Abstract
Novel 3,3-spiroanellated 5-aryl, 6-arylvinyl-substituted 1,2,4-trioxanes 19–34 have been synthesized and appraised for their antimalarial activity against multidrug-resistant Plasmodium yoelii nigeriensis in Swiss mice by oral route at doses ranging from 96 mg/kg × 4 days to 24 mg/kg × 4 days. The most active compound of the series (compound 25) provided 100% protection at 24 mg/kg × 4 days, and other 1,2,4-trioxanes 22, 26, 27, and 30 also showed promising activity. In this model, β-arteether provided 100 and 20% protection at 48 mg/kg × 4 days and 24 mg/kg × 4 days, respectively, by oral route. Compound 25 displayed a similar in vitro pharmacokinetic profile to that of reference drug β-arteether. The activity results demonstrated the importance of an aryl moiety at the C-5 position on the 1,2,4-trioxane pharmacophore.
Keywords: 1,2,4-trioxane; antimalarial; in vivo; orally active
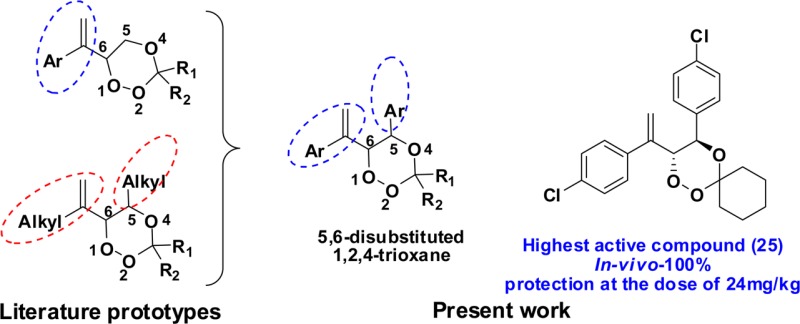
Synthetic trioxanes containing a 1,2,4-trioxane pharmacophore of artemisinin have been a key area of research since the discovery of the antimalarial lead molecule artemisinin from Artemisia annua.1−1e Malaria is of serious concern in many tropical areas of the world, and the situation has worsened due to drug resistance to common chemotherapeutic agents.2 Artemisinin and its derivatives have been thoroughly investigated for their efficacy against malaria parasite and also their peroxide specific mode of action.3−11c Since identification of the peroxide group-specific antimalarial activity of artemisinin, many synthetic peroxides have been synthesized and tested for their efficacy and were found to show promising antimalarial activity as compared to artemisinin.12−18 For the synthesis and assessment of the antimalarial activity of the 1,2,4-trioxane skeleton, two major prototypes are reported in the literature (Figure 1): (I) aryl vinyl substitution at C-6 position19−24 and (II) alkyl vinyl substitution at C-6 and alkyl/cycloalkyl substitution at C-5 position.25−27 So far, synthesized trioxanes have a substitution at C-6 because of the ease of synthesis via ene reaction of allylic alcohols with singlet oxygen. A substitution corresponding to the phenyl vinyl part of the molecule has been the subject of an extensive study by Singh et al.,19−24 where they have synthesized a number of compounds based on allylic alcohols derived from wittig/reformatsky products of various phenyl methyl ketones. They have experimental results to support the importance of an aryl vinyl substitution at C-6 of the 1,2,4-trioxane moiety, along with effects of different substituents in the aromatic ring. The results from Singh et al. motivated us to keep the C-6 phenyl vinyl part as such in our 1,2,4-trioxane (prototype III, Figure 1). For prototype II, Griesbeck et al. utilized diastereoselective photooxygenation to synthesize racemic threo-β-hydroperoxy alcohols that were in turn used to synthesize diastereomerically pure aliphatic C-5,6-substituted 1,2,4-trioxane and reported in vitro antimalarial activities against the K1 strain of P. falciparum.(25−27) On the basis of the in vitro activity profile of some of these Griesbeck's 1,2,4-trioxanes, O'Neill et al. reported the synthesis of C-5,6-alkylsubstituted 1,2,4-trioxanes by stereoselective photooxygenation of chiral allylic alcohols.28 From Figure 1, it is clear that approach I is attractive because of promising in vivo activity results, while approach II is attractive for in vitro activity results of C-5,6-dialkyl-substituted 1,2,4-trioxanes. Taking in to consideration the importance of a phenyl substituent on the synthetic 1,2,4-trioxanes evident from work by Singh et al. and in vitro activity results of C-5,6-disubstituted 1,2,4-trioxanes, we envisage a new route for aryl substitution at C-5 and aryl vinyl substitution at C-6 positions of the 1,2,4-trioxane moiety to assess their in vivo antimalarial potential. (Figure 1, prototype III).
