Abstract
Inclusions comprising the microtubule (MT)-stabilizing protein, tau, are found within neurons in the brains of patients with Alzheimer’s disease and related neurodegenerative disorders that are broadly referred to as tauopathies. The sequestration of tau into inclusions is believed to cause a loss of tau function, such that MT structure and function are compromised, leading to neuronal damage. Recent data reveal that the brain-penetrant MT-stabilizing agent, epothilone D (EpoD), improves cognitive function and decreases both neuron loss and tau pathology in transgenic mouse models of tauopathy. There is thus a need to identify additional MT-stabilizing compounds with blood–brain barrier (BBB) permeability and slow brain clearance, as observed with EpoD. We report here that the MT-stabilizing natural product, dictyostatin, crosses the BBB in mice and has extended brain retention. Moreover, a single administration of dictyostatin to mice causes prolonged stabilization of MTs in the brain. In contrast, the structurally related MT-stabilizer, discodermolide, shows significantly less brain exposure. Thus, dictyostatin merits further investigation as a potential tauopathy therapeutic.
Keywords: Blood−brain barrier, discodermolide, dictyostatin, microtubule, pharmacokinetics, tauopathy
A number of neurodegenerative disorders, including Alzheimer’s disease (AD), progressive supranuclear palsy, corticobasal degeneration, Pick’s disease, and related tauopathies are characterized by the presence of insoluble inclusions of hyperphosphorylated tau protein, which are referred to as neurofibrillary tangles (NFTs) and neuropil threads when found within the soma and neuritic processes of brain neurons, respectively.1,2 The normal function of tau is to bind and stabilize MTs.3,4 In the diseased brain of tauopathy patients, however, MT disengagement and aggregation of hyperphosphorylated tau are thought to result in reduced MT stabilization. As MTs play a critical role in the axonal transport of key neuronal components, an alteration of normal MT function could lead to neuron dysfunction and death. In this regard, there is evidence of impaired MT stabilization in AD brains,5,6 and tau transgenic mouse models of tauopathies that develop NFT-like inclusions have a reduction of MT density and axonal transport.7,8 Accordingly, it has been suggested that MT-stabilizing agents, such as the taxanes or functionally similar molecules, might serve to compensate for a loss of tau function in tauopathies.9,10 Evidence to support this concept was first provided with paclitaxel in a transgenic mouse model with predominantly brain stem and spinal cord tau pathology.11 In these mice, paclitaxel improved motor neuron function after drug uptake at neuromuscular junctions. Paclitaxel, however, does not effectively cross the blood–brain barrier (BBB),12 and thus is not useful for intervention in human tauopathies where tau inclusions are found largely in the brain.
Recently, our laboratories demonstrated that the brain-penetrant MT-stabilizing compound, epothilone D (EpoD, 1, Figure 1), is highly efficacious in both preventative13 and interventional8 studies using tau transgenic mice that develop AD-like tau deposits in their brains with age. In particular, doses of EpoD that were a fraction of those utilized in human oncology clinical trials increased MT density, reduced axonal dystrophy as well as neurodegeneration, and improved cognitive performance in this mouse model of tauopathy.8,13
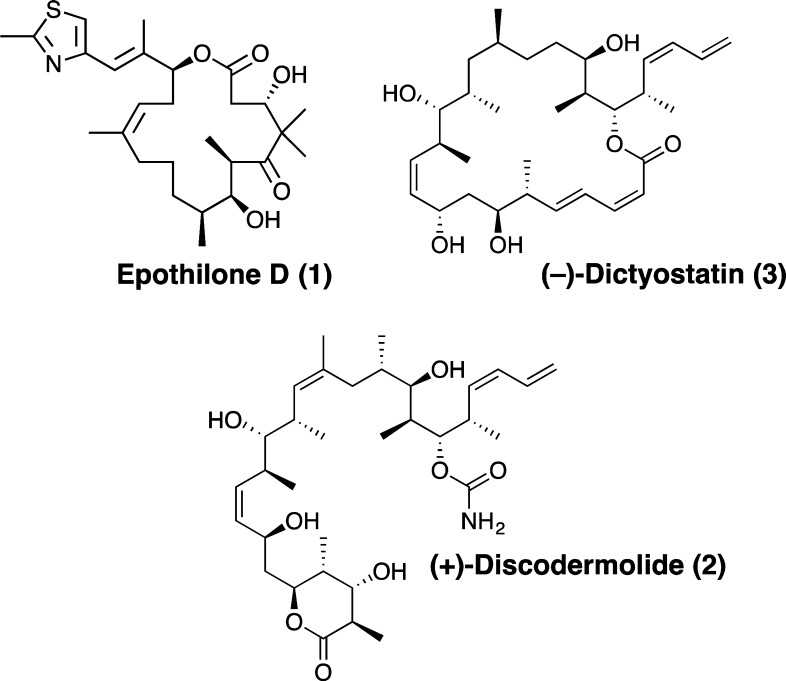
Figure 1.
