Abstract
In an effort toward the visualization of β-amyloid plaques by in vivo imaging techniques, we have conjugated an optimized derivative of the Pittsburgh compound B (PiB), a well-established marker of Aβ plaques, to DO3A-monoamide that is capable of forming stable, noncharged complexes with different trivalent metal ions including Gd3+ for MRI and 111In3+ for SPECT applications. Proton relaxivity measurements evidenced binding of Gd(DO3A-PiB) to the amyloid peptide Aβ1–40 and to human serum albumin, resulting in a two- and four-fold relaxivity increase, respectively. Ex vivo immunohistochemical studies showed that the DO3A-PiB complexes selectively target Aβ plaques on Alzheimer’s disease human brain tissue. Ex vivo biodistribution data obtained for the 111In-analogue pointed to a moderate blood–brain barrier (BBB) penetration in adult male Swiss mice (without amyloid deposits) with 0.36% ID/g in the cortex at 2 min postinjection.
Keywords: imaging agents, Gd3+, 111In3+, amyloid plaques, Alzheimer’s disease, Pittsburgh compound B
Alzheimer’s disease (AD) is a chronic neurodegenerative disorder leading to progressive decline of cognitive functions.1−3 Characteristic neuropathological findings associated with AD include the formation of extracellular senile plaques containing β-amyloid peptides and neurofibrillary tangles.4−6 Currently, no clinical imaging techniques are available for early detection of AD. Thus, the development of diagnostic agents for early in vivo visualization of the amyloid plaques is a critical issue, which would also be important for monitoring new therapies. Significant advances have been made in the field of nuclear imaging, in particular in positron emission tomography (PET). Small organic compounds such as derivatives of thioflavin T, benzoxazoles, and stilbenes have high binding affinities for the Aβ aggregates and some of them pass the blood–brain barrier (BBB). They inspired the development of amyloid-labeling nuclear imaging probes.7−1111C- and 18F-labeled derivatives of stilbene9 and the Pittsburgh compound-B (PiB)7,8 are promising PET tracers of AD; certain compounds are making their way into the clinics.8−10 Attempts have been also made to develop 99mTc-labeled Aβ SPECT probes, which, given the easier accessibility of 99mTc as compared to 18F, could provide a more convenient approach to the detection of AD. Small, neutral 99mTc-probes have been reported based on derivatives of biphenyl,12 benzothiazole aniline,13 chalcone,14 flavone,15 or pyridyl benzofurane.16 Some showed reasonable brain uptake and affinity toward β-amyloid plaques.16
Magnetic resonance imaging offers noninvasive mapping of structure and function with excellent spatial resolution. MR contrast can be enhanced with paramagnetic contrast agents (CAs), ideally with specific and selective delivery. Gd3+ complexes are the most widely used contrast materials.17 In the absence of any CA, the MR contrast in the amyloid plaques is associated to iron accumulation, which leads to hypointense spots in T2, T2* or susceptibility-weighted images.18 The detection of plaques weakly loaded with iron is more challenging, and exogenous contrast agents become necessary. So far, the development of MRI contrast agents for the diagnosis of Alzheimer’s disease has been modest. Poduslo et al. visualized AD plaques with GdDTPA conjugated to a putrescine-modified human Aβ1–42 peptide able to cross the BBB and target amyloid deposits in the brain of AD transgenic mice. Given the large size of the probe, several days (weeks) were necessary to label the amyloid plaques in the transgenic mouse brain in vivo.19 Yang et al. detected amyloid deposition with iron oxide nanoparticles coupled to Aβ1–42 peptide after intracarotid mannitol treatment to enhance BBB permeability in transgenic mice.20
Multimodality imaging combines two or more methodologies to provide complementary information. Optimally, the imaging probes used in the different modalities should possess identical biodistribution enabling an overlap of the acquired images. Metal ions provide suitable probes for various imaging modalities. By the appropriate choice of a chelating agent, one can complex, with the same ligand, different metal ions that possess properties for these imaging modalities. Provided their charge is identical, these complexes are expected to have similar biodistribution and are therefore interesting candidates for multimodal imaging applications.