Figure 1.
Basis for the synthesis of 3,3-spiroanellated 5,6-disubstituted 1,2,4-trioxane.
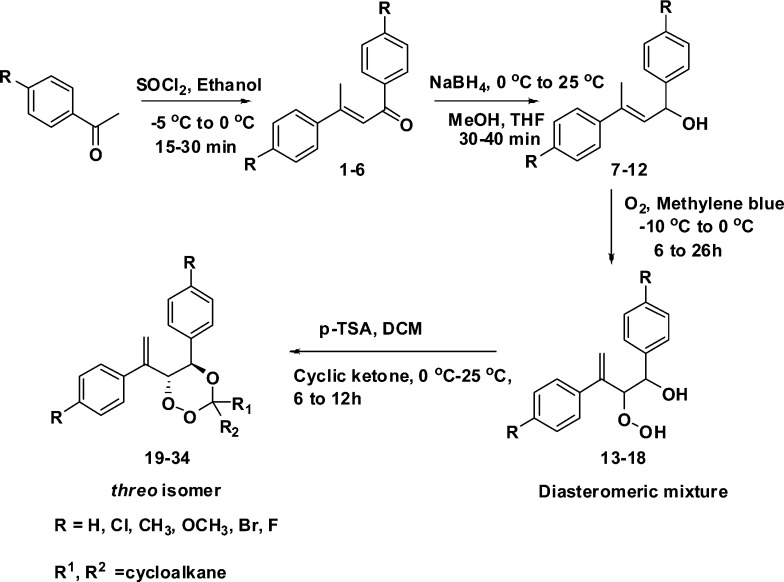
5,6-Disubstituted 1,2,4-trioxanes 19–34 were prepared by the procedure given in Scheme 1 and starts with the formation of homochalcones. The desired homochalcones 1–6 were synthesized by the thionyl chloride-catalyzed self-condensation of substituted acetophenones in ethanol in the temperature range from −5 to 0 °C (21–42% yields), following the reported procedure.29 Homochalcones 1–6 were reduced by sodium borohydride (NaBH4) in a mixture of methanol and tetrahydrofuran (THF) to give the corresponding allylic alcohol 7(30)–12 (55–66%) yields. Allylic alcohols are a very useful intermediates for the synthesis of 1,2,4-trioxanes via peroxyketalization of hydroperoxyalcohol. The synthesis of allylic alcohol-derived hydroperoxides has been the key step toward development of many synthetic 1,2,4-trioxanes. Photooxygenation of allylic alcohols 7–12 in acetonitrile (CH3CN) and chloroform (CHCl3) with methylene blue as a sensitizer furnished a diasteromeric mixture of threo (major) and erythro (minor) β-hydroperoxyalcohol 13–18 (44–66%). The threo-selectivity is explained on the basis of a hydroxyl-directing effect of allylic alcohol, which is in turn strongly affected by competing hydrogen bond acceptors. Peroxyketalization of hydroperoxyalcohol 13–15, 17, and 18 with cyclopentanone and cyclohexanone was carried out in the presence of catalytic acid to furnish exclusively the threo-1,2,4-trioxanes 19–23 (17–26%) and 24–28 (15–28%), respectively. Similarly Peroxyketalization of hydroperoxyalcohol 13–18 with admantanone were carried out in presence of p-toulene sulfonic acid (PTSA) to furnish the threo-1,2,4-trioxanes 29–34 (14–28%). The relative configuration of the product was determined to be threo based on 3JH5–H6 observed in the proton spectrum of product (3JH5–H6 = 9.6 Hz for compound 25).25,26 The corresponding peroxyketalization product from erythro (minor) β-hydroperoxyalcohol could not be isolated in all cases. This is the first report for the synthesis of 5-aryl, 6-aryl vinyl-substituted 1,2,4-trioxanes and their antimalarial activities.
