| Patent Application Title: | Monofluoro beta-secretase inhibitors | ||
| Patent Application Number: | WO 2013/054108 A1 | Publication date: | 18 April 2013 |
| Priority Application: | US 61/545334 | Priority date: | 10 October 2011 |
| Inventors: | Minidis, A.; Rahm, F.; Viklund, J. | ||
| Assignee Company: | Astrazeneca UK Limited; 2 Kingdom Street, London, Greater London W2 6BD, U.K. | ||
| Disease Area: | Aβ-related pathologies and β-amyloid angiopathy such as Alzheimer’s disease and Down’s syndrome | Biological Target: | β-secretase (BACE-1) |
| Summary: | The invention in this patent application relates to monofluoro compounds represented generally by formula I that are inhibitors of β-secretase [also known as beta site amyloid cleaving enzyme (BACE or BACE-1)]. These compounds may potentially be used for treatment and/or prevention of amyloid β (Aβ)-related pathologies and β-amyloid angiopathy such as Down’s syndrome, Alzheimer’s disease, and other related disorders. | ||
| BACE is a membrane bound type 1 protein that is abundantly expressed in brain tissue. Its activity is implicated in the generation of Aβ peptide from APP, and it is believed to be the rate-limiting step in the production of Aβ. Thus, inhibition of BACE activity with