Abstract

Glycogen phosphorylase inhibitors are considered as potential antidiabetic agents. 3-(β-d-Glucopyranosyl)-5-substituted-1,2,4-triazoles were prepared by acylation of O-perbenzoylated N1-tosyl-C-β-d-glucopyranosyl formamidrazone and subsequent removal of the protecting groups. The best inhibitor was 3-(β-d-glucopyranosyl)-5-(2-naphthyl)-1,2,4-triazole (Ki = 0.41 μM against rabbit muscle glycogen phosphorylase b).
Keywords: 1,2,4-Triazole; C-glucopyranosyl derivative; bioisoster; glycogen phosphorylase; inhibitor
Inhibitors of enzymes are among classics of medicinal chemistry, and many drug molecules’ activity is due to decreasing the efficiency of these catalytic proteins.1 In a chemical biological approach, finding an enzyme inhibitor is the result of a good match of the biological and chemical spaces represented by a binding site of an enzyme and a small molecule, respectively, fitting to each other with considerable strength. Among several methods to design inhibitors, bioisosteric replacement of structural elements of existing molecules is widely applied and in many cases results in higher activity or other advantageous property of the new compound.2
Glycogen phosphorylase (GP) is the main regulatory enzyme of glycogen metabolism. GP, catalyzing the rate determining step of glycogen degradation in the liver by phosphorolysis, is directly responsible for the regulation of blood glucose levels. Therefore, GP has been a validated target in combating noninsulin-dependent or type 2 diabetes mellitus (T2DM), and its inhibitors are considered as potential antidiabetic agents. The biochemical and pharmacological background of this research has been thoroughly summarized in several reviews of the past decade; therefore, the reader is kindly referred to those papers.3−5 Furthermore, possible application of GP inhibitors in intervention of other diseased states associated with GP activity (e.g., cardiovascular disorders,6 ischemic lesions,7,8 and tumorous growth7) has also been under investigations.
Several classes of compounds9,10 were shown to be inhibitors of GP. The most widely studied group of molecules is that of glucose derivatives,11,12 which bind primarily to the active site of GP.13 The best glucose derivatives are submicromolar inhibitors of rabbit muscle GPb, the prototype of GPs.14 Glucopyranosylidene-spiro-thiohydantoin (Ki = 29.8 μM against rat liver GP) was shown to exert considerable in vivo blood sugar diminishing activity.15
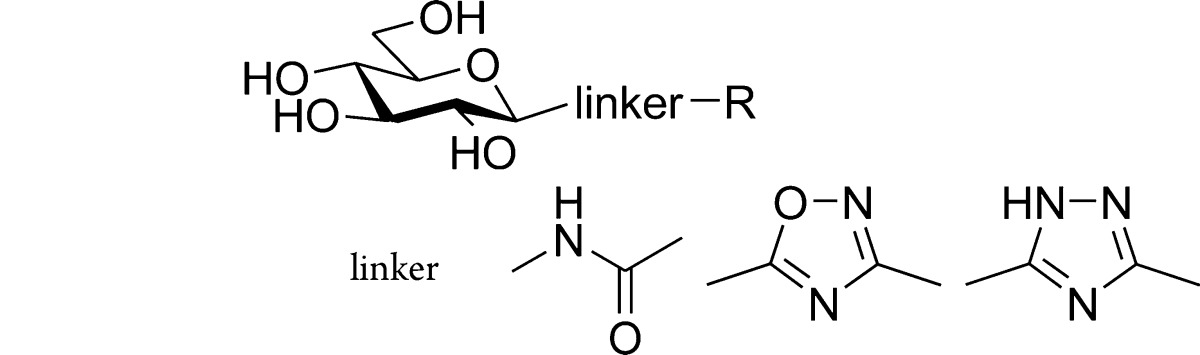
N-Acyl-β-d-glucopyranosylamines (compounds 1 in Chart 1) were among the first GP inhibitors,16 and many analogous derivatives were investigated.17−20 In this series, N-(2-naphthoyl)-β-d-glucopyranosylamine (1R = 2-naphthyl) was the best inhibitor,18 which also served as a lead structure for bioisosteric replacements. As illustrated in Chart 1, enzymatic tests21 as well as crystallographic studies19 revealed high similarity of amide (1) and 1,2,3-triazole (2) type inhibitors both in binding strength and structural features of the enzyme–inhibitor complexes. Kinetic tests of bioisosteric oxadiazoles22,233–5 demonstrated that the constitution of the heterocycle had a strong bearing on the inhibition: the most efficient inhibitor in these series was 5-(β-d-glucopyranosyl)-3-(2-naphthyl)-1,2,4-oxadiazole (5), which had a similar efficiency to that of 1.