Scheme 1. Synthesis of 3,3-Spiroanellated 5,6-Disubstituted 1,2,4-Trioxanes (19–34).
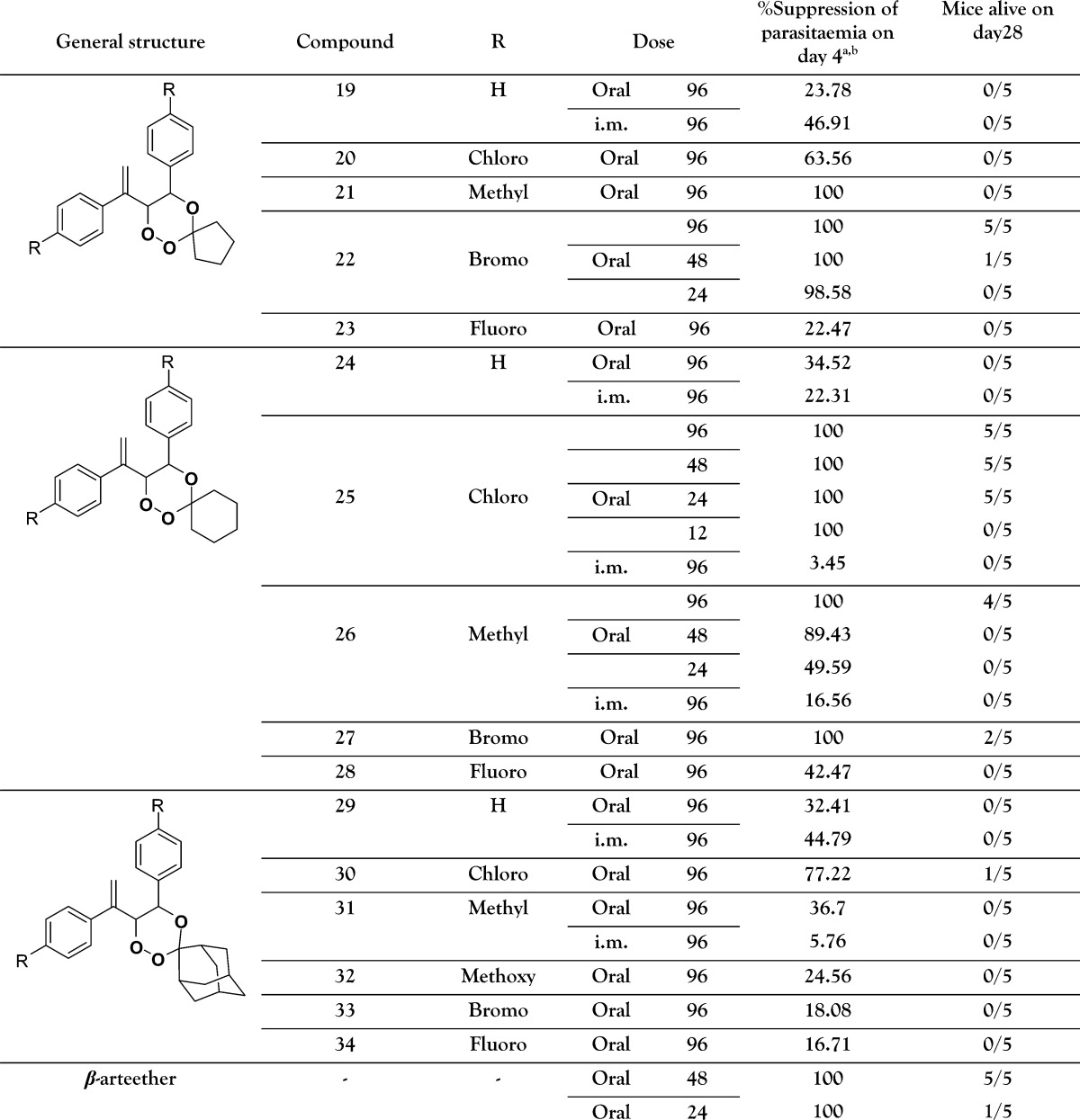
Compounds 19–34 were screened for antimalarial activity against multidrug-resistant Plasmodium yoelii nigeriensis in Swiss mice via oral route using Peter's procedure.31 In this model, β-arteether (standard drug) showed 100% suppression of parasitemia31 at 48 mg/kg × 4 days, and all of the treated mice survived beyond day 28. At 24 mg/kg × 4 days, β-arteether provides only 20% protection to the treated mice. Therefore, all of the trioxanes were initially tested at 96 mg/kg × 4 days orally, double the effective dose of β-arteether. Compounds 22, 25, and 26 provided 100% protection31b at 96 mg/kg × 4 days dose and hence were further screened at lower doses of 48, 24, and 12 mg/kg × 4 days. The results are summarized in Table 1.
Table 1. In Vivo Antimalarial Activity of Compounds 19–34 against Multidrug-Resistant P. yoelii nigeriensis in Swiss Mice by oral and im Routes.
Percent suppression = [(c – t)/c] × 100, where c = parasitaemia in control group, and t = parasitaemia in treated group.
100% suppression of parasitaemia means that the number of parasites, if present, is below the detection limit.
As evident from Table 1, some of the tested compounds displayed activity comparable with or better than that of β-arteether by oral route. Compound 25, the most active compound of the series, showed 100% parasite suppression on day 4 and 100% survival on day 28 of treatment at a dose of 24 mg/kg × 4 days, while at 12 mg/kg × 4 days, 100% suppression of parasitaemia on day 4 was shown but provided poor protection in terms of survival of the treated mice. Compound 25 is twice as active as β-arteether by oral route.
