Abstract
Flaviviruses form a large family of enveloped viruses affecting millions of people over the world. To date, no specific therapy was suggested for the infected people, making the treatment exclusively symptomatic. Several attempts were performed earlier for the design of fusion inhibitors for mosquito-borne flaviviruses, whereas for the tick-borne flaviviruses such design had not been performed. We have constructed homology models of envelope glycoproteins of tick-transmitted flaviviruses with the detergent binding pocket in the open state. Molecular docking of substituted 1,4-dihydropyridines and pyrido[2,1-b][1,3,5]thiadiazines was made against these models, and 89 hits were selected for the in vitro experimental evaluation. Seventeen compounds showed significant inhibition against tick-borne encephalitis virus, Powassan virus, or Omsk hemorrhagic fever virus in the 50% plaque reduction test in PEK cells. These compounds identified through rational design are the first ones possessing reproduction inhibition activity against tick-borne flaviviruses.
Keywords: Antiviral compounds; flavivirus; 1,4-dihydropyridines; pyrido[2,1-b][1,3,5]thiadiazines
The Flavivirus genus includes a range of viral pathogens causing severe diseases in humans.1−4 These viruses are transmitted by arthropod vectors to mammalian hosts. Apparently the most studied among flaviviruses are the mosquito-borne viruses such as dengue virus (DENV), West Nile virus (WNV), yellow fever virus (YFV), and Japanese encephalitis virus (JEV). Tick-borne flaviviral diseases such as tick-borne encephalitis, being a serious health concern in Russia and Europe,4,5 and Omsk hemorrhagic fever4,6 and Powassan encephalitis,4,7,8 showing low incidence and local importance in Russia, US, and Canada, are associated with severe symptoms. Nevertheless, their causing agents are less studied. The situation is additionally complicated by unavailability of specific drugs affecting flaviviruses. Despite that vaccines are available against tick-borne encephalitis virus (TBEV), YFV, and JEV,9 they can be used successfully only as prophylaxis measure before the infection. Other treatment options include symptomatic treatment schemes and immunoglobulin therapy of tick-borne encephalitis cases,10 several immunomodulating agents (e.g., jodantipyrin11 and luromarin12) are on a preclinical stage, but their applicability in the course of flaviviral infection is limited. Moreover, in the case of Omsk hemorrhagic fever virus (OHFV), Powassan virus (POWV), and other locally important flaviviruses, specific vaccines development would never be done due to a small number of cases. Thus, other more specific and effective ways of treatment of tick-borne flaviviral infections are urgently needed.
Inhibition of the virus entry and replication is a widely accepted strategy in the design of antivirals.13−15 The entry of flavivirus particle into the host cell via receptor-mediated endocytosis requires the fusion of viral and cellular membranes. This process is ruled by pH decrease in the endosome and mediated mostly by envelope glycoprotein E. During the fusion E protein dimers initially arranged in a herringbone pattern on the virion surface undergo a large-scale conformational rearrangement leading to the formation of protein spikes, which attack the host cell membrane.16 A detergent n-octyl-β-d-glucoside (β-OG) binding pocket has been identified in one of DENV E protein ectodomain structures (PDB ID 1OKE(17)), whereas in the other structures this pocket is closed by the kl loop.18 It was assumed that this pocket can be exploited as a putative site for DENV fusion inhibitors. As far as flaviviral E proteins are rather similar,1 this hypothesis has been extended to the other genus members. Blind docking studies against this pocket led to a successful identification of DENV, WNV, and YFV replication inhibitors.19−24 In all these cases, docking was performed into 1OKE structure, whereas activity was tested with different assays. Binding of the inhibitors in the β-OG pocket was shown by NMR.20 According to a Monte Carlo simulation, the open state of β-OG pocket may be preferred over the closed one in the dynamic native conditions.25 Alternative binding sites were also searched for on the surface of the DENV E ectodomain dimer, and several inhibitors were identified.26 A teicoplanin analogue LCTA-949 has been shown to prevent cell entry of various flaviviruses, being the most effective against TBEV, but no data is available on the mode of action of this molecule.27
We tried to identify small molecule compounds able to inhibit the reproduction of tick-borne flaviviruses (namely, TBEV, POWV, and OHFV) via interaction with the envelope protein E in the β-OG pocket. To achieve this goal, we constructed homology models of E proteins for the selected viruses with β-OG pocket in the open state. Subsequently, a virtual screening campaign of a compound library was performed, and hit compounds were selected according to the scores and visual analysis of the binding modes. In vitro assays of the hits revealed several compounds that had shown inhibition of viral reproduction. These data confirmed the validity of the chosen methodology and could be used for further design and development of novel antiflaviviral drugs.