Structures of epothilone D, discodermolide, and dictyostatin.
Importantly, the low doses of EpoD employed in these studies did not elicit any of the toxicities typically seen with higher doses of MT-stabilizing drugs used for treating cancer patients. These data, which were subsequently validated in two additional tau transgenic models,14 indicate that CNS active MT-stabilizing agents may be therapeutically useful for the prevention and/or treatment of AD and related tauopathies.
To date, EpoD is the only BBB-permeable MT-stabilizing agent that has been examined in tauopathy models, and this drug is now undergoing evaluation in AD patients (BMS-241027; clinicaltrials.gov). Given the potential promise of EpoD for the treatment of AD and related tauopathies, it would be desirable to identify additional CNS-active MT-stabilizing agents that might be considered as alternative candidates to EpoD for AD treatment. Although several members of the epothilone class have been identified that can cross the BBB, the brain penetration of most other classes of MT-stabilizing natural products has not been reported.15 This led us to investigate two structurally related MT-stabilizing natural products, discodermolide (2, Figure 1) and dictyostatin (3, Figure 1).16,17
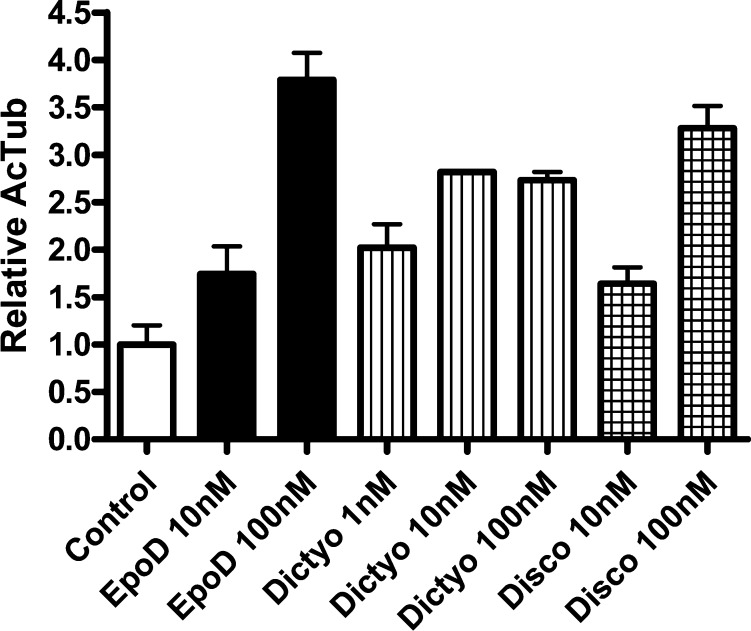
Discodermolide and dictyostatin are among the most potent MT-stabilizing agents reported to date.18−20 Like the taxanes and the epothilones, discodermolide and dictyostatin are thought to promote MT stabilization by interacting with β-tubulin in proximity to the taxane-binding site.18−20 Different modeling21 and NMR22−24 studies have also indicated that the bioactive U-shaped conformation of discodermolide closely resembles the preferred conformation of dictyostatin in solution, which further suggests that these two structurally related natural products may share a similar mode of action. Interestingly, despite the functional similarities observed between the different classes of taxane-binding site MT-stabilizing natural products, some striking differences have been reported. For example, discodermolide and dictyostatin have been found to retain antiproliferative activities against several taxane-resistant cell lines.19,25,26 In addition, cell-free studies indicate significant morphologic differences in the structure of MTs obtained with epothilones and discodermolide.27 The possible implications of these differences, particularly with respect to axonal transport and neurodegenerative diseases, have not been investigated. Thus, to evaluate the potential of 2 and 3 to be alternatives to EpoD for tauopathy treatment, we examined the plasma and brain pharmacokinetic (PK) properties of these natural products. Furthermore, the MT-stabilizing properties of 2 and 3 were evaluated in comparison with EpoD in a cell-based (HEK293) MT-stabilization assay,12 which measures compound-induced elevation of acetylated α-tubulin AcTub, a known biomarker of stable MTs.28,29 Finally, on the basis of the high brain exposure that was observed after dosing mice with dictyostatin (vide infra), we ascertained whether this compound could elicit increased AcTub levels in the mouse brain.