Chart 1. Selected Inhibitors of Glycogen Phosphorylase and Their Efficiencya.
a Ki [μM] against RMGPb for R = 2-naphthyl. bA Ki value of 2.4 μM was measured independently by Oikonomakos and co-workers.22
Other investigations on C-glucopyranosyl heterocycles with condensed rings showed that benzothiazole 7 was much less efficient than benzimidazole 8.24 An X-ray crystallographic study of the RMGPb–8 complex revealed a specific H-bond between NH of the heterocycle and the main chain C=O of His377,25 and the stronger binding of 8 was attributed to this interaction, which cannot exist in the case of 7.
On the basis of these preliminaries, synthesis and study of 1,2,4-triazoles of type 6 were envisaged anticipating that the H-bond donor capacity of this heterocycle would result in stronger inhibitors of GP.
3-Glycosyl-5-substituted-1,2,4-triazoles were described in the literature mainly with furanoid rings in reactions of C-glycofuranosyl (thio)formimidates with hydrazide or amidrazone reagents26−28 or transforming a 2,5-anhydro-d,l-allonolactone derivative with aminoguanidine.29 3-Glycopyranosyl-5-substituted-1,2,4-triazoles could not be located in the literature; the only C-glycopyranosyl-1,2,4-triazoles were 1,3,5-trisubstituted derivatives obtained from glycosyl cyanides with 1-aza-2-azoniaallene salts30 or with hydrazonoyl chlorides in the presence of Yb(OTf)3.31
Synthesis of the desired 3-glucopyranosyl-5-substituted-1,2,4-triazoles of type 6 was planned by adaptation of a literature protocol32 in which acylation of N1-tosylamidrazones gave 3,5-disubstituted-1-tosyl-1,2,4-triazoles. Removal of the N-tosyl group was foreseen under conditions usually applied for N-desulfonylation of nitrogen heterocycles.33
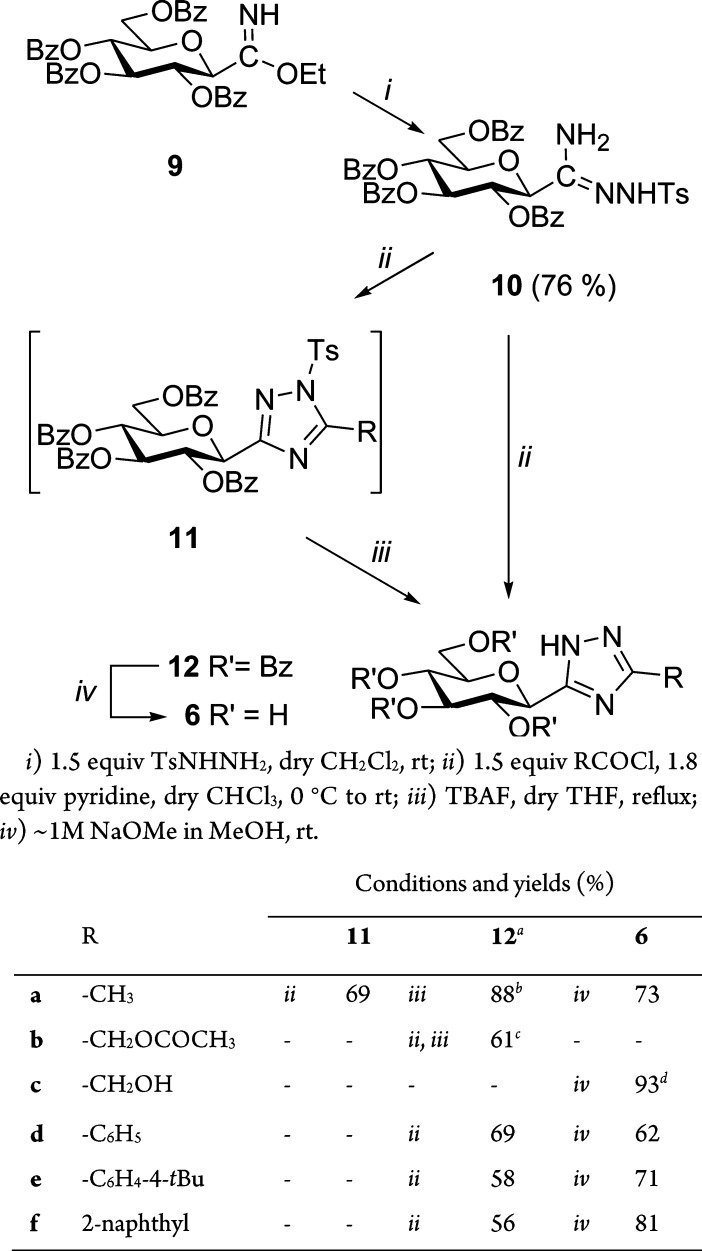
