Abstract
N-mono/dimethylated TE2A tetraazamacrocycles (MM-TE2A and DM-TE2A) were synthesized in high yields. Both Cu-MM/DM-TE2A complexes showed increased kinetic stability compared to that of Cu-TE2A, whereas Cu-DM-TE2A showed even higher in vitro stability than that of Cu-ECB-TE2A. MM-TE2A and DM-TE2A were quantitatively radiolabeled with 64Cu ions and showed rapid clearance from the body to emerge as a potential efficient bifunctional chelator.
Keywords: Bifunctional chelator, copper complex, imaging agent, radiopharmaceutical
Advances in metal-based radiopharmaceuticals are driving research and development for medical diagnosis and therapy.1−3 Several copper radioisotopes (60Cu, 61Cu, 62Cu, 64Cu, and 67Cu) have attractive physical properties for medical applications, and various radioactive copper labeled bioconjugates are in the clinical trial pipeline.4−6 The successful development of Cu(II)-based radiopharmaceuticals is not only dependent on targeting biomolecules but also highly dependent on the proper choice of a bifunctional chelator (BFC) coordinating radioactive Cu(II) ions.7−9
Enormous efforts to construct an ideal BFC have been seen in recent decades. These BFCs should be radiolabeled with radioactive copper ions at a mild temperature with fast reaction kinetics, form stable complex with Cu(II), and possess rapid body clearance.
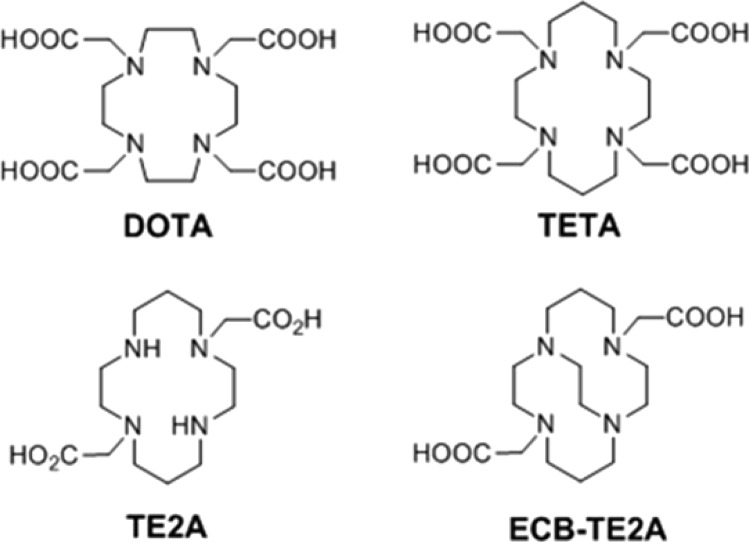
Various tetraazamacrocyclic BFCs containing N-acetic acid pendant arms have been utilized for Cu(II) complexation (Figure 1). The kinetic stability of BFC-Cu(II) complexes could indicate their in vivo stability more closely than thermodynamic stability.10 The order of stability for the BFC-Cu(II) complexes is Cu-ECB-TE2A ≫ Cu-TETA ≈ Cu-DOTA > Cu-EDTA.10,11 It is now well accepted that 64Cu-TETA and 64Cu-DOTA are prone to transchelation of 64Cu ions to proteins under physiological conditions resulting in slow body clearance of radioactivity.12,13 Ethylene cross-bridged (ECB)-TE2A shows excellent kinetic stability for Cu(II) ions in acid decomplexation experiments and equally good in vivo inertness. However, despite this high stability, ECB-TE2A suffers from shortcomings such as cumbersome synthesis (total synthesis time: 35 days and 45% overall yield from cyclam), and harsh radiolabeling conditions for 64Cu ions (1–2 h at 75–95 °C).14,15
Figure 1.
Commonly used bifunctional chelators for radioactive Cu radiolabeling.
