Four additional references have been added to the reference section, and the last column of Table 1 has been updated with the correct references.
Table 1. pKa Values of Monoacidic, Monobasic, and Dibasic Compounds Determined by the 96-Well UV Spectrophotometric Method.
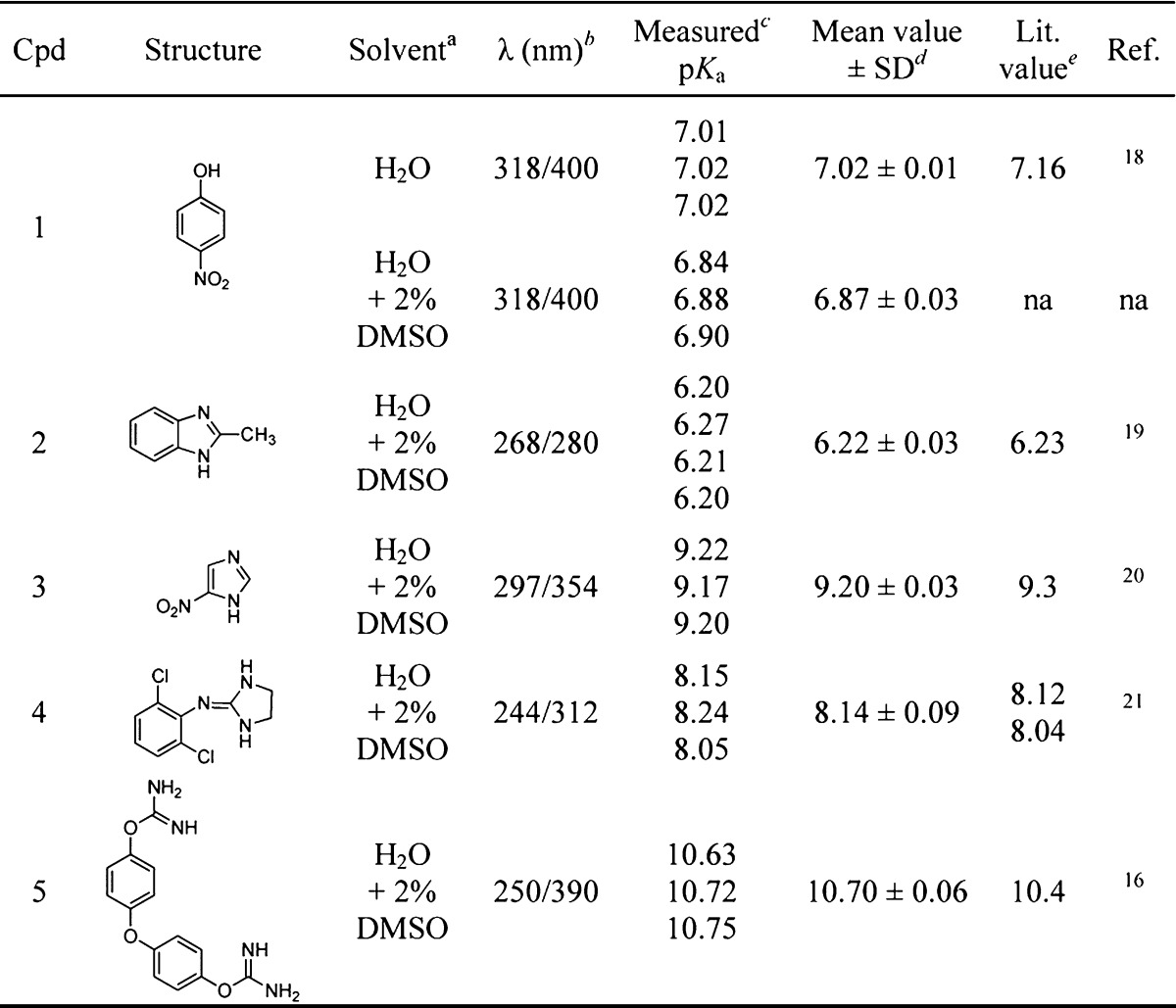
The use of 2% v/v DMSO as cosolvent did not alter significantly the pKa value of the test compounds. Working temperature = 30 °C. All pKa were measured at constant ionic strength (I = 0.1 M) and concentration (C = 0.2 mM).
Analytical wavelengths are determined at the maximum and minimum absorption values in the spectral difference plot.
Experiments were repeated at least three times.
Standard deviation.
Experimental pKa values at 25 °C in water.
■REFERENCES
(16) Nagle P.; Kahvedžić A.; McCabe T.; Rozas I.. On the protonated state of amidinium-like diaromatic derivatives: X-ray and UV studies. Struct. Chem. 2011, 1–9.
(17) Kinsella G. K.; Rodriguez F.; Watson G. W.; Rozas I.. Computational approach to the basicity of a series of [alpha]1-adrenoceptor ligands in aqueous solution. Bioorg. Med. Chem. 2007, 15, 2850–2855.
(18) Izutsu K.IUPAC: Acid–Base Dissociation Constants in Dipolar Aprotic Solvents; Blackewell Science: New York, 1990.
(19) Donkor K. K.; Kratochvil B.. Determination of thermodynamic aqueous acid–base stability constants for several benzimidazole derivatives. J. Chem. Eng. Data 1993, 38, 569–570.
(20) Schofield K.; Grimmett M. R.; Keene B. R. T.. The Azoles; Cambridge University Press: Cambridge, U.K., 1976.
(21) Tam K. Y.; Takács-Novák K.. Multi-wavelength spectrophotometric determination of acid dissociation constants: a validation study. Anal. Chim. Acta 2001, 434, 157–167.