To start the syntheses, O-perbenzoylated β-d-glucopyranosyl formimidate349 was reacted with tosylhydrazide to give the necessary tosylamidrazone 10 in good yield (Scheme 1). Reaction of 10 with acetyl chloride furnished tosyl-triazole 11a, which was N-detosylated by tetrabutylammonium fluoride (TBAF) to 12a. With acetoxyacetyl chloride 10 gave a mixture of 11b and 12b indicating that the N-tosyl group is prone to splitting off under the acylation conditions. The crude mixture of 11b and 12b was treated with TBAF to produce 12b in 61% yield for the two steps. Acylations of 10 with aromatic acid chlorides were accompanied by complete N-detosylation thereby simplifying the preparation of 12d–f, which were obtained in good yields. Removal of the O-acyl protecting groups was effected under Zemplén conditions to give test compounds 6a and 6c–f in good to excellent yields.
Scheme 1. Synthesis of 3-(β-d-Glucopyranosyl)-5-substituted-1,2,4-triazoles (6).
From 10. bFrom 11a. cThe crude mixture obtained from amidrazone 10 and acetoxyacetyl chloride was treated by TBAF. dFrom 12b.
3-(β-d-Glucopyranosyl)-5-substituted-1,2,4-triazoles 6 were assayed against RMGPb as described earlier,35 and the kinetic results, showing the compounds to be competitive inhibitors, are summarized in Table 1. Methyl (6a) and hydroxymethyl (6c) derivatives proved weak inhibitors in the micromolar range and were significantly less efficient than the parent amides 1a and 1c, respectively. Appending unsubstituted aromatic groups to the 1,2,4-triazole ring as in 6d and 6f led to a remarkable strengthening of the inhibition. While 1,2,4-oxadiazoles 5d and 5f were practically equipotent with the corresponding amides 1d and 1f, triazoles 6d and 6f inhibited the enzyme by ∼1 order of magnitude stronger, respectively. This indicated that the possibility for the formation of a H-bond was advantageous for the binding, rendering compound 6f to one of the most efficient glucose analogue inhibitors of GP known to date. Introduction of a t-butyl substituent in the 4-position of the phenyl group as in 6e resulted in a much weaker inhibitor. This observation may reveal that the active site of GP, where these compounds may bind to, can not accommodate a bulky aliphatic moiety.
Table 1. Inhibitiona of RMGPb by Compounds 6 and Comparison to Other Nonclassical Bioisosteres.