We have reported that TE2A (1,8-N,N′-bis-(carboxymethyl)-1,4,8,11-tetraazacyclotetradecane) is a better chelator for Cu(II) ions than TETA (1,4,8,11-tetraazacyclotetradecane-1,4,8,11-tetraacetic acid) and DOTA (1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid) in terms of high kinetic stability and easy radiolabeling.16 TE2A forms a stable Cu(II) complex by attaining a strong N4 in-plane ligand field in the trans configuration, whereas the Cu-TETA complex prefers N2O2 coordination in an equatorial plane, even in the same trans configuration.17−19
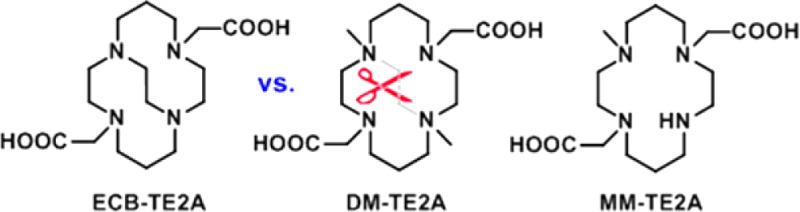
Here, we report the synthesis and physical characterization of N-dimethyl TE2A (DM-TE2A), which is a structural analogue of ECB-TE2A with a broken ethylene cross-bridge. In addition, we also synthesized N-monomethyl TE2A (MM-TE2A) to determine if there is any systematic change in stability depending on the N-alkylation number (Figure 2).
Figure 2.
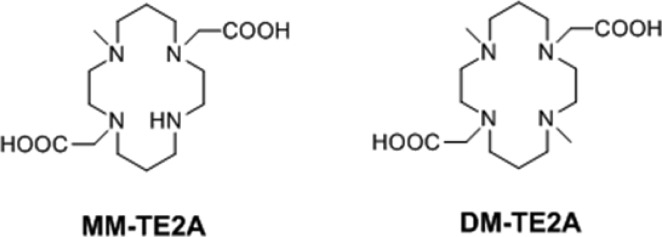
Structures of MM-TE2A and DM-TE2A.
DM-TE2A was first synthesized by Chapman et al. and Comparone et al. in the 1990s, but their synthetic methodologies possess some drawbacks such as lower overall synthesis yield (12.1% and 11.7%, respectively), tedious column chromatographic purification, and sluggish reaction conditions.18,20 Although the Cu(II) complex was also reported, no further detailed physical characterization, particularly stability experiments, have been carried out.
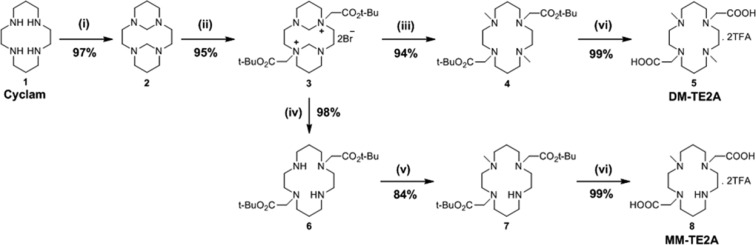
DM-TE2A and MM-TE2A were synthesized in four and five steps from cyclam, respectively, as shown in Scheme 1. Intermediates 3 and 6 were synthesized for DM-TE2A and MM-TE2A, respectively, modifying a method published previously.21 Intermediate 3 was treated with NaBH4 at room temperature to yield dimethylated trans-disubstituted cyclam 4 for DM-TE2A synthesis.22 Then, the dimethylated trans-disubstituted cyclam 4 was converted to DM-TE2A as a trifluoroacetic acid (TFA) salt after being treated with TFA. For MM-TE2A synthesis, compound 6 was stirred with CH3I at room temperature for 1 day to obtain monomethylated trans-disubstituted cyclam 7, which was hydrolyzed using the same TFA treatment to yield MM-TE2A as TFA salt. Dimethylation on the secondary amines of trans-disubstituted cyclam 6 was tried using excess MeI but resulted in only a monomethylated compound as a major product instead of the desired dimethylated analogue. All intermediates and final chelators were fully characterized by 1H-/13C NMR and high-resolution mass spectroscopy (see Supporting Information).
Scheme 1. Synthesis of DM-TE2A and MM-TE2A.
(i) HCHO, H2O, RT, 2 h; (ii) BrCH2CO2t-Bu, MeCN, RT, 1 d; (iii) NaBH4, EtOH, 1 d; (iv) 3 M NaOH, RT, 3 h; (v) MeI, CHCl3, RT, 1 d; (vi) TFA/CH2Cl2 (1:1), RT, 1 d.