Our objective was to design, synthesize, and investigate novel ligands with capability of (i) efficiently complexing metal ions adapted to different imaging modalities, including Gd3+ for MRI, 111In3+ for SPECT, or 68Ga3+ for PET; (ii) labeling Aβ plaques via specific binding; and (iii) delivering the complexes across the BBB. We have conjugated a PiB derivative21 to the metal chelating unit DO3A-monoamide. PiB derivatives are usually noncharged analogues of thioflavin T with excellent BBB permeability. DOTA4– derivatives are among the best chelators for many metal ions. They form highly stable and inert complexes, important for safe in vivo use. In our ligand design, the formation of neutral complexes with trivalent metal ions and a short linkage between the metal binding and the amyloid-recognition sites were essential elements to facilitate BBB permeability.
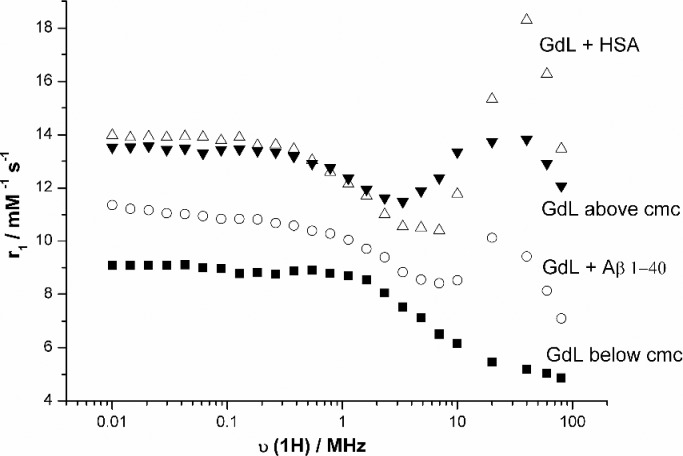
The synthesis of Ln(DO3A-PiB) complexes is summarized in Scheme 1 (see Supporting Information). Magnetic field dependent nuclear magnetic relaxation dispersion (NMRD) profiles are commonly used to characterize MRI contrast agents.17 The NMRD curve of Gd(DO3A-PiB) recorded at 0.2 mM concentration is characteristic of a small-molecular weight complex (Figure 1).
Scheme 1. Synthesis of Ln(DO3A-PiB).
Reagents and conditions: (a) THF, NEt3, 4-nitrobenzoyl chloride, 0°C, 96%; (b) chlorobenzene, Lawesson’s reagent, reflux, 85%; (c) 10% NaOH, K3Fe(CN)6, reflux, 52%; (d) SnCl2, EtOH, reflux, 92%; (e) ClCH2COCl, K2CO3, CH3CN, r.t., 86%; (f) tBu3DO3A, K2CO3, CH3CN, r.t., 88%; (g) dichloromethane, TFA, 45°C, 79%; (h) GdCl3, pH 7.
Figure 1.
1H NMRD profiles of Gd(DO3A-PiB) at 0.2 mM (■), 5 mM (▼), 0.2 mM in the presence of 0.2 mM Aβ1–40 (○), and 0.2 mM in the presence of 0.2 mM HSA (△) (0.05 M HEPES; pH 7.4, T = 310 K).