Compound 22, the next best compound, showed 20% protection at 48 mg/kg × 4 days, while at 24 mg/kg × 4 days, 98.58% suppression of parasitaemia on day 4 was shown and provided poor protection in terms of survival of the treated mice. Compound 26 showed 80% protection at 96 mg/kg × 4 days dose, whereas only 89.43% suppression of parasitaemia on day 4 was observed at 48 mg/kg × 4 days dose. Compound 27 showed 100% suppression of parasitaemia on day 4 at 96 mg/kg and provided 40% protection in terms of survival of the treated mice. The compounds 22, 26, 27, and 30 were inactive at further lower doses. Activity results given in Table 1 and a comparative study in Figure 1(27,32) clearly indicated that aryl substitution at C-5 resulted in highly active 1,2,4-trioxanes where the best active compound, that is, compound 25, was twice as active as the reference drug β-arteether and 3.75 times more active as compared to the compound A (Figure 1) without C-5 aryl substitution. Biologically the most promising molecule of this series, compound 25 was assessed for its biopharmaceutical properties (Table 2). It could be categorized as a class II drug as per the Biopharmaceutical Classification System based on its low solubility and high permeability.33 It was found to be highly protein bound, 50% metabolically stable after 30 min on incubation with rat liver microsomes, and was not an inhibitor of rat CYPs 3A, 2D4, 1A2, or 2C11 (IC50 > 100 μM) (Supporting Information, Experimental Section). When compared to the reference drug, β-arteether, compound 25 showed a similar in vitro pharmacokinetic profile; however, it was found to be less metabolically stable. The biopharmaceutical properties obtained meet most of the criteria set by MMV Compound Progression Criteria 2008; thus, it may be a potential candidate for lead selection and optimization.34,35
Table 2. Biopharmaceutical Properties of Compound 25.
