 Dec 22;372(6508):727–728. doi: 10.1038/372727a0. [DOI] [PubMed] [Google Scholar]
- Michalovitz D., Halevy O., Oren M. Conditional inhibition of transformation and of cell proliferation by a temperature-sensitive mutant of p53. Cell. 1990 Aug 24;62(4):671–680. doi: 10.1016/0092-8674(90)90113-s. [DOI] [PubMed] [Google Scholar]
- Mundlos S., Pelletier J., Darveau A., Bachmann M., Winterpacht A., Zabel B. Nuclear localization of the protein encoded by the Wilms' tumor gene WT1 in embryonic and adult tissues. Development. 1993 Dec;119(4):1329–1341. doi: 10.1242/dev.119.4.1329. [DOI] [PubMed] [Google Scholar]
- Nyman U., Hallman H., Hadlaczky G., Pettersson I., Sharp G., Ringertz N. R. Intranuclear localization of snRNP antigens. J Cell Biol. 1986 Jan;102(1):137–144. doi: 10.1083/jcb.102.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S., Schalling M., Bernard A., Maheswaran S., Shipley G. C., Roberts D., Fletcher J., Shipman R., Rheinwald J., Demetri G. The Wilms tumour gene WT1 is expressed in murine mesoderm-derived tissues and mutated in a human mesothelioma. Nat Genet. 1993 Aug;4(4):415–420. doi: 10.1038/ng0893-415. [DOI] [PubMed] [Google Scholar]
- Park S., Tomlinson G., Nisen P., Haber D. A. Altered trans-activational properties of a mutated WT1 gene product in a WAGR-associated Wilms' tumor. Cancer Res. 1993 Oct 15;53(20):4757–4760. [PubMed] [Google Scholar]
- Pelletier J., Bruening W., Kashtan C. E., Mauer S. M., Manivel J. C., Striegel J. E., Houghton D. C., Junien C., Habib R., Fouser L. Germline mutations in the Wilms' tumor suppressor gene are associated with abnormal urogenital development in Denys-Drash syndrome. Cell. 1991 Oct 18;67(2):437–447. doi: 10.1016/0092-8674(91)90194-4. [DOI] [PubMed] [Google Scholar]
- Pritchard-Jones K., Fleming S., Davidson D., Bickmore W., Porteous D., Gosden C., Bard J., Buckler A., Pelletier J., Housman D. The candidate Wilms' tumour gene is involved in genitourinary development. Nature. 1990 Jul 12;346(6280):194–197. doi: 10.1038/346194a0. [DOI] [PubMed] [Google Scholar]
- Rauscher F. J., 3rd, Morris J. F., Tournay O. E., Cook D. M., Curran T. Binding of the Wilms' tumor locus zinc finger protein to the EGR-1 consensus sequence. Science. 1990 Nov 30;250(4985):1259–1262. doi: 10.1126/science.2244209. [DOI] [PubMed] [Google Scholar]
- Reddy J. C., Morris J. C., Wang J., English M. A., Haber D. A., Shi Y., Licht J. D. WT1-mediated transcriptional activation is inhibited by dominant negative mutant proteins. J Biol Chem. 1995 May 5;270(18):10878–10884. doi: 10.1074/jbc.270.18.10878. [DOI] [PubMed] [Google Scholar]
- Sardet C., Vidal M., Cobrinik D., Geng Y., Onufryk C., Chen A., Weinberg R. A. E2F-4 and E2F-5, two members of the E2F family, are expressed in the early phases of the cell cycle. Proc Natl Acad Sci U S A. 1995 Mar 14;92(6):2403–2407. doi: 10.1073/pnas.92.6.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector D. L., Fu X. D., Maniatis T. Associations between distinct pre-mRNA splicing components and the cell nucleus. EMBO J. 1991 Nov;10(11):3467–3481. doi: 10.1002/j.1460-2075.1991.tb04911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector D. L. Macromolecular domains within the cell nucleus. Annu Rev Cell Biol. 1993;9:265–315. doi: 10.1146/annurev.cb.09.110193.001405. [DOI] [PubMed] [Google Scholar]
- Wang Z. Y., Qiu Q. Q., Deuel T. F. The Wilms' tumor gene product WT1 activates or suppresses transcription through separate functional domains. J Biol Chem. 1993 May 5;268(13):9172–9175. [PubMed] [Google Scholar]