molecules such as those described in this patent application could reduce the production of Aβ and may potentially slow formation of amyloid plaques and the progression of Alzheimer’s disease and other related disorders involving deposition of Aβ or its fragments. | |||
| Important Compound Classes: | 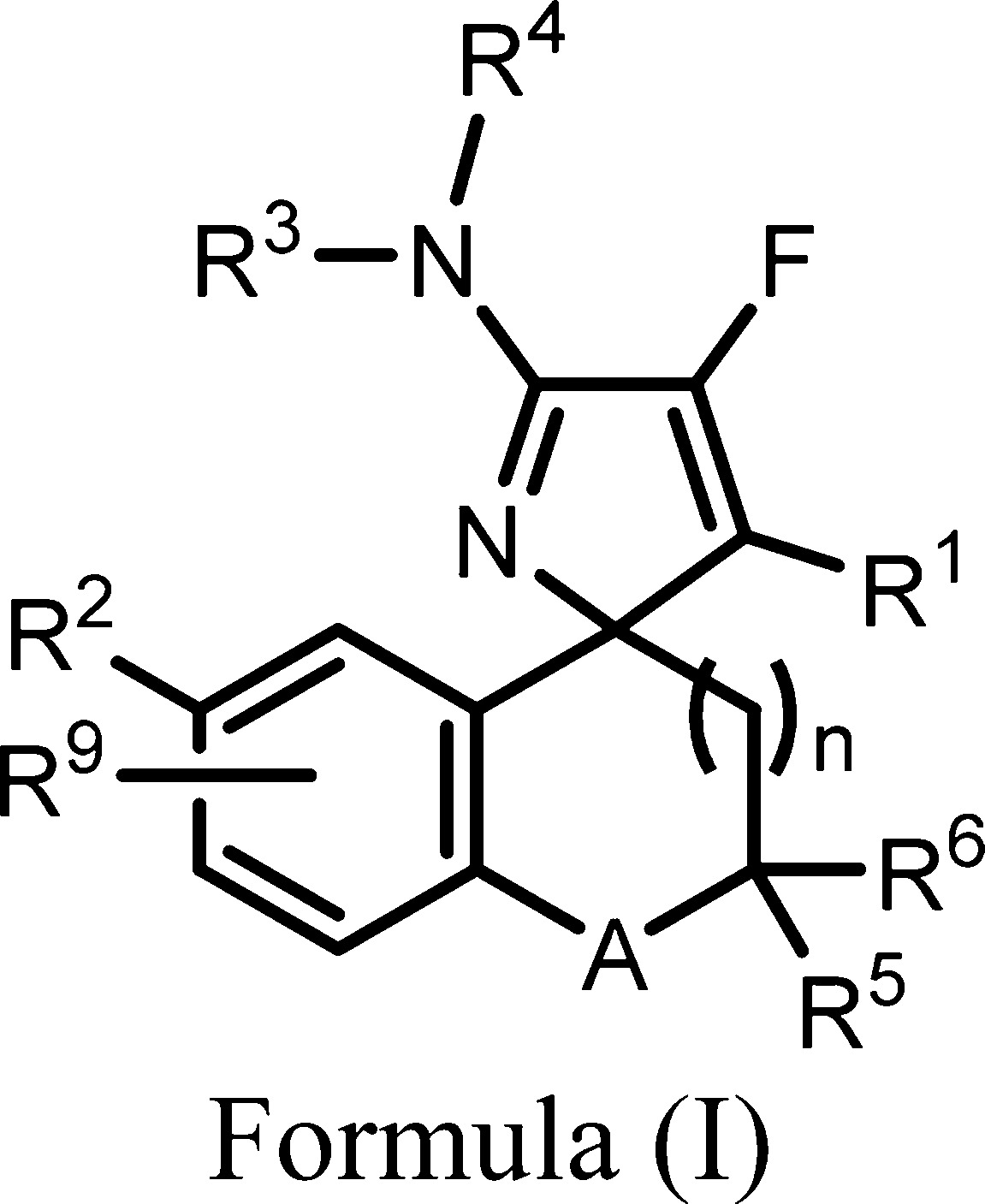 |
||
| Key Structures: | Four representative structures of the compounds of formula I: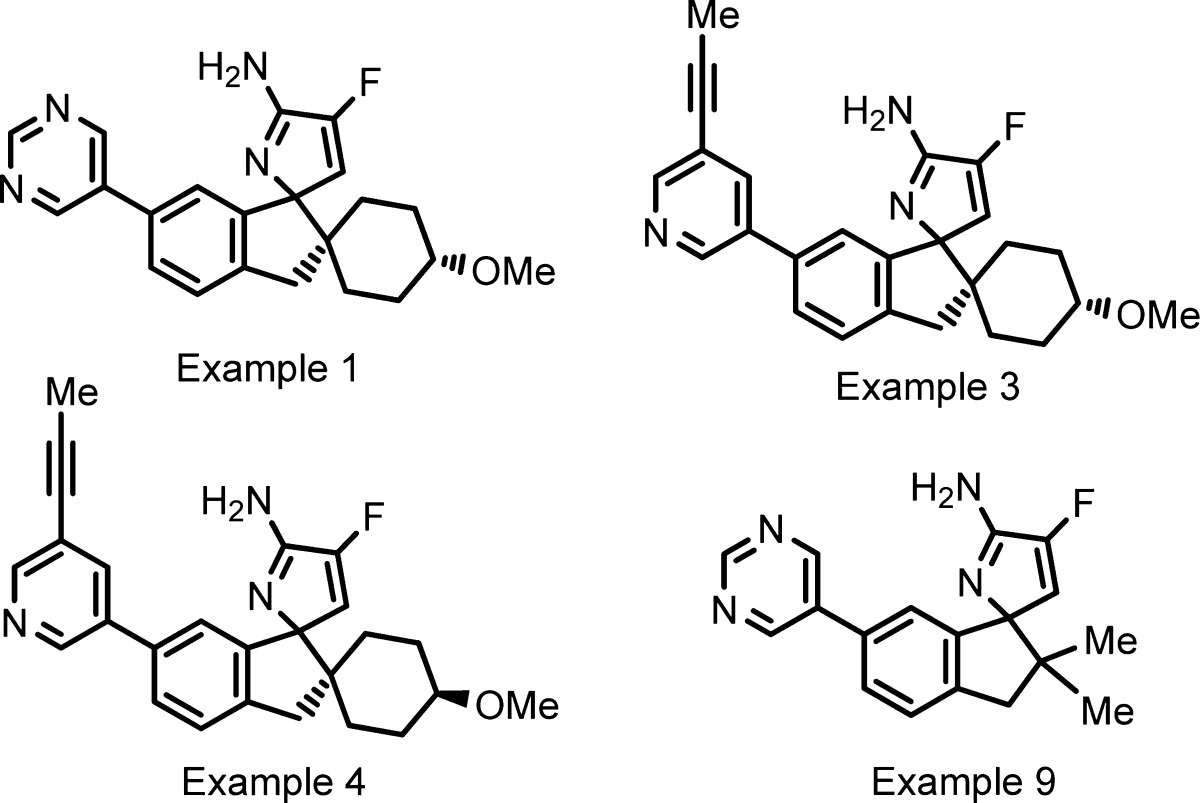
|
||
| Biological Assay: |
|
||
| Biological Data: | The inventors stated that typical IC50 values for the tested compounds are in the range of about 0.01 to about 100 000 nM. The reported values for the four examples above are listed in the following table: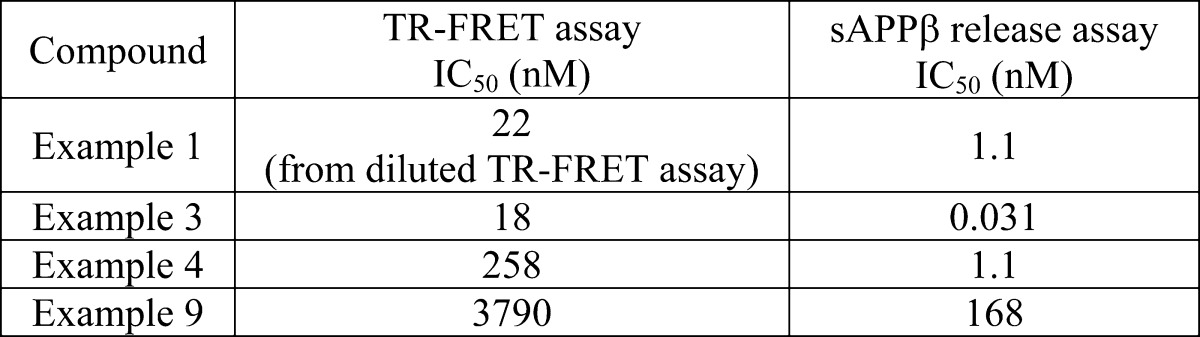
|
||
| Claims: | Claims 1–8: composition of matter, variations of formula I | ||
| Claim 9–10: composition of matter, specific examples of the compounds of formula I listed by chemical name | |||
| Claim 11: pharmaceutical composition | |||
| Claims 12–15: claiming compounds for use as medicament and treating applicable conditions and diseases | |||
| Claims 16–19: methods of treatment or preventing relevant conditions and diseases | |||
The authors declare no competing financial interest.