By employing efficient synthetic routes, DM-TE2A and MM-TE2A were synthesized with overall yields of 85% and 75% from cyclam, respectively, in a short synthesis time. Substantial improvement in the synthesis of DM-TE2A was achieved in terms of high synthesis yield (<13% vs 85%) as well as simple purification. MM-TE2A is first reported here.
MM-/DM-TE2A were refluxed with Cu(ClO4)2·6H2O in methanol solution to give Cu-MM-/DM-TE2A complexes at 89% and 85% yields. Acidic decomplexation and cyclic voltammetry experiments were carried out to verify kinetic stability of the newly synthesized copper complexes. Drastic acidic conditions (12 M HCl, 90 °C) were used to assess the stability of the Cu(II) complexes, and their degradation pattern was monitored by high performance liquid chromatography (HPLC) (Figure 3a,b). The current stability data were compared with those of Cu-TE2A and Cu-ECB-TE2A.16,21 Approximately 2.7% and 1.6% of the intact Cu-MM-/DM-TE2A complexes were found at 4 and 14 h after incubation, respectively, whereas most of the Cu-TE2A complex degraded in <1 h in the same acid decomplexation experiment. The Cu-DM-TE2A showed even higher robustness than Cu-ECB-TE2A under this harsh acidic condition (Table 1). Only 2.9% of the intact Cu-ECB-TE2A complex was found at 8 h postheating, compared to 9.1% of Cu-DM-TE2A at the same time point (Table 1).21 These acidic decomplexation studies clearly showed that the further alkylation on the TE2A N-atoms dramatically increased in vitro kinetic stability.
Figure 3.
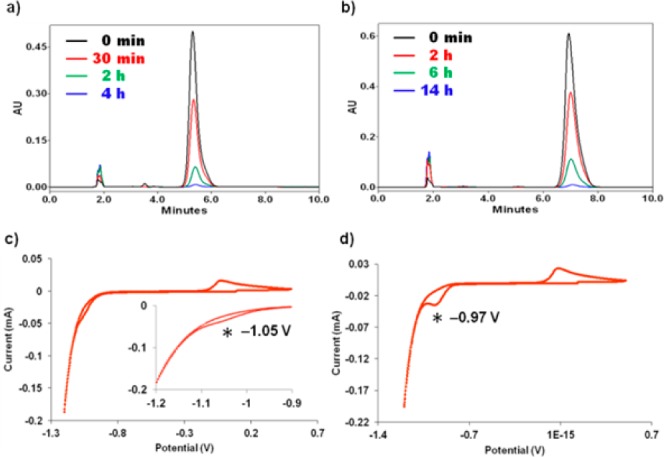
Time-dependent UV-HPLC chromatograms of Cu-MM-TE2A (a) and Cu-DM-TE2A (b) during acidic decomplexation in 12 M HCl at 90 °C. Cyclic voltammograms (scan rate 100 mV/s, 0.2 M phosphate buffer, pH 7) of Cu-MM-TE2A (c) and Cu-DM-TE2A (d).
Table 1. Acidic Decomplexation Study of Cu(II) Complexes.
| % of the intact Cu(II) complex |
||||
|---|---|---|---|---|
| time (h) | Cu-MM-TE2A | Cu-DM-TE2A | Cu-TE2A | Cu-ECB-TE2A |
| 0 | 100 | 100 | 100 | 100 |
| 0.5 | 56.3 | 9.7 | ||
| 1 | 35.8 | 0.6 | 54.9 | |
| 2 | 13.1 | 61.9 | 35.6 | |
| 3 | 6.1 | 23.8 | ||
| 4 | 2.7 | 32.2 | 15.2 | |
| 6 | 18.2 | 6.5 | ||
| 8 | 9.1 | 2.9 | ||
| 10 | 5.1 | |||
| 12 | 2.5 | |||
| 14 | 1.6 | |||
Both Cu-MM-/DM-TE2A complexes yielded irreversible reduction voltammograms with similar reduction potential values (Figure 3c,d). In comparison with Cu-TE2A and Cu-ECB-TE2A, very similar irreversible cyclic voltammograms were measured in the same scanning conditions (Table 2).21 Even though the reduction potential values of the Cu-MM-/DM-TE2A complexes were slightly shifted to positive values compared to those of TE2A and ECB-TE2A, the differences were <100 mV.