| drug-likeness property | compound 25 | β-arteether36 |
|---|---|---|
| solubility, distilled water (S) | 13 μM | practically insoluble |
| distribution coefficient (Log D), pH 7.4 | 4.23 | 3.6 |
| in situ permeability (Peff, cm/s) | 11.28 ± 1.68 × 10–5 | NA |
| plasma protein binding (% free) | 0.8 | 1–2 |
| metabolic stability, | ||
| half-life (T1/2, min) | 23.00 | 47.36 |
| clearance (CLint, μL/min/mg) | 60.26 | 36.58 |
In conclusion, the C-5 aryl and C-6 arylvinyl-substituted 1,2,4-trioxanes were synthesized using a simple methodology starting from self-condensation products of acetophenones, that is, homochalcones, in the least possible number of steps. Compound 25 was identified as the most active compound of the series, which is twice as active as β-arteether. The activity results demonstrated the importance of an aryl moiety at the C-5 position on the 1,2,4-trioxane pharmacophore. Further work on C-5-substituted 1,2,4-trioxanes with better aqueous solubility, oral bioavailability, and efficacy is under progress.
Acknowledgments
R.M., D.A., M.R., N.K.N., and A.S. are thankful to CSIR, New Delhi, for fellowship. We are thankful to the Anoop K. Srivastava for providing technical support during the course of work and SAIF-CDRI, Lucknow, for providing spectral data. The manuscript bears CDRI communication no. 8341.
Glossary
Abbreviations
- NaBH4
sodium borohydride
- THF
tetrahydrofuran
- CH3CN
acetonitrile
- CHCl3
chloroform
- PTSA
p-toulene sulfonic acid
Supporting Information Available
Detailed spectroscopic data, 1H and 13C NMR and mass spectra of synthesized compounds, and experimental procedures. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
Supplementary Material
References
- For reviews on artemisinin and its analogues, see the following:; Chaturvedi D.; Goswami A.; Pratim Saikia P.; Barua N. C.; Rao P. G. Artemisinin and its derivatives: A novel class of antimalarial and anti-cancer agents. Chem. Soc. Rev. 2010, 39, 435–454. [DOI] [PubMed] [Google Scholar]
- Muraleedharan K. M.; Avery M. A. Progress in the development of peroxide-based antiparasitic agents. Drug Discovery Today 2009, 14, 793–803. [DOI] [PubMed] [Google Scholar]
- Jefford C. W. New developments in synthetic peroxidic drugs as artemisinin mimics. Drug Discovery Today 2007, 12, 487–494. [DOI] [PubMed] [Google Scholar]
- Tang Y.; Dong Y.; Vennerstrom J. L. Synthetic peroxides as antimalarials. Med. Res. Rev. 2004, 24, 425–448. [DOI] [PubMed] [Google Scholar]
- Klayman D. L. Qinghaosu (artemisinin): An antimalarial drug from China. Science 1985, 228, 1049–1055. [DOI] [PubMed] [Google Scholar]
- WHO. 10 Facts on Malaria; www.who.int/features/factfiles/malaria.