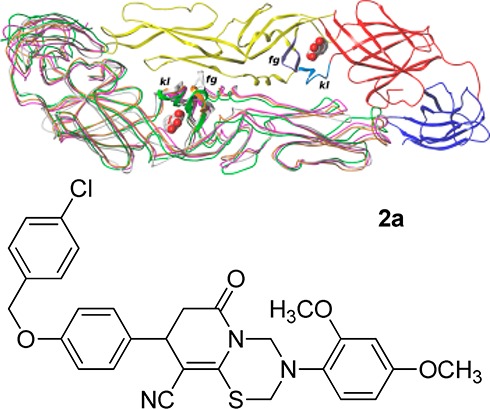
Homology models of E proteins were constructed for the representative strains of TBEV (Absettarov), POWV (Powassan24), and OHFV (Nikitina) (Figure 1). The homology models were built in Modeller 9.1028 based on the TBEV E protein dimer template structure (PDB ID 1SVB(18)) for the whole sequence except the kl loop, which was modeled based on 1OKE template.17 All models built possessed good stereochemical quality.
Figure 1.
Superposition of E protein structures and models for DENV (subunit 1 colored by domains (red, domain I; yellow, domain II; blue, domain III), subunit 2 colored magenta), TBEV (white), POWV (green), and OHFV (orange). Loops kl and fg are colored in subunit 1 and shown as ribbons in subunit 2. β-OG molecules are shown spacefilled.
Given the absence of known compounds targeting the TBEV, POWV, and OHFV E proteins, we chose a library of elongated heterocyclic compounds synthesized in ChemEx laboratory for further virtual screening (VS) studies. The molecular docking of the library containing 5886 compounds was performed with FRED 2.2.5.29 The predicted binding modes for POWV E protein were scored with six scoring functions implemented in FRED. Top 500 hit lists were analyzed for each scoring function, and the compounds included into at least three hit lists were selected for further analysis along with top compounds selected by each scoring function; the total number of compounds was 201. These compounds were then docked into TBEV and OHFV E protein models. Predicted binding modes of the compounds were analyzed visually, and compounds with unacceptable binding modes (e.g., forming hydrogen bond donor–donor or acceptor–acceptor interactions) were excluded along with compounds belonging to scarcely represented classes. The final hit list consisted of 89 compounds belonging to two related classes, 1 and 2.
The Hantzsch-type 1,4-dihydropyridines 1a–q were synthesized in two steps by analogy with known procedures.30,31 First, the reaction of cyanothioacetamide 3 with aromatic aldehydes and acetoacetanilides 4 in the presence of 1.5-fold excess of N-methylmorpholine gave 1,4-dihydropyridine-2-thiolates 5. The next step included regioselective S-alkylation by treatment with alkyl chlorides to give target compounds 1a–q (Scheme 1.a).
Scheme 1.a.