The results of the cell-based studies, highlighted in Figure 2, confirmed that, as expected, discodermolide, dictyostatin, and EpoD all produced a dose-dependent elevation in AcTub. Notably, although EpoD generated the largest absolute increase of AcTub at the highest concentration used in the assay (100 nM), dictyostatin produced the greatest AcTub elevation at 10 nM, as well as a significant AcTub increase at 1 nM.
Figure 2.
AcTub MT-stabilization assay in HEK293 cells: Epothilone D (EpoD), dictyostatin (Dictyo), and discodermolide (Disco). Error bars represent SEM, with n = 4/condition.
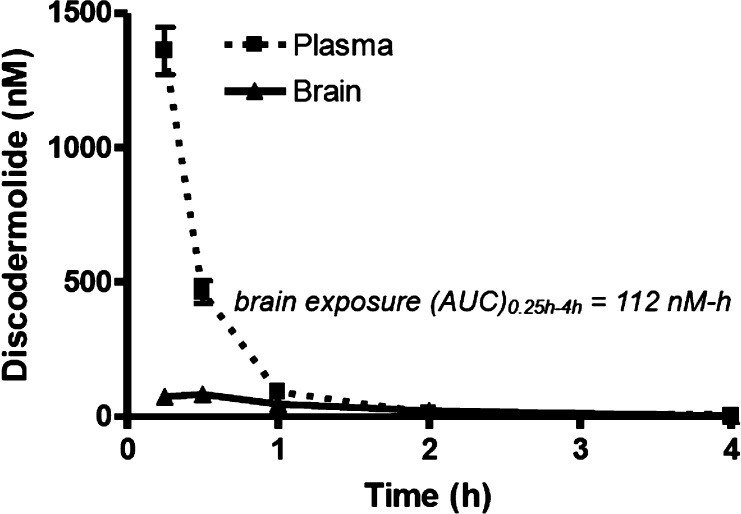
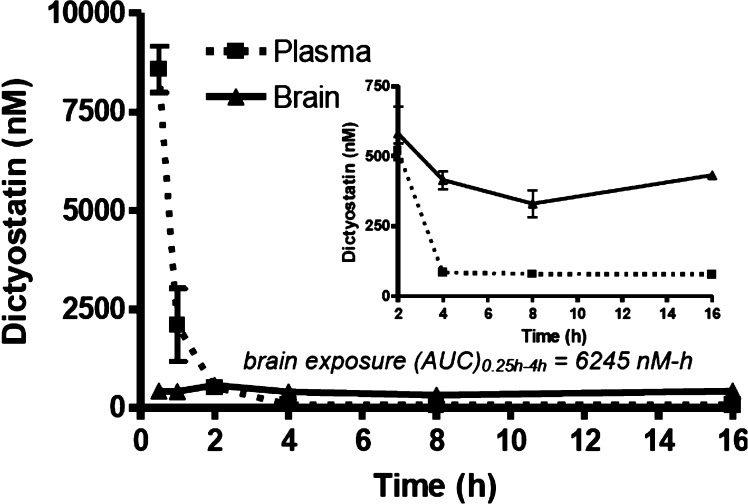
To examine the PK properties, discodermolide or dictyostatin were dosed via i.p. administration to CD1 mice (5 mg/kg), and blood and brain tissue were obtained from groups of animals at multiple time points after dosing. The concentrations of the two MT-stabilizing agents were then determined in these samples via LC–MS/MS analysis. As shown in Figures 3 and 4, in spite of the structural similarities between discodermolide and dictyostatin, the PK properties of these two compounds appeared to be significantly different, particularly as it pertains to brain retention and exposure. Indeed, the brain exposure of dictyostatin (AUC0.5–16h = 6245 nM-h) was much greater than that observed with discodermolide (AUC0.25–4h = 112 nM-h), indicating a possible role for the macrocyclic structure of 3 in determining overall brain penetration.