- Luo X. D.; Shen C. C. The chemistry, pharmacology, and clinical applications of qinghaosu (artemisinin) and its derivatives. Med. Res. Rev. 1987, 7, 29–52. [DOI] [PubMed] [Google Scholar]
- Cumming J. N.; Ploypradith P.; Posner G. H. Antimalarial activity of artemisinin (qinghaosu) and related trioxanes: mechanism(s) of action. Adv. Pharmacol. 1997, 37, 253–297. [DOI] [PubMed] [Google Scholar]
- Bhattacharya A. K.; Sharma R. P. Recent developments on the chemistry and biological activity of artemisinin and related antimalarials–an update. Heterocycles 1999, 51, 1681–1745. [Google Scholar]
- Borstnik K.; Paik I. H.; Shapiro T. A.; Posner G. H. Antimalarial chemotherapeutic peroxides: artemisinin, yingzhaosu A and related compounds. Int. J. Parasitol. 2002, 32, 1661–1667. [DOI] [PubMed] [Google Scholar]
- Ploypradith P. Development of artemisinin and its structurally simplified trioxane derivatives as antimalarial drugs. Acta Trop. 2004, 89, 329–342. [DOI] [PubMed] [Google Scholar]
- O'Neill P. M.; Posner G. H. A medicinal chemistryperspective on artemisinin and related endoperoxides. J. Med. Chem. 2004, 47, 2945–2964. [DOI] [PubMed] [Google Scholar]
- Asthana O. P.; Srivastava J. S.; Valecha N. Current status of the artemisinin derivatives in the treatment of malaria with focus on arteether. J. Parasitic Dis. 1997, 211, 1–12. [Google Scholar]
- Jambou. R.; Legrand E.; Niang M.; Khim N.; Lim P.; Volney B.; Ekala M. T.; Bouchier C.; Esterre P.; Fandeur T.; Mercereau-Puijalon O. Resistance of Plasmodium falciparum field isolates to in vitro artemether and point mutations of the SERCA-type PfATPase6. Lancet 2005, 366, 1960–1963. [DOI] [PubMed] [Google Scholar]
- Peters W.; Robinson B. L.; Tovey G.; Rossier J. C.; Jefford C. W. The chemotherapy of rodent malaria. XLVIII. The activities of some synthetic 1,2,4-trioxanes against chloroquine-sensitive and chloroquine-resistant parasites. Part 1: Studies leading to the development of novel cis-fused cyclopenteno derivatives. Ann. Trop. Med. Parasitol. 1993, 87, 1–7. [DOI] [PubMed] [Google Scholar]
- Peters W.; Robinson B. L.; Rossier J. C.; Misra D.; Jefford C. W.; Rossiter J. C. The chemotherapy of rodent malaria. XLIX. The activities of some synthetic 1,2,4-trioxanes against chloroquine-sensitive and chloroquine-resistant parasites. Part 2: Structure-activity studies on cis-fused cyclopenteno-1,2,4-trioxanes (fenozans) against drug-sensitive and drug-resistant lines of Plasmodium berghei and P. yoelii ssp. NS in vivo. Ann. Trop. Med. Parasitol 1993, 87, 9–16. [DOI] [PubMed] [Google Scholar]
- Peters W.; Robinson B. L.; Tovey G.; Rossier J. C.; Jefford C. W. The chemotherapy of rodent malaria. L. The activities of some synthetic 1,2,4-trioxanes against chloroquine- sensitive and chloroquine-resistant parasites. Part 3: Observations on 'Fenozan-50F', a difluorinated 3,3′-spirocyclopentane 1,2,4- trioxane. Ann. Trop. Med. Parasitol. 1993, 87, 111–123. [DOI] [PubMed] [Google Scholar]