Upon binding of Gd(DO3A-PiB) to Aβ plaques, higher relaxivity is expected since the complex becomes immobilized. Indeed, in the presence of the amyloid peptide Aβ1–40 (cGd = cAβ1–40 = 0.2 mM), the relaxivity of Gd(DO3A-PiB) increases considerably at magnetic fields where the effect of slower rotation is most pronounced, evidencing an interaction. The binding affinity of Gd(DO3A-PiB) to Aβ1–40 was evaluated by surface plasmon resonance measurements (Figure S9, Supporting Information), which yielded Kd = (180 ± 10) μM, a value considerably higher than that for small benzothiazole derivatives (Kd < 1 μM).22 Given their similar charge and the fact that the binding interaction mainly involves the PiB moiety, similar binding affinity is expected also for the Eu3+ and In3+ analogues. Gd(DO3A-PiB) binds to serum albumin as well, causing a remarkable increase of relaxivity at intermediate fields (Figures 1 and S1–S4, Supporting Information). Albumin binding leads to a prolonged lifetime of the agent in the blood pool that, given the slower elimination from the body, can be useful for an MRI probe, though unfavorable for nuclear imaging probes due to longer radiation exposure and higher nonspecific signal. Strong HSA binding can be also detrimental for the BBB permeability of the agent.23 The binding affinity of Gd(DO3A-PiB) to HSA has been assessed by proton relaxation enhancement measurements and yielded Kd = 110 ± 20 μM (Figures S1 and S2, Supporting Information).24 The NMRD curve recorded at 5 mM Gd(DO3A-PiB) concentration shows the relaxivity peak at intermediate magnetic fields, typical of slowly tumbling systems thus evidencing the formation of micellar aggregates. A critical micellar concentration, cmc = 1.90 ± 0.02 mM, was determined by relaxometry25 (Figure S3, Supporting Information).
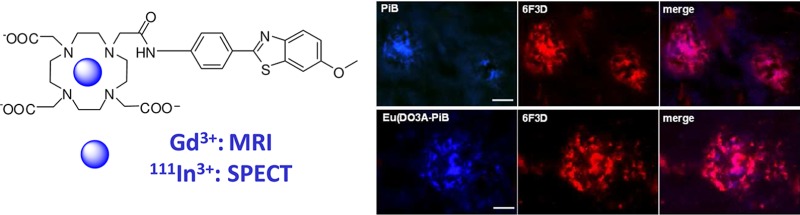
We tested the capability of Eu(DO3A-PiB) to bind amyloid aggregates on postmortem human brain tissue of AD patients. The staining with Eu(DO3A-PiB) revealed the presence of various amyloid deposits. The distribution pattern and intensity of the senile plaques were similar for PiB, thioflavin-S, and Eu(DO3A-PiB) (Figures S6 and S7, Supporting Information). Eu(DO3A-PiB) seems to have lower affinity for neurofibrillary tangles, being thus more specific to Aβ deposits than PiB (Figure 2). To confirm that PiB and Eu(DO3A-PiB) labeling is specific for the amyloid deposits, we performed a double fluorescence staining experiment using the routinely employed 6F3D antibody (Figure 3). The staining of PiB and Eu(DO3A-PiB) is strong in the focal deposits, while the surrounding crown is slightly less positive, probably due to its partially fibrillar morphology. Some of the less intense Aβ positive structures were not labeled by PiB or Eu(DO3A-PiB).
Figure 2.
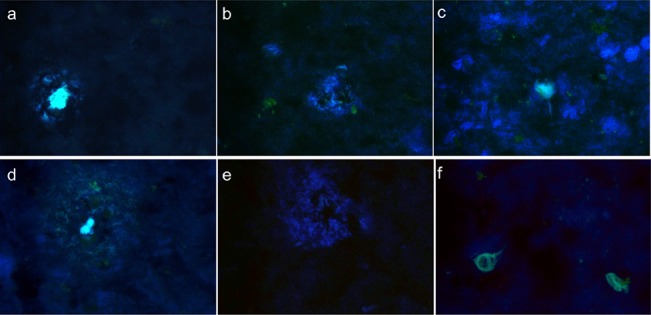
Positive staining of an Alzheimer tissue with PiB (a–c) and Eu(DO3A-PiB) (d–f). Senile plaques (left), diffuse deposits (middle), and neurofibrillary tangles (right) are put into evidence by the PIB and Eu(DO3A-PiB) staining.
Figure 3.
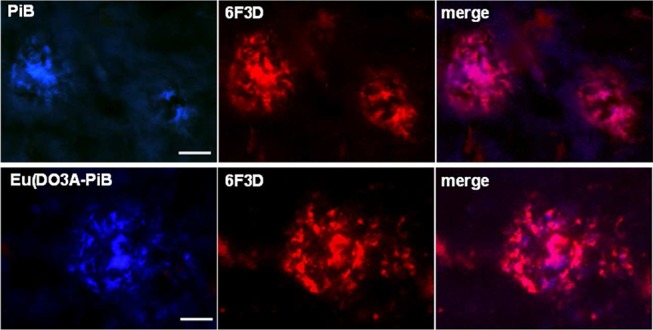
Micrographs illustrating the colocalization of PiB or Eu(DO3A-PiB) and 6F3D-antibody labeling of amyloid deposits of postmortem human brain tissue of an AD patient: the colocalization is confirmed by the magenta color in the merge images; scale bar = 20 μm.