Table 2. Reduction Potentials and Partition Coefficients of the Cu(II) Complexes.
| copper(II) complexes | Ered (V) vs Ag/AgCl | log Pa |
|---|---|---|
| Cu-DM-TE2A | –0.97 (irrev) | –3.05 ± 0.06 |
| Cu-MM-TE2A | –1.05 (irrev) | –3.19 ± 0.05 |
| Cu-TE2A | –1.05 (irrev)16 | –3.36 ± 0.02 |
| Cu-ECB-TE2A | –1.07 (irrev)21 | –3.15 ± 0.01 |
Log P values were measured using 64Cu-labeled complexes.
Thus, N-methylation seemed to have a limited effect on the electrochemical behavior of the TE2A Cu(II) complex derivatives. Furthermore, both Cu-MM/DM-TE2A complexes were expected to show similar inertness as Cu-TE2A and Cu-ECB-TE2A to reduce Cu(II) to Cu(I) and subsequent demetalation of the Cu(I) ions from chelators under physiological conditions.
After analyzing the robustness of the synthesized Cu(II) complexes, 64Cu radiolabeling of the BFCs was attempted under a variety of conditions, i.e., different base and buffers, pH (5–9.5), and temperature (25–99 °C) (Scheme 2). The radiolabeling yield was measured by radio-thin-layer chromatography and reconfirmed by radio-HPLC. Both MM- and DM-TE2A were quantitatively radiolabeled with 64CuCl2 within 1 h by the Cs2CO3 treatment as a base at 50 °C (Figure 4). Even though DM-TE2A could also be quantitatively radiolabeled in 0.1 M NaHCO3 buffer (pH 9.5) in 1 h, a 95 °C temperature incubation was required. We did not observe any radiolabeled peak at a lower temperature (<40 °C) under buffered conditions. MM-TE2A showed lower labeling yield (∼70%) than DM-TE2A at the same buffer conditions (0.1 M NaHCO3, pH 9.5, 1 h, 95 °C). These harsh radiolabeling conditions were striking when considering the structural similarity of MM-/DM-TE2A and TE2A. The quantitative radiolabeling of TE2A with 64CuCl2 was achieved in simple buffer within 20 min at 30 °C. The 64Cu-radiolabeling conditions of MM- and DM-TE2A chelators more closely resembled those of ECB-TE2A. This experiment showed that the 64Cu-radiolabeling conditions can be highly influenced by just one or two N-alkylations in the cyclam backbone.
Scheme 2. 64Cu Radiolabeling of MM-TE2A (R′ = Me; R″ = H) and DM-TE2A (R′ and R″ = Me).
Figure 4.
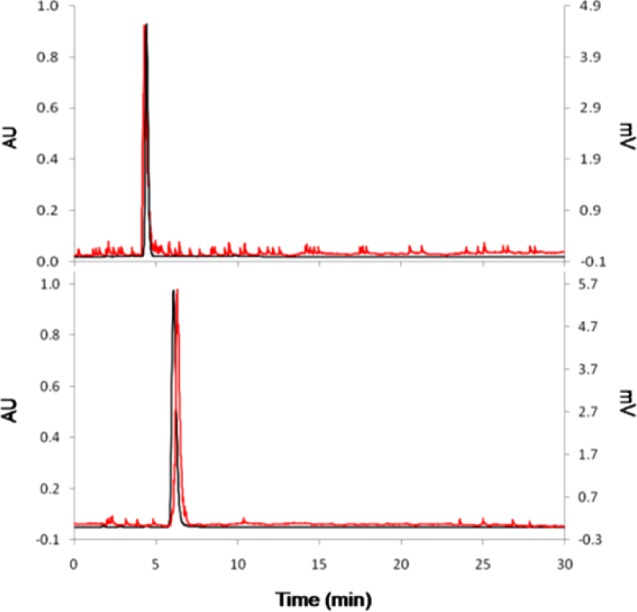
UV-HPLC (280 nm, black) and radio-HPLC chromatogram (red) of 64Cu-MM-TE2A (top) and 64Cu-DM-TE2A (bottom).