Pyridothiadiazines 2a–e were obtained in three steps starting from cyanothioacetamide 3 and Meldrum’s acid 6 by analogy with the known procedure.32 The stable adducts 7 were cyclized in ethanol under reflux to furnish 4,5-dihydropyridone-2-thiolates 8.33,34 Double Mannich-type aminomethylation of these intermediates was achieved by treatment with 1 equiv of a primary amine and an excess of formaldehyde under short-term heating to form compounds 2 as sole products.
Prior to in vitro studies, cytotoxicity of the compounds was assessed in porcine embryo kidney (PEK) cell line (Table 1); acute (24 h) and chronic (7 days) toxicities were evaluated. For the majority of compounds, CC50 values were much over 10 μM. It was considered as generally acceptable for the first compounds in the class.
Table 1. Structures and Antiviral Activity of the Compounds That Passed Preliminary Assays.
| IC50, μM |
||||||||
|---|---|---|---|---|---|---|---|---|
| compd | Ar | R | R1 | CC50, μM (acute) | CC50, μM (chronic) | TBEV | POWV | OHFV |
| 1a | 2-furyl | 4-EtOC6H4 | 4-nBuC6H4NH | 64 | 14 | 2.5 ± 0.5 | >10 | >10 |
| 1b | 2-furyl | 4-H2NSO2C6H4 | 4-EtC6H4NH | >250a | 153 | >10 | >10 | 10 ± 7.8 |
| 1c | 2-furyl | 4-ClC6H4 | 4-EtC6H4NH | 26 | 27 | >10 | >10 | >10 |
| 1d | 2-furyl | 4-EtC6H4 | 4-EtC6H4NH | 57 | 33 | >10 | >10 | >10 |
| 1e | 2-furyl | 2-MeC6H4 | 2-naphthyl-NH | >250 | >250 | >10 | >10 | 5.3 ± 0.1 |
| 1f | 2-furyl | Ph | 3,4-Me2C6H3 | >250 | 19 | >10 | >10 | 3.2 ± 0.8 |
| 1g | 2-furyl | 2,6-Me2C6H3 | 2-benzothiazolyl-NH | >250 | 7 | >10 | >10 | 7.1 ± 0.1 |
| 1h | 2-furyl | 2-benzothiazolyl | 2-benzothiazolyl-NH | >250 | 52 | >10 | >10 | 2.5 ± 0.9 |
| 1i | 2-furyl | 2-benzothiazolyl | 4-iPrC6H4NH | >250 | 29 | >10 | >10 | 2.5 ± 0.1 |
| 1j | 5-Me-2-furyl | 2-MeOC6H4 | 4-BrC6H4NH | 248 | 34 | >10 | >10 | 3.7 ± 0.4 |
| 1k | 2-thienyl | Ph | Ph | >250 | 17 | >10 | >10 | >10 |
| 1l | 2-thienyl | 2-MeOC6H4 | 4-MeC6H4NH | >250 | 97 | >10 | >10 | 5.5 ± 0.9 |
| 1m | Ph | 4-ClC6H4 | 3-MeC6H4NH | >250 | 41 | 2.0 ± 0.4 | >10 | >10 |
| 1n | Ph | 4-ClC6H4 | 4-PhOC6H4NH | >250 | 38 | 2.8 ± 0.6 | >10 | >10 |
| 1o | Ph | 4-ClC6H4 | 2-naphthyl-NH | 111 | 20 | >10 | >10 | >10 |
| 1p | 2-FC6H4 | 2-MeC6H4 | 4-MeOC6H4NH | >250 | 89 | >10 | >10 | 7.2 ± 0.5 |
| 1q | 4-HO-3-MeOC6H3 | Ph | 4-EtC6H4NH | 114 | 31 | >10 | >10 | 1.8 ± 0.4 |
| 2a | 4-(4-ClC6H4CH2O)C6H4 | 2,4-(MeO)2C6H3 | 109 | 39 | 0.07 ± 0.02 | 1.3 ± 0.1 | >10 | |
| 2b | 3-BnOC6H4 | 2-EtOC6H4 | >250 | 116 | 2.6 ± 0.4 | 2.2 ± 0.3 | >10 | |
| 2c | 3-BnOC6H4 | 4-EtC6H4 | >250 | 236 | >10 | >10 | >11.9 | |
| 2d | 4-BnOC6H4 | 4-nBuC6H4 | >250 | 35 | 1.9 ± 0.4 | >10 | >10 | |
| 2e | 4-BnO-3-MeOC6H3 | 4-MeOC6H4 | >250 | 53 | 0.09 ± 0.01 | >10 | >10 | |
The 250 μM concentration is the highest concentration studied.