The BBB permeability of a compound is related to its (i) lipophilicity, expressed by the water/octanol partition coefficient, log Poct/water, (ii) molecular weight (MW), and (iii) plasma pharmacokinetics.23 Low MW amphiphilic molecules with log Poct/water ≈ 2 have optimal BBB penetration. For Gd(DO3A-PiB), log Poct/water = −0.15 was obtained, a value lower than that for phenylbenzothiazole derivatives (Table 1).7,21,26 It is similar to those reported for neutral In(DTPA-bisamide) complexes bearing hydrophobic chains,27 and higher than log Poct/water = −2.86 for the double-charged In(DTPA)2–. The molecular weight of Gd(DO3A-PiB), MW = 842, is above the optimal values,28 although peptide analogues with a MW > 1000 Da were shown to cross the BBB.29,30
Table 1. Molecular Weight (MW) and Lipophilicity (log POct/H2O) of Phenylbenzothiazole (BTA) Derivatives.

In order to evaluate the penetration of our complexes across the BBB, we have performed in vivo biodistribution experiments with the 111In-analogue in adult male Swiss mice without amyloid deposits in their brain. The radiolabeling of the ligand has been carried out in isotonic ammonium acetate at pH 7.0, by adding a solution of 111InCl3 (specific activity 15.38 TBq/mg), to obtain 1:1 111In3+ complexes (10% ligand excess, 100 °C, 30 min). Thin layer chromatography indicated >96% radiochemical purity (Figure S7, Supporting Information). The complex remained stable in vitro with 2% degradation at 22 h after radiolabeling. The in vivo biodistribution of 111In(DO3A-PiB) at 2 and 30 min (Table 2) showed the highest uptake in kidney suggesting urinary elimination. The radioactive signal was also elevated in lungs 2 min after tracer injection, then decreased at the 30 min time point. This transient high lung uptake reflects the large blood volume of this blood-rich organ. In the brain, the % ID/g in the cortex and cerebellum at 2 min was 0.36 and 0.5, while at 30 min, it was 0.11 and 0.21, respectively. The clearance measured by the ratio of % ID/g values at 2 and 30 min is 3.3, comparable to that for PiB.2199mTc-based β-amyloid tracers had similar or higher brain uptake (0.1–1.4% ID/g at 2 min and 0.1–1.08% ID/g at 30 min).12−16 Given the higher molecular weight of 111In(DO3A-PiB), the brain % ID/g values are remarkable. They are similar to the cerebral biodistribution of some SPECT radioligands, including PE2I31 and CLINDE,32 developed for brain imaging of the dopaminergic system and neuroinflammation. Therefore, the BBB crossing of 111In(DO3A-PiB) might be sufficient for nuclear imaging amyloid deposits in rodent transgenic models overexpressing Aβ aggregates. BBB permeability sufficient for MRI detection with the Gd3+ analogue will be certainly difficult to attain. However, an MRI agent specifically binding to amyloid deposits can be useful in animal studies where a BBB opening is feasible. These agents can complete the use of nonspecific Gd-complexes like GdDTPA applied in intracerebroventricular injection protocols or can be useful in ex vivo MRI to stain Alzheimer brain tissues.33
Table 2. Distribution of 111In(DO3A-PiB) in Swiss Mice 2 and 30 min after Tracer Injection; the Data Represent the Mean ± SD Percent Injected Dose Per Gram (% ID/g, n = 4).