Figure 3.
Plasma and brain concentrations of discodermolide after a single 5 mg/kg i.p. administration. Error bars represent SEM, with n = 3/time-point.
Figure 4.
Plasma and brain concentrations of dictyostatin after a single 5 mg/kg i.p. administration. The inset shows the compound levels in plasma and brain from 2 h to 16 h. Error bars represent SEM, with n = 3/time-point.
As revealed in Figure 3, discodermolide levels in the plasma declined rapidly after administration, with essentially all compound cleared by 2 h. Relatively little discodermolide partitioned into brain tissue, with a total brain AUC0.25–4h to plasma AUC0.25–4h ratio of 0.26. Dictyostatin was also cleared relatively quickly from plasma after dosing (Figure 4), although the plasma exposure was considerably greater than for discodermolide (5536 nM-h vs 439 nM-h). Interestingly, although the brain concentrations of dictyostatin were much lower than the plasma concentrations shortly after dosing, by 2 h the brain and plasma levels were comparable and thereafter the amount of dictyostatin in the brain exceeded that in plasma (Figure 4; inset). In fact, the brain concentration of dictyostatin stayed relatively constant at ∼400 nM from 4 to 16 h (Table 1). Notably, similarly long brain retentions have been reported for EpoD13 and epothilone B.30 However, our studies demonstrate that the concentration of dictyostatin in the brain 16 h after a single 5 mg/kg dose is greater than that observed at 16 h after a comparable dose of EpoD (Table 1). Given that dictyostatin, like EpoD, exhibits a low unbound fraction in plasma (0.9% ± 0.6%) and after incubation with brain homogenates (0.5% ± 0.02%), the free dictyostatin concentration in brain after a 5 mg/kg dose is estimated to be in the low nM range.
Table 1. Comparison of Plasma and Brain Concentrations of Dictyostatin and EpoD after a Single 5 mg/kg i.p. Dosea.
| 4 h |
16 h |
|||
|---|---|---|---|---|
| compd | brain (nM) | plasma (nM) | brain (nM) | plasma (nM) |
| 3 | 412 ± 54 | 83 ± 4 | 383 ± 87 | 77 ± 5 |
| 1 | 161 ± 14 | 19 ± 2 | 111 ± 9 | <LOD |
Values represent the average and SD of n = 3 mice/time-point/compound (<LOD = below limit of detection).
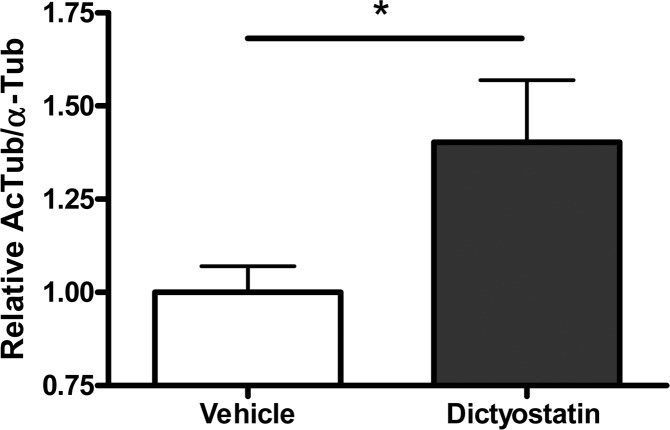
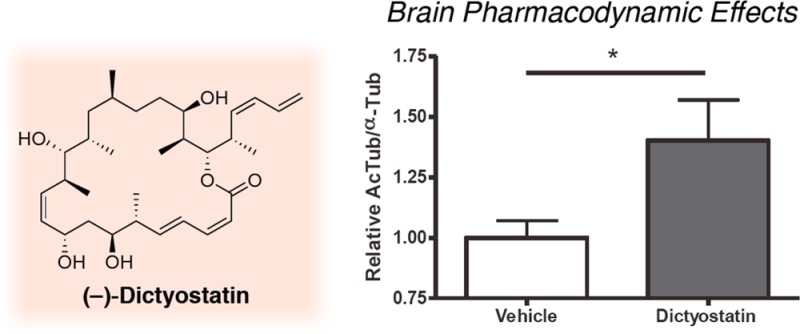
The long residence time of dictyostatin in the brain suggests that this compound may exert a prolonged effect on MT stabilization in the brain. Thus, to evaluate dictyostatin-induced MT-stabilization in the brain, we measured the relative AcTub levels in the brain of WT mice after compound and vehicle administration. Our previous studies with EpoD revealed that an increase in this pharmacodynamic (PD) marker of stable MTs can be seen one week after dosing.12 Notably, as illustrated in Figure 5, a single 5 mg/kg i.p. administration of dictyostatin also resulted in MT-stabilization in the brain one week after dosing, as revealed by the statistically significant elevation in the brain AcTub level of compound-treated animals relative to vehicle-treated controls.