Lipophilicity of the radiolabeled MM/DM-TE2A complexes was measured using the octanol/water method (Table 2).21 As expected, 64Cu-TE2A showed the most negative value (−3.36), and lipophilicity increased in 64Cu-MM-TE2A and 64Cu-DM-TE2A (−3.19 and −3.05, respectively) as the secondary amines were converted to tertiary amines by N-methylation. The log P value of 64Cu-ECB-TE2A fell in-between those of 64Cu-MM-TE2A and 64Cu-DM-TE2A (−3.15).
64Cu-MM/DM-TE2A did not show any signs of decomplexation for up to 24 h in the serum stability test (fetal bovine serum, 37 °C).
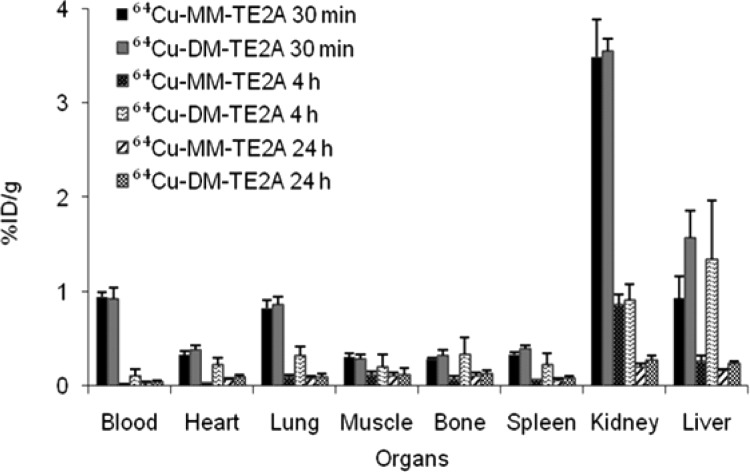
Finally, the in vivo stability and clearance pattern of 64Cu-MM-/DM-TE2A were monitored by biodistribution experiments in Balb/c mice (Figure 5).23 Both radiocomplexes rapidly cleared from the body. The highest uptake of 64Cu-MM-TE2A was observed in kidneys at 30 min (3.49 ± 0.40% of injected dose per gram, % ID/g) but decreased dramatically to 0.85 ± 0.36 and 0.16 ± 0.02% ID/g at 4 and 24 h postinjection, respectively. Less than one-third of kidney uptake was seen in the liver at 30 min (0.93 ± 0.24% ID/g), indicating that 64Cu-MM-TE2A is excreted mainly by the renal track. Very minimal 64Cu-MM-TE2A activity was observed in nonclearance organs such as blood, heart, muscle, bone, and spleen at 24 h (<0.08% ID/g).
Figure 5.
Biodistribution data of 64Cu-MM-TE2A and 64Cu-DM-TE2A at 30 min, 4 h, and 24 h postinjection in Balb/c mice (n = 5).
A similar renal excretion pattern was observed for 64Cu-DM-TE2A, but the hepatobilliary excretion portion via the liver was higher than that of 64Cu-MM-TE2A at all time points. Additionally, uptake in the lung, heart, and spleen also increased for 64Cu-DM-TE2A compared to that of 64Cu-MM-TE2A. This observation could be the consequence of higher lipophilicity of 64Cu-DM-TE2A than that of 64Cu-MM-TE2A. It is well documented that small lipophilic molecules show persistent uptake in the liver, lung, heart, and spleen along with elevated uptake in kidneys.24,25 However, liver uptake of 64Cu-DM-TE2A also decreased dramatically from 1.43 ± 0.54% ID/g at 4 h to 0.42 ± 0.03% ID/g at 24 h.
The blood, liver, and kidney uptake of 64Cu-MM-/DM-TE2A at 24 h was compared with that of 64Cu-ECB-TE2A because the late time biodistribution data could be a good indicator of in vivo Cu(II) complex stability (Table 3).12,2664Cu-MM-TE2A showed the lowest values in all three organs and 64Cu-DM-TE2A showed the highest uptake in the liver and kidney, which seemed to have some correlation with their lipophilicity. However, the uptake differences of the three complexes were rather small and comparable with each other.