| % ID/g | 2 min | 30 min |
|---|---|---|
| kidney | 12.53 ± 1.07 | 4.23 ± 0.42 |
| cerebellum | 0.50 ± 0.07 | 0.21 ± 0.02 |
| cortex | 0.36 ± 0.03 | 0.11 ± 0.01 |
| heart | 7.36 ± 0.18 | 1.51 ± 0.21 |
| liver | 4.90 ± 0.12 | 7.65 ± 1.59 |
| blood | 14.59 ± 0.23 | 4.23 ± 0.45 |
| lung | 11.42 ± 0.77 | 1.44 ± 0.14 |
In conclusion, we synthesized a novel amyloid targeted ligand, which is able to efficiently complex different metal ions adapted to various imaging modalities, including Gd3+ for MRI and 111In3+ for SPECT. The complexes bind the amyloid peptide Aβ1–40. This results in elevated relaxivity of the Gd3+ analogue due to reduced rotational motion, which will lead to an increased contrast in T1-weighted images upon binding to senile plaques. The complexes show affinity for amyloid deposits in postmortem human tissue. The biodistribution experiments in normal mice proved moderate brain uptake.
Glossary
Abbreviations
- AD
Alzheimer’s disease
- CA
contrast agent
- MRI
magnetic resonance imaging
- PET
positron emission tomography
- SPECT
single-photon emission computed tomography
- BBB
blood brain barrier
- HSA
human serum albumin
- MW
molecular weight
- PiB
Pittsburgh compound B
Supporting Information Available
Synthesis, immunohistochemistry, relaxometric studies, and experimental procedures. This material is available free of charge via the Internet at http://pubs.acs.org.
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of this manuscript.
We acknowledge financial support of the F.C.T. Portugal (Ph.D. grant SFRH/BD/46370/2008 to A.F.M.), the French-Portuguese PESSOA project, and the European Action TD1004 “Theragnostics Imaging and Therapy” and TD1007 “PET-MRI”.
The authors declare no competing financial interest.
Supplementary Material
References
- Evans D. A.; Funkenstein H. H.; Albert M. S. Prevalence of Alzheimers-disease in a community population of older persons: higher than previously reported. JAMA 1989, 262, 2551–2556. [PubMed] [Google Scholar]
- Fratiglioni L.; Winblad B.; von Strauss E. Prevention of Alzheimer’s disease and dementia. Major findings from the Kungsholmen project. Physiol. Behav. 2007, 92, 98–104. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed; American Psychiatric Association: Washington, DC, 2000. [Google Scholar]
- Braak H.; Braak E. Neuropathological staging of Alzheimer-related changes. Acta Neuropathol. 1991, 82, 239–259. [DOI] [PubMed] [Google Scholar]
- Hardy J.; Selkoe D. J. Medicine: The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science 2002, 297, 353–356. [DOI] [PubMed] [Google Scholar]
- Rauk A. The chemistry of Alzheimer’s disease. Chem. Soc. Rev. 2009, 38, 2698–2715. [DOI] [PubMed] [Google Scholar]
- Mathis C. A.; Bacskai B. J.; Kajdasz S. T.; McLellan M. E.; Frosch M. P.; Hyman B. T.; Holt D. P.; Wang Y.; Huang G. F.; Debnath M. L.; Klunk W. E. A lipophilic thioflavin-T derivative for positron emission tomography (PET) imaging of amyloid in brain. Bioorg. Med. Chem. Lett. 2002, 12, 295–298. [DOI] [PubMed] [Google Scholar]
- Vandenberghe R.; Van Laere K.; Ivanoiu A.; Salmon E.; Bastin C.; Triau E.; Hasselbalch S.; Law I.; Andersen A.; Korner A.; Minthon L.; Garraux G.; Nelissen N.; Bormans G.; Buckley C.; Owenius R.; Thurfjell L.; Farrar G.; Brooks D. J. 18F-Flutemetamol amyloid imaging in Alzheimer disease and mild cognitive impairment: a phase 2 trial. Ann. Neurol. 2010, 68, 319–29. [DOI] [PubMed] [Google Scholar]