Figure 5.
Relative AcTub-to-α-Tub levels in the brains of mice 7 days after a single i.p. administration of vehicle (DMSO) or dictyostatin (5 mg/kg). The mean AcTub/α-Tub values for the dictyostatin-treated mice were normalized to those of the mice that received vehicle, with 3 mice for each treatment. Error bars represent SEM.
The observed differential between brain and plasma clearance of dictyostatin, which was reported also for EpoD,12,13 coupled with the ability of dictyostatin to produce prolonged PD effects in the brain, may be particularly desirable for the treatment of CNS diseases, such as tauopathies. Indeed, the favorable efficacy and safety profile of EpoD in mouse tauopathy models is likely to relate at least in part to the fact that the drug is cleared very slowly from the brain relative to the plasma.12,13 Thus, the PK and PD results presented here suggest that dictyostatin and possibly other related congeners hold considerable promise as additional brain-penetrant MT-stabilizing compounds for evaluation in mouse models of tauopathy and perhaps ultimately as clinical candidates for human tauopathies.
Glossary
Abbreviations
- AcTub
acetyl–tubulin
- AD
Alzheimer’s disease
- BBB
blood–brain barrier
- EpoD
epothilone D
- MT
microtubule
- NFT
neurofibrillary tangle
Supporting Information Available
Experimental details concerning the pharmacokinetic and pharmacodynamics studies. This material is available free of charge via the Internet at http://pubs.acs.org.
Author Contributions
The manuscript was written by K.R.B. and C.B. and reviewed by all authors. N.M.G., M.J.J., and Y.Y. made experimental contributions; K.B., C.B., and A.B.S. directed research; and V.M.-Y.L., J.Q.T., and I.P. provided resources. All authors have given approval to the final version of the manuscript.
These studies were funded by the Nathan Bilger Fund, The Rotary Coins for Alzheimer’s Research Trust Fund, and the Marian S. Ware Alzheimer Program.
The authors declare no competing financial interest.
Supplementary Material
References
- Ballatore C.; Lee V. M.-Y.; Trojanowski J. Q. Tau-mediated neurodegeneration in Alzheimer’s disease and related disorders. Nat. Rev. Neurosci. 2007, 8, 663–672. [DOI] [PubMed] [Google Scholar]
- Lee V. M.-Y.; Goedert M.; Trojanowski J. Q. Neurodegenerative tauopathies. Annu. Rev. Neurosci. 2001, 24, 1121–1159. [DOI] [PubMed] [Google Scholar]
- Drechsel D. N.; Hyman A. A.; Cobb M. H.; Kirschner M. W. Modulation of the dynamic instability of tubulin assembly by the microtubule-associated protein tau. Mol. Biol. Cell 1992, 3, 1141–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustke N.; Trinczek B.; Biernat J.; Mandelkow E. M.; Mandelkow E. Domains of tau protein and interactions with microtubules. Biochemistry 1994, 33, 9511–9522. [DOI] [PubMed] [Google Scholar]
- Hempen B.; Brion J. P. Reduction of acetylated alpha-tubulin immunoreactivity in neurofibrillary tangle-bearing neurons in Alzheimer’s disease. J. Neuropathol. Exp. Neurol. 1996, 55, 964–972. [DOI] [PubMed] [Google Scholar]
- Cash A. D.; Aliev G.; Siedlak S. L.; Nunomura A.; Fujioka H.; Zhu X.; Raina A. K.; Vinters H. V.; Tabaton M.; Johnson A. B.; Paula-Barbosa M.; Avíla J.; Jones P. K.; Castellani R. J.; Smith M. A.; Perry G. Microtubule reduction in Alzheimer’s disease and aging is independent of tau filament formation. Am. J. Pathol. 2003, 162, 1623–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara T.; Hong M.; Zhang B.; Nakagawa Y.; Lee M. K.; Trojanowski J. Q.; Lee V. M.-Y. Age-dependent emergence and progression of a tauopathy in transgenic mice overexpressing the shortest human tau isoform. Neuron 1999, 24, 751–762. [DOI] [PubMed] [Google Scholar]