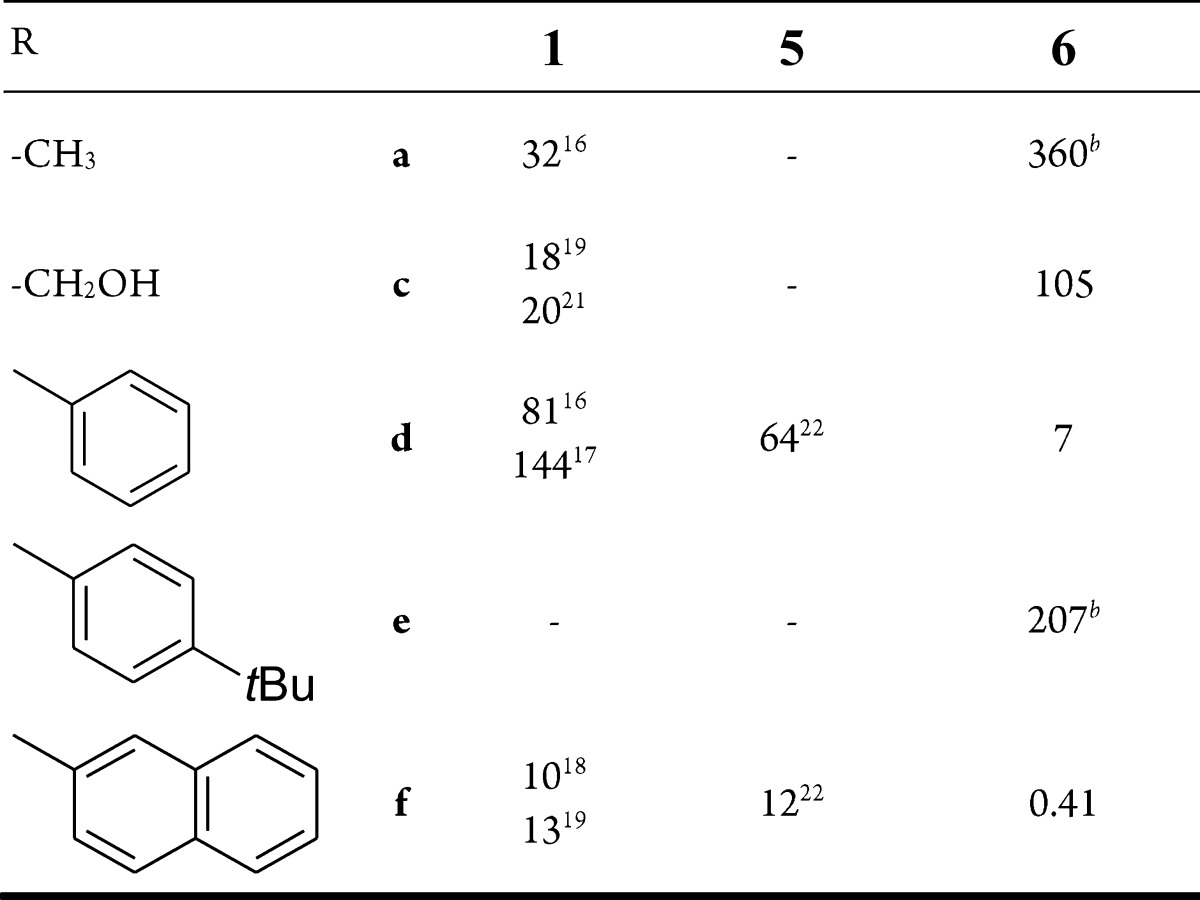
Ki [μM]
Calculated from the IC50 value by using a web-based tool.36
Further studies to establish the binding peculiarities of these inhibitors by X-ray crystallographic investigation of the enzyme–inhibitor complexes as well as molecular dockings to predict other efficient derivatives based on this skeleton are in progress.
In conclusion, a new method was elaborated for the synthesis of hitherto unknown 3-(β-d-glucopyranosyl)-5-substituted-1,2,4-triazoles. These compounds inhibited rabbit muscle GPb, and the 5-(2-naphthyl) derivative with its submicromolar inhibition proved one of the best inhibitors of the enzyme.
Supporting Information Available
Representative synthetic procedures, enzyme kinetic measurements, and compound characterization. This material is available free of charge via the Internet at http://pubs.acs.org.