Preliminary assessment of antiviral activity of hit compounds in 10 μM concentration was studied in 50% plaque reduction test (PRT50) in PEK cell line against selected strains of TBEV, POWV, or OHFV. Twenty-two compounds showing over 50% antiviral activity in the preliminary tests were promoted for the IC50 determination stage performed as the same assay with sequential dilutions of the studied compound starting from 10 μM. Tested compounds showed dose-dependent inhibition of viral reproduction. PRT50 IC50 values are given in Table 1. Detailed computational and experimental procedures and compound characterization data are provided in the Supporting Information.
Compounds from 1,4-dihydropyridine series 1 have shown the inhibitory activity against OHFV or TBEV (Table 1). These inhibitors were ranked high in the hit lists for the corresponding viruses, showing high selectivity despite 93% sequence identity between E protein ectodomains. Compound 1q, the most active against OHFV, was the only one in the series 1 containing hydroxy and methoxy groups in the Ar substituent. According to the docking results, this moiety occupied a cavity between Tyr49 and the kl loop (Figure 2). The presence of many aromatic and/or hydroxyl bearing residues in this region (Ser47, Tyr49, Thr279, and Tyr281) suggested that binding of such moiety is favored there. The presence of hydrogen bond acceptors in the variable moieties was the only requirement that seems to be important for the compound potency; the only active compound in the series 1 without such groups was 1m.
Figure 2.
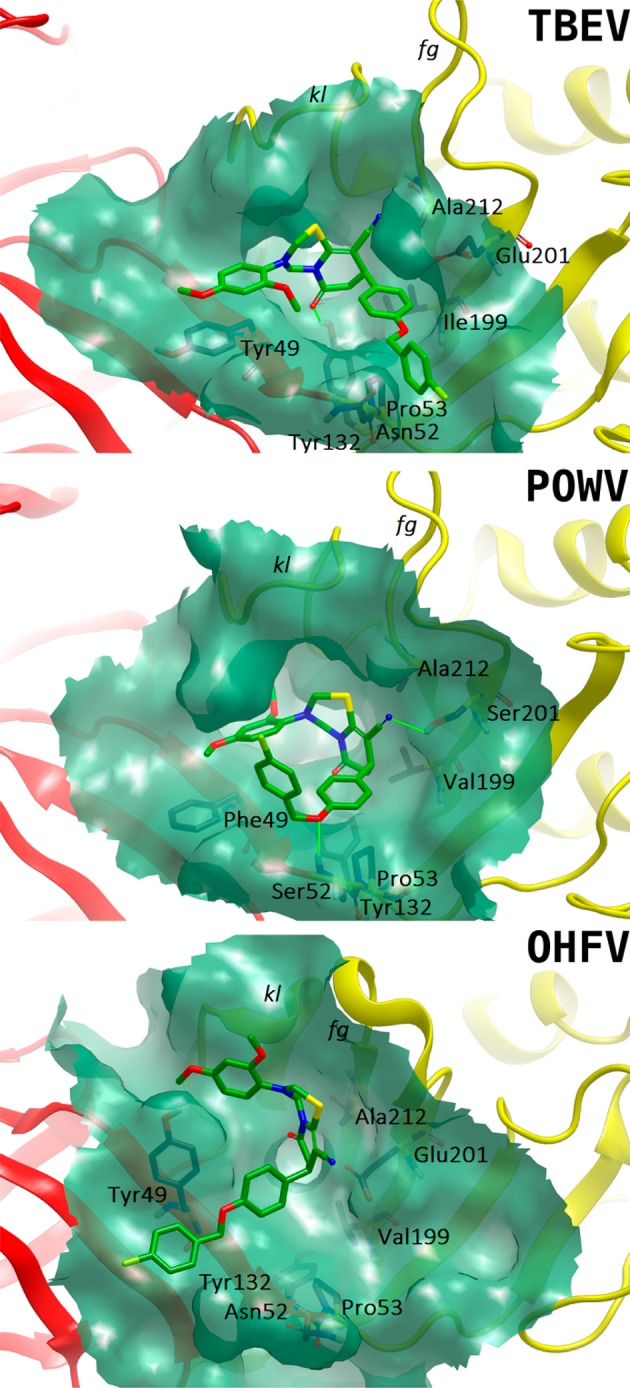
Putative binding modes of 2a in the β-OG pockets according to the docking results. Hydrogen bonds are shown as green lines.