- Zhang B.; Carroll J.; Trojanowski J. Q.; Yao Y.; Iba M.; Potuzak J. S.; Hogan A. M.; Xie S. X.; Ballatore C.; Smith A. B.; Lee V. M.; Brunden K. R. The microtubule-stabilizing agent, epothilone d, reduces axonal dysfunction, neurotoxicity, cognitive deficits, and Alzheimer-like pathology in an interventional study with aged tau transgenic mice. J. Neurosci. 2012, 32, 3601–3611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee V. M.-Y.; Daughenbaugh R.; Trojanowski J. Q. Microtubule stabilizing drugs for the treatment of Alzheimer’s disease. Neurobiol. Aging 1994, 15Suppl 2S87–S89. [DOI] [PubMed] [Google Scholar]
- Trojanowski J. Q.; Smith A. B.; Huryn D.; Lee V. M.-Y. Microtubule-stabilizing drugs for therapy of Alzheimer’s disease and other neurodegenerative disorders with axonal transport impairments. Expert Opin. Pharmacother. 2005, 6, 683–686. [DOI] [PubMed] [Google Scholar]
- Zhang B.; Maiti A.; Shively S.; Lakhani F.; McDonald-Jones G.; Bruce J.; Lee E. B.; Xie S. X.; Joyce S.; Li C.; Toleikis P. M.; Lee V. M.-Y.; Trojanowski J. Q. Microtubule-binding drugs offset tau sequestration by stabilizing microtubules and reversing fast axonal transport deficits in a tauopathy model. Proc. Natl. Acad. Sci. U.S.A. 2005, 102, 227–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunden K. R.; Yao Y.; Potuzak J. S.; Ferrer N. I.; Ballatore C.; James M. J.; Hogan A. M.; Trojanowski J. Q.; Smith A. B.; Lee V. M. The characterization of microtubule-stabilizing drugs as possible therapeutic agents for Alzheimer’s disease and related tauopathies. Pharmacol. Res. 2011, 63, 341–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunden K. R.; Zhang B.; Carroll J.; Yao Y.; Potuzak J. S.; Hogan A. M.; Iba M.; James M. J.; Xie S. X.; Ballatore C.; Smith A. B.; Lee V. M.; Trojanowski J. Q. Epothilone D improves microtubule density, axonal integrity, and cognition in a transgenic mouse model of tauopathy. J. Neurosci. 2010, 30, 13861–13866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barten D. M.; Fanara P.; Andorfer C.; Hoque N.; Wong P. Y. A.; Husted K. H.; Cadelina G. W.; DeCarr L. B.; Yang L.; Liu L.; Fessler C.; Protassio J.; Riff T.; Turner H.; Janus C. G.; Sankaranarayanan S.; Polson C.; Meredith J. E.; Gray G.; Hanna A.; Olson R. E.; Kim S.-H.; Vite G. D.; Lee F. Y.; Albright C. F. Hyperdynamic microtubules, cognitive deficits, and pathology are improved in tau transgenic mice with low doses of the microtubule-stabilizing agent BMS-241027. J. Neurosci. 2012, 32, 7137–7145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballatore C.; Brunden K. R.; Huryn D. M.; Trojanowski J. Q.; Lee V. M. Y.; Smith A. B. Microtubule stabilizing agents as potential treatment for Alzheimer’s disease and related neurodegenerative tauopathies. J. Med. Chem. 2012, 55, 8979–8996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunasekera S. P.; Gunasekera M.; Longley R. E.; Schulte G. K. Discodermolide: a new bioactive polyhydroxylated lactone from the marine sponge Discodermia dissoluta. J. Org. Chem. 1990, 55, 4912–4915. [Google Scholar]
- Pettit G. R.; Cichacz Z. A.; Gao F.; Boyd M. R.; Schmidt J. M. Isolation and structure of the cancer cell growth inhibitor dictyostatin 1. J. Chem. Soc., Chem. Commun. 1994, 1111–1112. [Google Scholar]