This work was supported by the Hungarian Scientific Research Fund (OTKA CK77712, CNK80709, and PD105808) as well as TÁMOP 4.2.1./B-09/1/KONV-2010-0007 and TÁMOP-4.2.2.A-11/1/KONV-2012-0025 projects implemented through the New Hungary Development Plan, cofinanced by the European Social Fund. T.D. thanks the Hungarian Academy of Sciences for a János Bolyai research fellowship.
The authors declare no competing financial interest.
Supplementary Material
References
- Smith H. J.; Simons C.. Enzymes and Their Inhibition, Drug Development; CRC Press: Boca Raton, FL, 2005. [Google Scholar]
- Lima L. M. A.; Barreiro E. J. Bioisosterism: a useful strategy for molecular modification and drug design. Curr. Med. Chem. 2005, 12, 23–49. [DOI] [PubMed] [Google Scholar]
- Kurukulasuriya R.; Link J. T.; Madar D. J.; Pei Z.; Richards S. J.; Rohde J. J.; Souers A. J.; Szczepankiewicz B. G. Potential drug targets and progress towards pharmacologic inhibition of hepatic glucose production. Curr. Med. Chem. 2003, 10, 123–153. [DOI] [PubMed] [Google Scholar]
- Ross S. A.; Gulve E. A.; Wang M. H. Chemistry and biochemistry of type 2 diabetes. Chem. Rev. 2004, 104, 1255–1282. [DOI] [PubMed] [Google Scholar]
- Agius L. New hepatic targets for glycaemic control in diabetes. Best Pract. Res., Clin. Endocrinol. Metab. 2007, 21, 587–605. [DOI] [PubMed] [Google Scholar]
- Baker D. J.; Greenhaff P. L.; Timmons J. A. Glycogen phosphorylase inhibition as a therapeutic target: a review of the recent patent literature. Expert Opin. Ther. Patents 2006, 16, 459–466. [Google Scholar]
- Henke B. R.; Sparks S. M. Glycogen phosphorylase inhibitors. Mini-Rev. Med. Chem. 2006, 6, 845–857. [DOI] [PubMed] [Google Scholar]
- Guan T.; Qian Y. S.; Tang X. Z.; Huang M. H.; Huang L. F.; Li Y. M.; Sun H. B. Maslinic acid, a natural inhibitor of glycogen phosphorylase, reduces cerebral ischemic injury in hyperglycemic rats by GLT-1 up-regulation. J. Neurosci. Res. 2011, 89, 1829–1839. [DOI] [PubMed] [Google Scholar]
- Somsák L.; Czifrák K.; Tóth M.; Bokor É.; Chrysina E. D.; Alexacou K. M.; Hayes J. M.; Tiraidis C.; Lazoura E.; Leonidas D. D.; Zographos S. E.; Oikonomakos N. G. New inhibitors of glycogen phosphorylase as potential antidiabetic agents. Curr. Med. Chem. 2008, 15, 2933–2983. [DOI] [PubMed] [Google Scholar]
- Loughlin W. A. Recent advances in the allosteric inhibition of glycogen phosphorylase. Mini-Rev. Med. Chem. 2010, 10, 1139–1155. [DOI] [PubMed] [Google Scholar]
- Praly J. P.; Vidal S. Inhibition of glycogen phosphorylase in the context of type 2 diabetes, with focus on recent inhibitors bound at the active site. Mini-Rev. Med. Chem. 2010, 10, 1102–1126. [DOI] [PubMed] [Google Scholar]
- Somsák L. Glucose derived inhibitors of glycogen phosphorylase. C. R. Chim. 2011, 14, 211–223. [Google Scholar]
- Chrysina E. D.; Chajistamatiou A.; Chegkazi M. From structure-based to knowledge-based drug design through X-ray protein crystallography: sketching glycogen phosphorylase binding sites. Curr. Med. Chem. 2011, 18, 2620–2629. [DOI] [PubMed] [Google Scholar]