Pyridothiadiazines 2 showed inhibitory activity against TBEV and POWV (Table 1); they were generally more potent despite their lower abundance in the hit lists compared to 1,4-dihydropyridines. Compounds 2a and 2b showed significant activity against both TBEV and POWV despite the limited similarity between these viruses. The only inactive compound in this series, 2c, was ranked high only against the OHFV E protein. Subtle differences in the 2e structure compared to 2a led to the elimination of anti-POWV activity in 2e but did not affect submicromolar anti-TBEV activity. Such ambiguities could be attributed to the fact that racemic mixtures were studied because molecular docking simulations showed some preference for a specific enantiomer over another one: (R)-isomers were usually ranked higher for series 2.
Structure–activity relationships for the compounds seem to be rather obscure, being more complicated by the absence of a consistent binding mode of the compounds: even similar compounds are predicted to interact with the proteins in different orientations (Figure 2). The compounds, protecting the cells against OHFV, do not protect the cells against TBEV and POWV, and vice versa. It should be attributed to E protein peculiarities resulting in different cellular tropism and therefore clinical outcome. These peculiarities also reveal themselves in the docking results: usually, compounds active against certain viruses form more hydrogen bonds with the corresponding E proteins (Figure 2) and are ranked higher against them. Because of the significant similarity of OHFV and TBEV and the compounds themselves, this situation is counterintuitive and may be attributed to a rather small number of compounds assessed in this study and evaluation of activity only for racemates.
Whether the mode of interaction of the identified inhibitors with the flavivirus envelope proteins is realized as predicted, i.e., via the binding in the β-OG pocket, cannot be unambiguously established in this experiment. Even a nonspecific mode of binding cannot be unanticipated. Rigorous study of the reproduction inhibition mechanism and the binding mode will be reported elsewhere.
Our virtual screening campaign against homology models of the envelope proteins from tick-borne flaviviruses resulted in the identification of the small molecule compounds preventing TBEV, POWV, and OHFV reproduction in the host cells. These compounds belong to the series of 1,4-dihydropyridines, showing activity against TBEV or OHFV, and pyridothiadiazines, showing submicromolar activity against TBEV and micromolar against POWV. Further optimization of these compounds guided by in vitro and in vivo studies will be performed with the aim to obtain drugs for flaviviral fevers and encephalitises treatment.
Acknowledgments
The authors are grateful to Dr. Mstislav I. Lavrov and Dr. Alexey D. Averin (Department of Chemistry, Lomonosov Moscow State University) for assistance with compound characterisation and to Sergey A. Pisarev for manuscript reading and discussion. Free academic licence for OpenEye software is provided by OpenEye Scientific Software, Inc.