- Hung D. T.; Chen J.; Schreiber S. L. (+)-Discodermolide binds to microtubules in stoichiometric ratio to tubulin dimers, blocks taxol binding and results in mitotic arrest. Chem. Biol. 1996, 3, 287–293. [DOI] [PubMed] [Google Scholar]
- Kowalski R. J.; Giannakakou P.; Gunasekera S. P.; Longley R. E.; Day B. W.; Hamel E. The microtubule-stabilizing agent discodermolide competitively inhibits the binding of paclitaxel (Taxol) to tubulin polymers, enhances tubulin nucleation reactions more potently than paclitaxel, and inhibits the growth of paclitaxel-resistant cells. Mol. Pharmacol. 1997, 52, 613–622. [PubMed] [Google Scholar]
- Madiraju C.; Edler M. C.; Hamel E.; Raccor B. S.; Balachandran R.; Zhu G.; Giuliano K. A.; Vogt A.; Shin Y.; Fournier J. H.; Fukui Y.; Bruckner A. M.; Curran D. P.; Day B. W. Tubulin assembly, taxoid site binding, and cellular effects of the microtubule-stabilizing agent dictyostatin. Biochemistry 2005, 44, 15053–15063. [DOI] [PubMed] [Google Scholar]
- Martello L. A.; LaMarche M. J.; He L.; Beauchamp T. J.; Smith A. B. III; Horwitz S. B. The relationship between taxol and (+)-discodermolide: synthetic analogs and modeling studies. Chem. Biol. 2001, 8, 843–855. [DOI] [PubMed] [Google Scholar]
- Smith A. B.; LaMarche M. J.; Falcone-Hindley M. Solution structure of (+)-discodermolide. Org. Lett. 2001, 3, 695–698. [DOI] [PubMed] [Google Scholar]
- Canales A.; Matesanz R.; Gardner N. M.; Andreu J. M.; Paterson I.; Díaz J. F.; Jiménez-Barbero J. The bound conformation of microtubule-stabilizing agents: NMR insights into the bioactive 3D structure of discodermolide and dictyostatin. Chem.—Eur. J. 2008, 14, 7557–7569. [DOI] [PubMed] [Google Scholar]
- Sánchez-Pedregal V. M.; Kubicek K.; Meiler J.; Lyothier I.; Paterson I.; Carlomagno T. The tubulin-bound conformation of discodermolide derived by NMR studies in solution supports a common pharmacophore model for epothilone and discodermolide. Angew. Chem., Int. Ed. 2006, 45, 7388–7394. [DOI] [PubMed] [Google Scholar]
- Isbrucker R. A.; Cummins J.; Pomponi S. A.; Longley R. E.; Wright A. E. Tubulin polymerizing activity of dictyostatin-1, a polyketide of marine sponge origin. Biochem. Pharmacol. 2003, 66, 75–82. [DOI] [PubMed] [Google Scholar]
- Vollmer L. L.; Jiménez M.; Camarco D. P.; Zhu W.; Daghestani H. N.; Balachandran R.; Reese C. E.; Lazo J. S.; Hukriede N. A.; Curran D. P.; Day B. W.; Vogt A. A simplified synthesis of novel dictyostatin analogues with in vitro activity against epothilone B-resistant cells and antiangiogenic activity in zebrafish embryos. Mol. Cancer Ther. 2011, 10, 994–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gertsch J.; Meier S.; Müller M.; Altmann K.-H. Differential effects of natural product microtubule stabilizers on microtubule assembly: single agent and combination studies with taxol, epothilone B, and discodermolide. ChemBioChem 2009, 10, 166–175. [DOI] [PubMed] [Google Scholar]
- Laferriere N.; MacRae T.; Brown D. Tubulin synthesis and assembly in differentiating neurons. Biochem. Cell Biol. 1997, 75, 103–117. [PubMed] [Google Scholar]
- Black M.; Baas P.; Humphries S. Dynamics of alpha-deacetylation in intact neurons. J. Neurosci. 1989, 9, 358–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly T.; Wartmann M.; Brueggen J.; Allegrini P. R.; Floersheimer A.; Maira M.; McSheehy P. M. Pharmacokinetic profile of the microtubule stabilizer patupilone in tumor-bearing rodents and comparison of anti-cancer activity with other MTS in vitro and in vivo. Cancer Chemother. Pharmacol. 2008, 62, 1045–1054. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.