Table 3. Selected Organ Biodistribution (% ID/g) of 64Cu-MM-TE2A, 64Cu-DM-TE2A, and 64Cu-ECB-TE2A at 24 h Postinjection in Balb/c Mice (n = 5).
| 64Cu-BFC complex | blood | kidney | liver |
|---|---|---|---|
| 64Cu-MM-TE2A | 0.019 ± 0.003 | 0.162 ± 0.021 | 0.168 ± 0.035 |
| 64Cu-DM-TE2A | 0.053 ± 0.004 | 0.382 ± 0.031 | 0.426 ± 0.033 |
| 64Cu-ECB-TE2A | 0.055 ± 0.010 | 0.280 ± 0.026 | 0.297 ± 0.038 |
These biodistribution data suggest that the 64Cu-DM-TE2A and 64Cu-MM-TE2A complexes cleared rapidly with minimum transchelation of 64Cu ions from the chelators to the biomolecules.12,26
Notably, even though DM-TE2A and ECB-TE2A share structural similarity, their core coordination spheres of Cu(II) complexes of the two chelators are very different.27 DM-TE2A forms a Cu(II) complex in the trans-III configuration, in which the Cu(II) ion exhibits coordination with four short bonds to nitrogen in a ring plane and two longer bonds to oxygen in axial positions.18 In contrast, the Cu-ECB-TE2A complex has a cis-V configuration with Jahn–Teller elongation along a N–Cu–O axis.14
A different conjugation strategy will be employed when MM- and DM-TE2A are conjugated with biomolecules. The additional functional group is to be introduced on the remaining secondary amine for facile conjugation of MM-TE2A with biomolecules, while one of two acetate groups of DM-TE2A will be used for amide bond formation with amine group of biomolecules.28 Cross-bridged monoamides, model compounds of peptide-conjugated ECB-TE2A, showed high in vivo stability and fast body clearance.29 On the basis of high structural similarity between ECB-TE2A and DM-TE2A, high in vivo stability of 64Cu-radiolabeled DM-TE2A-bioconjugate is also expected. However, all further conjugation using MM/DM-TE2A and following in vivo stability of conjugates should be evaluated by appropriate experiments.
In summary, two non-cross-bridged TE2A derivatives showing high kinetic stability were synthesized in an efficient manner. MM- and DM-TE2A showed high similarity with ECB-TE2A rather than TE2A in terms of high kinetic stability and harsh radiolabeling conditions. Easy synthesis, high stability of the Cu complex, and quantitative radiolabeling yield with 64Cu ions make MM/DM-TE2A a good candidate as a potential BFC. Our results clearly demonstrate that there is still room for developing a better chelator for 64Cu-radiolabeling by simple structural fine-tuning of non-cross-bridged tetraazamacrocyclic compounds.
Supporting Information Available
Experimental procedures and detailed characterization for synthesis of MM/DM-TE2A, Cu(II) complexation, acidic decomplexation, cyclic voltametry, 64Cu radiolabeling, in vitro serum stability experiments, partition coefficient, and comparative biodistribution experiments of 64Cu-MM/DM/ECB-TE2A. This material is available free of charge via the Internet at http://pubs.acs.org.
Author Contributions
# These authors contributed equally to this work.
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (No. 20090081817, 2012-0006386, 20090078235, and 2013R1A2A2A01012250), and Brain Korea 21 Project. This research was also partially supported by Kyungpook National University Research Fund, 2012. The Korea Basic Science Institute (Daegu) is acknowledged for the NMR and MS measurements.
The authors declare no competing financial interest.