- Chrysina E. D. The prototype of glycogen phosphorylase. Mini-Rev. Med. Chem. 2010, 10, 1093–1101. [DOI] [PubMed] [Google Scholar]
- Docsa T.; Czifrák K.; Hüse C.; Somsák L.; Gergely P. The effect of glucopyranosylidene-spiro-thiohydantoin on the glycogen metabolism in liver tissues of streptozotocin-induced and obese diabetic rats. Mol. Med. Rep. 2011, 4, 477–481. [DOI] [PubMed] [Google Scholar]
- Watson K. A.; Mitchell E. P.; Johnson L. N.; Cruciani G.; Son J. C.; Bichard C. J. F.; Fleet G. W. J.; Oikonomakos N. G.; Kontou M.; Zographos S. E. Glucose analogue inhibitors of glycogen phosphorylase: from crystallographic analysis to drug prediction using GRID force-field and GOLPE variable selection. Acta Crystallogr. 1995, D51, 458–472. [DOI] [PubMed] [Google Scholar]
- Somsák L.; Kovács L.; Tóth M.; Ősz E.; Szilágyi L.; Györgydeák Z.; Dinya Z.; Docsa T.; Tóth B.; Gergely P. Synthesis of and a comparative study on the inhibition of muscle and liver glycogen phosphorylases by epimeric pairs of d-gluco- and d-xylopyranosylidene-spiro-(thio)hydantoins and N-(d-glucopyranosyl) amides. J. Med. Chem. 2001, 44, 2843–2848. [DOI] [PubMed] [Google Scholar]
- Györgydeák Z.; Hadady Z.; Felföldi N.; Krakomperger A.; Nagy V.; Tóth M.; Brunyánszky A.; Docsa T.; Gergely P.; Somsák L. Synthesis of N-(β-d-glucopyranosyl)- and N-(2-acetamido-2-deoxy-β-d-glucopyranosyl) amides as inhibitors of glycogen phosphorylase. Bioorg. Med. Chem. 2004, 12, 4861–4870. [DOI] [PubMed] [Google Scholar]
- Chrysina E. D.; Bokor É.; Alexacou K.-M.; Charavgi M.-D.; Oikonomakos G. N.; Zographos S. E.; Leonidas D. D.; Oikonomakos N. G.; Somsák L. Amide-1,2,3-triazole bioisosterism: the glycogen phosphorylase case. Tetrahedron: Asymm. 2009, 20, 733–740. [Google Scholar]
- Kónya B.; Docsa T.; Gergely P.; Somsák L. Synthesis of heterocyclic N-(β-d-glucopyranosyl)carboxamides for inhibition of glycogen phosphorylase. Carbohydr. Res. 2012, 351, 56–63. [DOI] [PubMed] [Google Scholar]
- Bokor É.; Docsa T.; Gergely P.; Somsák L. Synthesis of 1-(d-glucopyranosyl)-1,2,3-triazoles and their evaluation as glycogen phosphorylase inhibitors. Bioorg. Med. Chem. 2010, 18, 1171–1180. [DOI] [PubMed] [Google Scholar]
- Tóth M.; Kun S.; Bokor É.; Benltifa M.; Tallec G.; Vidal S.; Docsa T.; Gergely P.; Somsák L.; Praly J.-P. Synthesis and structure–activity relationships of C-glycosylated oxadiazoles as inhibitors of glycogen phosphorylase. Bioorg. Med. Chem. 2009, 17, 4773–4785. [DOI] [PubMed] [Google Scholar]
- Benltifa M.; Vidal S.; Fenet B.; Msaddek M.; Goekjian P. G.; Praly J.-P.; Brunyánszki A.; Docsa T.; Gergely P. In the search of glycogen phosphorylase inhibitors: 5-substituted 3-C-glucopyranosyl-1,2,4-oxadiazoles from β-d-glucopyranosyl cyanides upon cyclization of O-acyl-amidoxime intermediates. Eur. J. Org. Chem. 2006, 4242–4256. [Google Scholar]
- Hadady Z.; Tóth M.; Somsák L. C-(β-d-glucopyranosyl) heterocycles as potential glycogen phosphorylase inhibitors. Arkivoc 2004, (vii), 140–149. [Google Scholar]