- Camus V.; Payoux P.; Barré L.; Desgranges B.; Voisin T.; Tauber C.; La Joie R.; Tafani M.; Hommet C.; Chételat G.; Mondon K.; de La Sayette V.; Cottier J. P.; Beaufils E.; Ribeiro M. J.; Gissot V.; Vierron E.; Vercouillie J.; Vellas B.; Eustache F.; Guilloteau D. Using PET with 18F-AV-45 (florbetapir) to quantify brain amyloid load in a clinical environment. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 621–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthel H.; Gertz H. J.; Dresel S.; Peters O.; Bartenstein P.; Buerger K.; Hiemeyer F.; Wittemer-Rump S. M.; Seibyl J.; Reininger C.; Sabri O.; et al. Cerebral amyloid-β PET with florbetaben (18F) in patients with Alzheimer’s disease and healthy controls: a multicentre phase 2 diagnostic study. Lancet Neurol. 2011, 10, 424–435. [DOI] [PubMed] [Google Scholar]
- Nesterov E. E.; Skoch J.; Hyman B. T.; Klunk W. E.; Bacskai B. J.; Swager T. M. In vivo optical Imaging of amyloid aggregates in brain: Design of fluorescent markers. Angew. Chem., Int. Ed. 2005, 26, 5452–5456. [DOI] [PubMed] [Google Scholar]
- Zhuang Z. P.; Kung M. P.; Hou C.; Ploessl K.; Kung H. F. Biphenyls labeled with technetium 99m for imaging β-amyloid plaques in the brain. Nucl. Med. Biol. 2005, 32, 171–184. [DOI] [PubMed] [Google Scholar]
- Serdons K.; Verduyckt T.; Cleynhens J.; Terwinghe C.; Mortelmans L.; Bormans G.; Verbruggen A. Synthesis and evaluation of a [99mTc]-BAT-phenylbenzothiazole conjugate as a potential in vivo tracer for visualization of amyloid β. Bioorg. Med. Chem. Lett. 2007, 17, 6086–6090. [DOI] [PubMed] [Google Scholar]
- Ono M.; Ikeoka R.; Watanabe H.; Kimura H.; Fuchigami T.; Haratake M.; Saji H.; Nakayama M. Synthesis and evaluation of novel chalcone derivatives with 99mTc/Re complexes as potential probes for detection of β-amyloid plaques. ACS Chem. Neurosci. 2010, 1, 598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono M.; Ikeoka R.; Watanabe H.; Kimura H.; Fuchigami T.; Haratake M.; Saji H.; Nakayama M. 99mTc/Re complexes based on flavone and aurone as SPECT probes for imaging cerebral β-amyloid plaques. Bioorg. Med. Chem. Lett. 2010, 20, 5743–5748. [DOI] [PubMed] [Google Scholar]
- Cheng Y.; Ono M.; Kimura H.; Ueda M.; Saji H. Technetium-99m labeled pyridyl benzofuran derivatives as single photon emission computed tomography imaging probes for β-amyloid plaques in Alzheimer’s brains. J. Med. Chem. 2012, 55, 2279–2286. [DOI] [PubMed] [Google Scholar]
- Toth E.; Merbach A., Eds. The Chemistry of Contrast Agents in Medical MRI; John Wiley & Sons: Chichester, U.K., 2001. [Google Scholar]
- Jack C. R.; Garwood M.; Wengenack T. M.; Borowski B.; Curran G. L.; Lin J.; Adriany G.; Grohn I. H. J.; Grimm R.; Poduslo J. F. In vivo visualization of Alzheimer’s amyloid plaques by magnetic resonance imaging in transgenic mice without a contrast agent. Magn. Reson. Med. 2004, 52, 1263–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poduslo J. F.; Wengenack T. M.; Curran G. L.; Wisniewski T.; Sigurdsson E. M.; Macura S. I.; Borowski B. J.; Jack C. R. Jr. Molecular targeting of Alzheimer’s amyloid plaques for contrast-enhanced magnetic resonance imaging. Neurobiol. Dis. 2002, 11, 315–329. [DOI] [PubMed] [Google Scholar]