Supplementary Material
References
- Wadas T. J.; Wong E. H.; Weisman G. R.; Anderson C. J. Coordinating radiometals of copper, gallium, indium, yttrium, and zirconium for PET and SPECT imaging of disease. Chem. Rev. 2010, 110, 2858–2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeglis B. M.; Lewis J. S. A practical guide to the construction of radiometallated bioconjugates for positron emission tomography. Dalton Trans. 2011, 40, 6168–6195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya S.; Dixit M. Metallic radionuclides in the development of diagnostic and therapeutic radiopharmaceuticals. Dalton Trans. 2011, 40, 6112–6128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson B. M.; Donnelly P. S. Copper complexes of bis(thiosemicarbazones): from chemotherapeutics to diagnostic and therapeutic radiopharmaceuticals. Chem. Soc. Rev. 2011, 40, 3005–3018. [DOI] [PubMed] [Google Scholar]
- Anderson C. J.; Ferdani R. Copper-64 radiopharmaceuticals for PET imaging of cancer: advances in preclinical and clinical research. Cancer Biother. Radiopharm. 2009, 24, 379–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mewis R. E.; Archibald S. J. Biomedical applications of macrocyclic ligand complexes. Coord. Chem. Rev. 2010, 254, 1686–1712. [Google Scholar]
- Wadas T. J.; Wong E. H.; Weisman G. R.; Anderson C. J. Copper chelation chemistry and its role in copper radiopharmaceuticals. Curr. Pharm. Des. 2007, 13, 3–16. [DOI] [PubMed] [Google Scholar]
- Bartholomä M. D. Recent developments in the design of bifunctional chelators for metal-based radiopharmaceuticals used in positron emission tomography. Inorg. Chim. Acta 2012, 389, 36–51. [Google Scholar]
- Liu S. Bifunctional coupling agents for radiolabeling of biomolecules and target-specific delivery of metallic radionuclides. Adv. Drug Delivery Rev. 2008, 60, 1347–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodin K. S.; Heroux K. J.; Boswell C. A.; Wong E. H.; Weisman G. R.; Niu W.; Tomellini S. A.; Anderson C. J.; Zakharov L. N.; Rheingold A. L. Kinetic inertness and electrochemical behavior of copper(II) tetraazamacrocyclic complexes: possible implications for in vivo stability. Eur. J. Inorg. Chem. 2005, 2005, 4829–4833. [Google Scholar]
- Cole W. C.; DeNardo S. J.; Meares C. F.; McCall M. J.; DeNardo G. L.; Epstein A. L.; O’Brien H. A.; Moi M. K. Comparative serum stability of radiochelates for antibody radiopharmaceuticals. J. Nucl. Med. 1987, 28, 83–90. [PubMed] [Google Scholar]
- Bass L. A.; Wang M.; Welch M. J.; Anderson C. J. In vivo transchelation of copper-64 from TETA-octreotide to superoxide dismutase in rat liver. Bioconjugate Chem. 2000, 11, 527–532. [DOI] [PubMed] [Google Scholar]
- Boswell C. A.; Sun X.; Niu W.; Weisman G. R.; Wong E. H.; Rheingold A. L.; Anderson C. J. Comparative in vivo stability of copper-64-labeled cross-bridged and conventional tetraazamacrocyclic complexes. J. Med. Chem. 2004, 47, 1465–1474. [DOI] [PubMed] [Google Scholar]
- Wong E. H.; Weisman G. R.; Hill D. C.; Reed D. P.; Rogers M. E.; Condon J. S.; Fagan M. A.; Calabrese J. C.; Lam K.-C.; Guzei I. A.; Rheingold A. L. Synthesis and characterization of cross-bridged cyclams and pendant-armed derivatives and structural studies of their copper(II) complexes. J. Am. Chem. Soc. 2000, 122, 10561–10572. [Google Scholar]
- Wadas T. J.; Anderson C. J. Radiolabeling of TETA- and CB-TE2A-conjugated peptides with copper-64. Nat. Protoc. 2006, 1, 3062–3068. [DOI] [PubMed] [Google Scholar]