- Chrysina E. D.; Kosmopolou M. N.; Tiraidis C.; Kardarakis R.; Bischler N.; Leonidas D. D.; Hadady Z.; Somsák L.; Docsa T.; Gergely P.; Oikonomakos N. G. Kinetic and crystallographic studies on 2-(β-d-glucopyranosyl)-5-methyl-1,3,4-oxadiazole, -benzothiazole, and -benzimidazole, inhibitors of muscle glycogen phosphorylase b. Evidence for a new binding site. Protein Sci. 2005, 14, 873–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poonian M. S.; Nowoswiat E. F. Novel precursor for the synthesis of C-nucleoside analogues. Synthesis of the C-nucleoside analogues of ribavirin, bredinin, and related compounds. J. Org. Chem. 1980, 45, 203–208. [Google Scholar]
- Huynh-Dinh T.; Igolen J.; Bisagni E.; Marquet J. P.; Civier A. Synthesis of C-nucleosides. Part 14. 5(3)-Glycosyl-1,2,4-triazole-3(5)-carboxamides as analogues of ribavirin. J. Chem. Soc., Perkin Trans. 1 1977, 761–764. [DOI] [PubMed] [Google Scholar]
- Vanek T.; Farkas J.; Gut J. Synthesis of 3,5-disubstituted 1,2,4-triazole derivatives: an alternative preparation of the C-analogue of ribavirin. Collect. Czech. Chem. Commun. 1979, 44, 1334–1338. [Google Scholar]
- Just G.; Ramjeesingh M. C-Nucleosides and related compounds. 5. The synthesis of d,l-4(1β-ribofuranosyl)3-carboxamidopyrazole (V), d,l-5(1β-ribofuranosyl)2-amino-1,3,4-oxadiazole (VII) and d,l-5(1β-ribofuranosyl)2-amino-1,2,4-triazole (IX). Tetrahedron Lett. 1975, 985–988. [Google Scholar]
- Al-Masoudi N.; Hassan N. A.; Al-Soud Y. A.; Schmidt P.; Gaafar A.; Weng M.; Marino S.; Schoch A.; Amer A.; Jochims J. C. Syntheses of C- and N-nucleosides from 1-aza-2-azoniaallene and 1,3-diaza-2-azoniaallene salts. J. Chem. Soc., Perkin. Trans. 1 1998, 947–953. [Google Scholar]
- Al-Masoudi N. A.; Al-Soud Y. A.; Ali I. A. I. Synthesis of 1,2,4-triazole C-nucleosides from hydrazonyl chlorides and nitriles. Nucleosides, Nucleotides Nucleic Acids 2007, 26, 37–43. [DOI] [PubMed] [Google Scholar]
- Chouaieb H.; Ben Mosbah M.; Kossentini M.; Salem M. Novel method for the synthesis of 1,2,4-triazoles and 1,2,4-triazol-3-ones. Synth. Commun. 2003, 33, 3861–3868. [Google Scholar]
- Wuts P. G. M.; Greene T. W.. Greene’s Protective Groups in Organic Synthesis, 4th ed.; Wiley-Interscience: Hoboken, NJ, 2007. [Google Scholar]
- Bokor É.; Szilágyi E.; Docsa T.; Gergely P.; Somsák L. Synthesis of substituted 2-(β-d-glucopyranosyl)-benzimidazoles and their evaluation as inhibitors of glycogen phosphorylase. Carbohydr. Res. 2013, 10.1016/j.carres.2013.01.011. [DOI] [PubMed] [Google Scholar]
- Ősz E.; Somsák L.; Szilágyi L.; Kovács L.; Docsa T.; Tóth B.; Gergely P. Efficient inhibition of muscle and liver glycogen phosphorylases by a new glucopyranosylidene-spiro-thiohydantoin. Bioorg. Med. Chem. Lett. 1999, 9, 1385–1390. [DOI] [PubMed] [Google Scholar]
- Cer R. Z.; Mudunuri U.; Stephens R.; Lebeda F. J. IC50-to-Ki: a web-based tool for converting IC50 to Ki values for inhibitors of enzyme activity and ligand binding. Nucleic Acids Res. 2009, 37, W441–W445. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.