- Yang J.; Wadghiri Y. Z.; Hoang D. M.; Tsui W.; Sun Y. J.; Chung E.; Li Y. S.; Wang A.; de Leon M.; Wisniewski T. Detection of amyloid plaques targeted by USPIO-A beta 1-42 in Alzheimer’s disease transgenic mice using magnetic resonance microimaging. Neuroimage 2011, 55, 1600–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathis C. A.; Wang Y.; Holt D. P.; Huang G.-F.; Debnath M. L.; Klunk W. E. Synthesis and evaluation of C-11-labeled 6-substituted 2-arylbenzothiazoles as amyloid imaging agents. J. Med. Chem. 2003, 46, 2740–2754. [DOI] [PubMed] [Google Scholar]
- Yona R. L.; Mazères S.; Faller P.; Gras E. Thioflavin derivatives of amyloid-β fibrils: insights into structural features important for high-affinity binding. ChemMedChem 2008, 3, 63–66. [DOI] [PubMed] [Google Scholar]
- Mensch J.; Oyarzabal J.; Mackie C.; Augustijns P. In vivo, in vitro and in silico methods for small molecule transfer across the BBB. J. Pharm. Sci. 2009, 98, 4429–4468. [DOI] [PubMed] [Google Scholar]
- Aime S.; Fasano M.; Terreno E., Botta M.. Protein-Bound Metal Chelates. In The Chemistry of Contrast Agents in Medical Magnetic Resonance Imaging; Merbach A.E., Toth E., Eds.; Wiley: New York, 2001; Chapter 5, pp 194–241. [Google Scholar]
- Nicolle G. M.; Tóth É.; Eisenwiener K.-P.; Mäcke H. R.; Merbach A. E. From monomers to micelles: investigation of the parameters influencing proton relaxivity. J. Biol. Inorg. Chem. 2002, 7, 757–769. [DOI] [PubMed] [Google Scholar]
- Lin K.-S.; Debnath M. L.; Mathis C. A.; Klunk W. E. Synthesis and beta-amyloid binding properties of rhenium 2-phenylbenzothiazoles. Bioorg. Med. Chem. Lett. 2009, 19, 2258–2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prata M. I. M.; Santos A. C.; Bligh S. W. A.; Chowdhury A. H. M. S.; Geraldes C. F. G. C.; de Lima J. J. P. Characterization of In-111(3+) complexes of DTPA amide derivatives: Biodistribution and clearance studied by gamma imaging. Nucl. Med. Biol. 2000, 27, 605–610. [DOI] [PubMed] [Google Scholar]
- Levin V. Relationship of octanol–water partition-coefficient and molecular-weight to rat brain capillary-permeability. J. Med. Chem. 1980, 23, 682–684. [DOI] [PubMed] [Google Scholar]
- Bornebroek M.; Verzijlbergen J.; Haan J.; Van Scheyen E.; Verhoeff N.; Pauwels E.; Roos R. Potential for imaging cerebral amyloid deposits using I-123-labelled serum amyloid P component and SPET. Nucl. Med. Commun. 1996, 17, 929–933. [DOI] [PubMed] [Google Scholar]
- Saito Y.; Buciak J.; Yang J.; Pardridge W. M. Vector-mediated delivery of I-125 labeled beta-amyloid peptide A-beta(1–40) through the blood-brain-barrier and binding to Alzheimer-disease amyloid of the A-beta(1–40)/vector complex. Proc. Natl. Acad. Sci. U.S.A. 1995, 92, 10227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emond P.; Guilloteau D.; Chalon S. PE2I: a radiopharmaceutical for in vivo exploration of the dopamine transporter. CNS Neurosci. Ther. 2008, 14, 47–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arlicot N.; Katsifis A.; Garreau L.; Mattner F.; Vergote J.; Duval S.; Kousignian I.; Bodard S.; Guilloteau D.; Chalon S. Evaluation of CLINDE as potent translocator protein (18 kDa) SPECT radiotracer reflecting the degree of neuroinflammation in a rat model of microglial activation. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 2203–2211. [DOI] [PubMed] [Google Scholar]
- Petiet A.; Dhenain M. Improvement of microscopic MR imaging if amyloid plaques with targeting and non-targeting contrast agents. Curr. Med. Imaging Rev. 2011, 7, 1–8. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.