- Kepler J. A.; Philip A.; Lee Y. W.; Morey M. C.; Carroll F. I. 1,2,4-Trioxanes as potential antimalarial agents. J. Med. Chem. 1988, 31, 713–716. [DOI] [PubMed] [Google Scholar]
- Posner G. H.; Maxwell J. P.; O'Dowd H.; Krasavin M.; Xie S.; Shapiro T. A. Antimalarial sulfide, sulfone, and sulfonamide trioxanes. Bioorg. Med. Chem. 2000, 8, 1361–1370. [DOI] [PubMed] [Google Scholar]
- Posner G. H.; Jeon H. B.; Parker M. H.; Krasavin M.; Paik I.-H.; Shapiro T. A. Antimalarial simplified 3-aryltrioxanes: Synthesis and preclinical efficacy/toxicity testing in rodents. J. Med. Chem. 2001, 44, 3054–3058. [DOI] [PubMed] [Google Scholar]
- Posner G. H.; Jeon H. B.; Polypradith P.; Paik I.-H.; Borstnik K.; Xie S.; Shapiro T. A. Orally active, water-soluble antimalarial 3-aryltrioxanes: Short synthesis and preclinical efficacy testing in rodents. J. Med. Chem. 2002, 45, 3824–3828. [DOI] [PubMed] [Google Scholar]
- O'Neill P. M.; Mukhtar A.; Ward S. A.; Bickley J. F.; Davies J.; Bachi M. D.; Stocks P. A. Application of thiol-olefin cooxygenation methodology to a new synthesis of the 1,2,4-trioxane pharmacophore. Org. Lett. 2004, 6, 3035–3038. [DOI] [PubMed] [Google Scholar]
- Cabaret O. D.; Vical F. B.; Loup C.; Robert A.; Gornitzka H.; bonhoure A.; Vial H.; Magnaval J. F.; Seguela J- P.; Meunier B. Synthesis and antimalarial activity of trioxaquines derivatives. Chem.—Eur. J. 2004, 10, 1625–1636. [DOI] [PubMed] [Google Scholar]
- Slack R. D.; Jacobine A. M.; Gary G. H. Antimalarial peroxides: Advances in drug discovery and design. Med. Chem. Commun. 2012, 3, 281–297. [Google Scholar]
- Singh C.; Malik H.; Puri S. K. Orally Active 1, 2, 4-Trioxanes: Synthesis and Antimalarial Assessment of a New Series of 9-Functionalized 3-(1-Arylvinyl)-1,2,5-trioxaspiro[5.5]undecanes against Multi-Drug-Resistant Plasmodium yoelii nigeriensis in Mice. J. Med. Chem. 2006, 49, 2794–2803. [DOI] [PubMed] [Google Scholar]
- Singh C.; Verma V. P.; Naikade N. K.; Singh A. S.; Hassam M.; Puri S. K. Novel Bis- and Tris-1,2,4-trioxanes: Synthesis and Antimalarial Activity against Multidrug-Resistant Plasmodium yoelii in Swiss Mice. J. Med. Chem. 2008, 51, 7581–7592. [DOI] [PubMed] [Google Scholar]
- Singh C.; Gupta N.; Puri S. K. Synthesis of new 6-alkylvinyl/arylalkylvinyl substituted 1,2,4-trioxanes active against multidrug-resistant malaria in mice. Bioorg. Med. Chem. 2004, 12, 5553–5562. [DOI] [PubMed] [Google Scholar]
- Singh C.; Malik H.; Puri S. K. Orally active amino functionalized antimalarial 1,2,4-trioxanes. Bioorg. Med. Chem. Lett. 2004, 14, 459–462. [DOI] [PubMed] [Google Scholar]
- Singh C.; Kanchan R.; Srivastava D.; Puri S. K. 8-(1-Naphthalen-2-yl-vinyl)-6,7,10-trioxaspiro (4.5) decane, a new 1,2,4-trioxane effective against rodent and simian malaria. Bioorg. Med. Chem. Lett. 2006, 16, 584–586. [DOI] [PubMed] [Google Scholar]
- Singh C.; Hassam M.; Naikade N. K.; Verma V. P.; Singh A. S.; Puri S. K. Synthesis and Antimalarial Assessment of a New Series of Orally Active Amino-Functionalized Spiro 1,2,4-Trioxanes. J. Med. Chem. 2010, 53, 7587–7598. [DOI] [PubMed] [Google Scholar]