Glossary
Abbreviations
- β-OG
n-octyl-β-d-glucoside
- DENV
dengue virus
- JEV
Japanese encephalitis virus
- OHFV
Omsk hemorrhagic fever virus
- PEK
porcine embryo kidney
- POWV
Powassan virus
- TBEV
tick-borne encephalitis virus
- VS
virtual screening
- WNV
West Nile virus
- YFV
Yellow fever virus
Supporting Information Available
Detailed experimental procedures for the homology modeling, virtual screening, synthesis and purification of compounds, and antiviral assays. Preliminary experimental data for inactive compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
Author Contributions
The study was initiated and designed by D.I.O., L.I.K., G.G.K., and V.A.P. Computational study was performed by D.I.O., E.V.D., and E.G.R. Compounds were synthesized and characterized by V.V.D., K.A.F., S.G.K., and A.S.M. Biological assessment was performed by L.I.K. and Y.V.R. The study was supervised by V.A.P., G.G.K., V.M.P., and N.S.Z. All authors discussed and approved the publication of the manuscript.
iSCALARE laboratory is supported by the Grant of the Government of Russian Federation (decree 220 of April 09, 2010). The present work was partially supported by RFBR grants 11-03-01174a and 12-04-31317a and by the Program of Development of Lomonosov Moscow State University.
The authors declare no competing financial interest.
Author Status
# Vladimir M. Pentkovski deceased on December 24, 2012. We dedicate this paper to his memory.
Supplementary Material
References
- Lindebach B. D.; Thiel H.-J.; Rice C. M.. Flaviviridae: The Viruses and Their Replication. In Fields Virology, 5th ed.; Knippe D. M., Howley P. M., Eds.; Lippincott Williams and Wilkins and Wolters Kluwer Business Publishers: Philadelphia, PA, 2007; pp 1101–1152. [Google Scholar]
- Turtle L.; Griffiths M. J.; Solomon T. Encephalitis Caused by Flaviviruses. QJM 2012, 105, 219–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sips G. J.; Wilschut J.; Smit J. M. Neuroinvasive Flavivirus Infections. Rev. Med. Virol. 2011, 22, 69–87. [DOI] [PubMed] [Google Scholar]
- Gritsun T. S.; Nuttall P. A.; Gould E. A. Tick-Borne Flaviviruses. Adv. Virus Res. 2003, 61, 317–371. [DOI] [PubMed] [Google Scholar]
- Mansfield K. L.; Johnson N.; Phipps L. P.; Stephenson J. R.; Fooks A. R.; Solomon T. Tick-Borne Encephalitis Virus: A Review of an Emerging Zoonosis. J. Gen. Virol. 2009, 90, 1781–1794. [DOI] [PubMed] [Google Scholar]
- Růžek D.; Yakimenko V. V.; Karan L. S.; Tkachev S. E. Omsk Haemorrhagic Fever. Lancet. 2010, 376, 2104–2113. [DOI] [PubMed] [Google Scholar]
- Gholam B. I. A.; Puksa S.; Provias J. P. Powassan Encephalitis: A Case Report with Neuropathology and Literature Review. Can. Med. Assoc. J. 1999, 161, 1419–1422. [PMC free article] [PubMed] [Google Scholar]
- Raval M.; Singhal M.; Guerrero D.; Alonto A. Powassan Virus Infection: Case Series and Literature Review from a Single Institution. BMC Res. Notes 2012, 5, 594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz F. X.; Stiasny K. Flaviviruses and Flavivirus Vaccines. Vaccine 2012, 30, 4301–4306. [DOI] [PubMed] [Google Scholar]