- Pandya D. N.; Kim J. Y.; Park J. C.; Lee H.; Phapale P. B.; Kwak W.; Choi T. H.; Cheon G. J.; Yoon Y. R.; Yoo J. Revival of TE2A; a better chelate for Cu(II) ions than TETA?. Chem. Commun. 2010, 46, 3517–3519. [DOI] [PubMed] [Google Scholar]
- Helps I. M.; Parker D.; Chapman J.; Ferguson G. Selective N,N-functionalisation of cyclam: crystal structure of the Cu2+ complex of 1,4,8,11-tetra-azacyclotetradecane-1,8-diacetic acid and the tricyclic lactam 15,18-dioxo-1,5,8,12-tetra-azatricyclo[10.2.2.25.8]tetradecane. J. Chem. Soc., Chem. Commun. 1988, 16, 1094–1095. [Google Scholar]
- Chapman J.; Ferguson G.; Gallagher J. F.; Jennings M. C.; Parker D. Copper and nickel complexes of 1,8-disubstituted derivatives of 1,4,8,11-tetraazacyclotetradecane. J. Chem. Soc., Dalton Trans. 1992, 3, 345–353. [Google Scholar]
- Silversides J. D.; Allan C. C.; Archibald S. J. Copper(II) cyclam-based complexes for radiopharmaceutical applications: synthesis and structural analysis. Dalton Trans. 2007, 9, 971–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comparone A.; Kaden T. A. Copper(II) and nickel(II) complexes of trans-difunctionalized tetraaza macrocycles. Helv. Chim. Acta 1998, 81, 1765–1772. [Google Scholar]
- Pandya D. N.; Dale A. V.; Kim J. Y.; Lee H.; Ha Y. S.; An G. I.; Yoo J. New macrobicyclic chelator for the development of ultrastable 64Cu-radiolabeled bioconjugate. Bioconjugate Chem. 2012, 23, 330–335. [DOI] [PubMed] [Google Scholar]
- Royal G.; Dahaoui-Gindrey V.; Dahaoui S.; Tabard A.; Guilard R.; Pullumbi P.; Lecomte C. New synthesis of trans-disubstituted cyclam macrocycles: elucidation of the disubstitution mechanism on the basis of X-ray data and molecular modeling. Eur. J. Org. Chem. 1998, 1998, 1971–1975. [Google Scholar]
- Cai H.; Li Z.; Huang C. W.; Park R.; Shahinian A. H.; Conti P. S. An improved synthesis and biological evaluation of a new cage-like bifunctional chelator, 4-((8-amino-3,6,10,13,16,19-hexaazabicyclo[6.6.6]icosane-1-ylamino)methyl)benzoic acid, for 64Cu radiopharmaceuticals. Nucl. Med. Biol. 2010, 37, 57–65. [DOI] [PubMed] [Google Scholar]
- Bailey G. A.; Price E. W.; Zeglis B. M.; Ferreira C. L.; Boros E.; Lacasse M. J.; Patrick B. O.; Lewis J. S.; Adam M. J.; Orvig C. H2azapa: a versatile acyclic multifunctional chelator for (67)Ga, (64)Cu, (111)In, and (177)Lu. Inorg. Chem. 2012, 51, 12575–12589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard A. B.; Kronauge J. F.; Barbarics E.; Kiani S.; Treves S. T. Synthesis and biodistribution of a lipophilic 64Cu-labeled monocationic Copper(II) complex. Nucl. Med. Biol. 2002, 29, 289–294. [DOI] [PubMed] [Google Scholar]
- Yoo J.; Reichert D. E.; Welch M. J. Comparative in vivo behavior studies of cyclen-based copper-64 complexes: regioselective synthesis, X-ray structure, radiochemistry, log P, and biodistribution. J. Med. Chem. 2004, 47, 6625–6637. [DOI] [PubMed] [Google Scholar]
- Kent Barefield E. Coordination chemistry of N-tetraalkylated cyclam ligands: A status report. Coord. Chem. Rev. 2010, 254, 1607–1627. [Google Scholar]
- Sprague J. E.; Peng Y.; Sun X.; Weisman G. R.; Wong E. H.; Achilefu S.; Anderson C. J. Preparation and biological evaluation of copper-64-labeled Tyr3-octreotate using a cross-bridged macrocyclic chelator. Clin. Cancer Res. 2004, 10, 8674–8682. [DOI] [PubMed] [Google Scholar]
- Sprague J. E.; Peng Y.; Fiamengo A. L.; Woodin K. S.; Southwick E. A.; Weisman G. R.; Wong E. H.; Golen J. A.; Rheingold A. L.; Anderson C. J. Synthesis, characterization and in vivo studies of Cu(II)-64-labeled cross-bridged tetraazamacrocycle-amide complexes as models of peptide conjugate imaging agents. J. Med. Chem. 2007, 50, 2527–2535. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.