- Griesbeck A. G.; El-Idreesy T. T.; Fiege M.; Brun R. Synthesis of Antimalarial 1,2,4-Trioxanes via Photooxygenation of a chiral allylic alcohol. Org. Lett. 2002, 4, 4193–4195. [DOI] [PubMed] [Google Scholar]
- Griesbeck A. G.; El-Idreesy T. T.; Lex J. Singlet oxygen addition to chiral allylic alcohols and subsequent peroxyacetalization with β-naphthaldehyde: Synthesis of diastereomerically pure 3-β-naphthyl-substituted 1,2,4-trioxanes. Tetrahedron 2006, 62, 10615–10622. [Google Scholar]
- Griesbeck A. G.; El-Idreesy T. T.; Höinck L.-O.; Lex J.; Brun R. Novel spiroanellated 1,2,4-trioxanes with high in vitro antimalarial activities. Bioorg. Med. Chem. Lett. 2005, 15, 595–597. [DOI] [PubMed] [Google Scholar]
- Sabbani S.; Pensée L. L.; Bacsa J.; Hedenström E.; O'Neill P. M. Diastereoselective schenck ene reaction of singlet oxygen with chiral allylic alcohols; access to enantiomerically enriched 1,2,4-trioxanes. Tetrahedron 2009, 65, 8531–8537. [Google Scholar]
- Hu Z.; Liu J.; Li G.; Dong Z.; Li W. Synthesis of Asymmetric Triarylbenzenes by Using SOCl2-C2H5OH Reagent. J. Chin. Chem. Soc. 2004, 51, 581–584. [Google Scholar]
- Arai N.; Azuma K.; Nii N.; Ohkuma T. Highly Enantioselective Hydrogenation of Aryl Vinyl Ketones to Allylic Alcohols Catalyzed by the Tol-Binap/Dmapen Ruthenium (II) Complex. Angew. Chem., Int. Ed. 2008, 47, 7457–7460. [DOI] [PubMed] [Google Scholar]
- Peter's procedure: 100% suppression of parasitaemia means that the number of parasites, if at all present, is below the detection limit. The parasites present below the detection limit can multiply and eventually can be detected. In such cases, although the drug is providing near 100% suppression of the parasitemia, it will not provide full protection to the treated mice. Multidrug-resistant P. yoelii nigeriensis used in this study is resistant to chloroquine, mefloquine, and halofantrine.
- One hundred percent protection means that all of the treated mice survive till day 28. Similarly, 60% protection means only 60% of the treated survive till day 28.
- Singh C.; Misra D.; Saxena G.; Chandra S. In vivo potent antimalarial 1,2,4-trioxanes: synthesis and activity of 8-(α-arylvinyl)-6,7,10-trioxaspiro[4,5]decanes and 3-(α-arylvinyl)-1,2,5-trioxaspiro[5,5]undecanes against Plasmodium berghei in mice. Bioorg. Med. Chem. Lett. 1995, 5, 1913–1916. [Google Scholar]
- Ku M. S. Use of the Biopharmaceutical Classification System in Early Drug Development. AAPS J. 2008, 10, 208–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medicines For Malaria Venture (MMV). MMV Compound Progression Criteria; August, 2008; 8 pp.
- Bosman A.; Malaria R. B.. Review of Application for Inclusion of a Drug in the WHO Essential Drugs List, 2002; http://archives.who.int/eml/expcom/expcom12/arteether_review.doc.
- Grace J. M.; Aguilar A. J.; Trotman K. M.; Brewer T. G. Metabolism of β-arteether to dihydroqinghaosu by human liver microsomes and recombinant cytochrome P450. Drug Metab. Dispos. 1998, 264313–317. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.