- Pen’evskaia N. A.; Rudakov N. V. Efficiency of Use of Immunoglobulin Preparations for the Postexposure Prevention of Tick-Borne Encephalitis in Russia (A Review of Semi-Centennial Experience). Med. Parazitol. 2010, 1, 53–59. [PubMed] [Google Scholar]
- Khudoley V. N.; Saratikov A. S.; Lepekhin A. V.; Yavorskaya V. E.; Evstropov A. N.; Portnyagina E. V.; Pomogaeva A. D.; Beloborodova E. I.; Vnushinkaia M. A.; Schmidt E. V.; Krilova N. V.; Khunafina D. K.; Mezenzeva M. V.; Ershov F. I.; Raevski K. K.; Vlasova E. V.; Abdulova G. A.; Kropotkina E. A. Antiviral Activity of Jodantipyrin: An Anti-Inflammatory Oral Therapeutic with Interferon-Inducing Properties. Anti-Inflammatory Anti-Allergy Agents Med. Chem. 2008, 7, 106–115. [Google Scholar]
- Krylova N. V.; Leonova G. N.; Maistrovskaia O. S.; Popov A. M.; Artiukov A. A.; Kozlovskaia E. P. In Vitro Activity of Luromarin against Tick-Borne Encephalitis Virus. Antibiot. Khimioter. 2010, 55, 17–19. [PubMed] [Google Scholar]
- De Clercq E. Strategies in the Design of Antiviral Drugs. Nat. Rev. Drug Discovery 2002, 1, 13–25. [DOI] [PubMed] [Google Scholar]
- Stevens A. J.; Gahan M. E.; Mahalingam S.; Keller P. A. The Medicinal Chemistry of Dengue Fever. J. Med. Chem. 2009, 52, 7911–7926. [DOI] [PubMed] [Google Scholar]
- Teissier E.; Penin F.; Pécheur E.-I. Targeting Cell Entry of Enveloped Viruses as an Antiviral Strategy. Molecules 2011, 16, 221–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit J. M.; Moesker B.; Rodenhuis-Zybert I.; Wilschut J. Flavivirus Cell Entry and Membrane Fusion. Viruses 2011, 3, 160–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modis Y.; Ogata S.; Clements D.; Harrison S. C. A Ligand-Binding Pocket in the Dengue Virus Envelope Glycoprotein. Proc. Natl. Acad. Sci. U.S.A. 2003, 100, 6986–6991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey F. A.; Heinz F. X.; Mandl C.; Kunz C.; Harrison S. C. The Envelope Glycoprotein from Tick-Borne Encephalitis Virus at 2 Å Resolution. Nature 1995, 375, 291–298. [DOI] [PubMed] [Google Scholar]
- Yang J.-M.; Chen Y.-F.; Tu Y.-Y.; Yen K.-R.; Yang Y.-L. Combinatorial Computational Approaches to Identify Tetracycline Derivatives as Flavivirus Inhibitors. PLoS One 2007, 2, e428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z.; Khaliq M.; Suk J.-E.; Patkar C.; Li L.; Kuhn R. J.; Post C. B. Antiviral Compounds Discovered by Virtual Screening of Small-Molecule Libraries against Dengue Virus E Protein. ACS Chem. Biol. 2008, 3, 765–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z.; Khaliq M.; Zhou Z.; Post C. B.; Kuhn R. J.; Cushman M. Design, Synthesis and Biological Evaluation of Antiviral Agents Targeting Flavivirus Envelope Proteins. J. Med. Chem. 2008, 51, 4660–4671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampmann T.; Yennamalli R.; Campbell P.; Stoermer M. J.; Fairlie D. P.; Kobe B.; Young P. R. In Silico Screening of Small Molecule Libraries Using the Dengue Virus Envelope E Protein Has Identified Compounds with Antiviral Activity against Multiple Flaviviruses. Antiviral Res.. 2009, 84, 234–241. [DOI] [PubMed] [Google Scholar]
- Poh M. K.; Yip A.; Zhang S.; Priestle J. P.; Ma N. L.; Smit J. M.; Wilschut J.; Shi P.-Y.; Wenk M. R.; Schul W. A Small Molecule Fusion Inhibitor of Dengue Virus. Antiviral Res. 2009, 84, 260–266. [DOI] [PubMed] [Google Scholar]
- Mayhoub A. S.; Khaliq M.; Botting C.; Li Z.; Kuhn R. J.; Cushman M. An Investigation of Phenylthiazole Antiflaviviral Agents. Bioorg. Med. Chem. 2011, 19, 3845–3854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osolodkin D. I.; Kozlovskaya L. I.; Palyulin V. A.; Pentkovski V. M.; Karganova G. G.; Zefirov N. S. A Molecular Model and Monte Carlo Simulation of Flavivirus Envelope Building Block. Biochem. Biophys. Res. Commun. 2012, 425, 207–211. [DOI] [PubMed] [Google Scholar]
- Yennamalli R.; Subbarao N.; Kampmann T.; McGeary R. P.; Young P. R.; Kobe B. Identification of Novel Target Sites and an Inhibitor of the Dengue Virus E Protein. J. Comput.-Aided Mol. Des. 2009, 23, 333–341. [DOI] [PubMed] [Google Scholar]
- De Burghgraeve T.; Kaptein S. J. F.; Ayala-Nunez N. V.; Mondotte J. A.; Pastorino B.; Printsevskaya S. S.; de Lamballerie X.; Jacobs M.; Preobrazhenskaya M.; Gamarnik A. V.; Smit J. M.; Neyts J. An Analogue of Antibiotic Teicoplanin Prevents Flavivirus Entry In Vitro. PLoS One 2012, 7, e37244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Šali A.; Blundell T. L. Comparative Protein Modelling by Satisfaction of Spatial Restraints. J. Mol. Biol. 1993, 234, 779–815. [DOI] [PubMed] [Google Scholar]
- McGann M. FRED Pose Prediction and Virtual Screening Accuracy. J. Chem. Inf. Model. 2011, 51, 578–596. [DOI] [PubMed] [Google Scholar]
- Dyachenko V. D.; Krivokolysko S. G.; Nesterov V. N.; Litvinov V. P. Synthesis and Properties of N-Methylmorpholinium 4-Aryl-6-methyl-5-phenylcarbamoyl-3-cyano-1,4-dihydropyridine-2-thiolates. Molecular and Crystalline Structure of 2-Allylthio-6-methyl-5-phenylcarbamoyl-4-(2-chlorophenyl)-3-cyano-1,4-dihydropyridine. Chem. Heterocycl. Compd. 1996, 32, 1066–1074. [Google Scholar]
- Dyachenko V. D.; Krivokolysko S. G.; Litvinov V. P. Synthesis of N-Methylmorpholinium 6-Methyl-4-(2-thienyl)-5-phenylcarbamoyl-3-cyano-1,4-dihydropyridine-2-thiolate and Its Reaction with Various Functionally Substituted Methyl Halides. Chem. Heterocycl. Compd. 1997, 33, 577–582. [Google Scholar]
- Dotsenko V. V.; Krivokolysko S. G.; Chernega A. N.; Litvinov V. P. Synthesis and Structure of Pyrido[2,1-b][1,3,5]thiadiazine Derivatives. Dokl. Chem. 2003, 389No. 4–692–96. [Google Scholar]
- Nesterov V. N.; Krivokolysko S. G.; Dyachenko V. D.; Dotsenko V. V.; Litvinov V. P. Synthesis, Properties, and Structures of Ammonium 4-Aryl-5-cyano-2-oxo-1,2,3,4-tetrahydropyridine-6-thiolates. Russ. Chem. Bull. 1997, 46, 990–996. [Google Scholar]
- Krivokolysko S. G.; Dyachenko V. D.; Litvinov V. P. A New Method for the Synthesis of N-Methylmorpholinium 4-Aryl-5-cyano-2-oxo-1,2,3,4-tetrahydropyridine-6-thiolates and Their Properties. Russ. Chem. Bull. 1997, 46, 1758–1762. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.