Abstract
Antiangiogenic agents (AA) are cytostatic, and their utility in cancer chemotherapy lies in their combination with cytotoxic chemotherapeutic agents. Clinical combinations of vascular endothelial growth factor receptor-2 (VEGFR2) inhibitors with antitubulin agents have been particularly successful. We have discovered a novel, potentially important analogue, that combines potent VEGFR2 inhibitory activity (comparable to that of sunitinib) with potent antitubulin activity (comparable to that of combretastatin A-4 (CA)) in a single molecule, with GI50 values of 10–7 M across the entire NCI 60 tumor cell panel. It potently inhibited tubulin assembly and circumvented the most clinically relevant tumor resistance mechanisms (P-glycoprotein and β-III tubulin expression) to antimicrotubule agents. The compound is freely water-soluble as its HCl salt and afforded excellent antitumor activity in vivo, superior to docetaxel, sunitinib, or Temozolomide, without any toxicity.
Keywords: Antitubulin, antimitotic, antiangiogenic, VEGFR2 inhibition, combination chemotherapy
Antiangiogenic agents (AA) have defined a new paradigm for cancer treatment.1 The principal mediator of angiogenesis is the vascular endothelial growth factor (VEGF) and its receptor tyrosine kinase (RTK) VEGFR2.2 Rapid regrowth of vasculature in tumors after the removal of VEGFR2 inhibitors from the treatment regimen attests to their cytostatic mechanism.3 VEGFR2 inhibitors are known to cause regression of impaired tumor blood vessels but transiently normalize the surviving vessels, which have less leakiness and are more like normal blood vessels. Such treatment therefore can transiently increase blood flow in the surviving vasculature.4 Administering a cytotoxic agent during this transient tumor vasculature normalization provides improved drug delivery to the tumor, dependent on the surviving vasculature. However, administering separate antiangiogenic and cytotoxic agents may miss the timing window. Single entities with both antiangiogenic and cytotoxic components should allow the cytotoxicity to be manifested even at low concentrations in the tumor as soon as the antiangiogenic effect and vasculature normalization occur. Hence, these agents might not need the cytotoxic component to be as potent as conventional cytotoxic drugs and as such could perhaps avoid or reduce dose-limiting toxicities associated with conventional chemotherapy. Additionally, such single agents could circumvent pharmacokinetic problems of multiple agents, avoid drug–drug interactions, and be used at lower doses to minimize toxicities and tumor cell resistance.
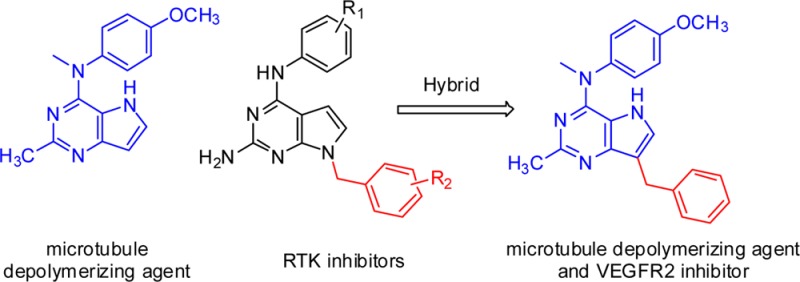
It has been our long-standing interest to design single entities with multiple targets.5−8 We chose inhibition of tubulin as the mechanism for the cytotoxic effect because antitubulin agents are some of the most effective drugs in cancer chemotherapy.9 Additionally, evidence for the remarkable clinical success of VEGFR2 inhibitors in combination with antitubulin agents is abundant in the literature.10 Structural similarities of antitubulin11 agents and RTK inhibitors12 previously reported from our laboratory provided the rationale to incorporate structural features of both antitubulin activity and RTK inhibitory activity in single molecules to explore the possibility of providing dual inhibitory activities in single compounds. We11 previously reported the design, synthesis, and antitumor activity of pyrrolo[3,2-d]pyrimidine (1; Figure 1), which inhibits tubulin assembly. Substituted 7-benzyl pyrrolo[2,3-d]pyrimidines with general structure 2 (Figure 1) were reported as antiangiogenic, antimetastatic, and antitumor agents.9 Substituents on the benzyl group and the location of the benzyl group dictate RTK inhibitory activity in pyrrolo[2,3-d]pyrimidines. Hence, having determined the antitubulin effects of 1, it was of interest to engineer RTK inhibitory activity without loss of antitubulin activity by incorporating the 7-benzyl group onto the pyrrolo[3,2-d]pyrimidine scaffold. The results of this hybrid design afforded compound 3 (Figure 1). Compounds 4 and 5 were designed to evaluate the importance of the 4′-OCH3 and the 4-NCH3 moieties, respectively. Compound 6 was designed by incorporating a 2′-OCH3 group to explore the effect of substituents at this position. The antimitotic activity of 3 also prompted its evaluation against cell lines which overexpress the multidrug resistance protein P-glycoprotein (Pgp) and the β-tubulin isoform βIII. Tumors with either of these proteins are associated with significant resistance to antitubulin agents, especially with resistance to paclitaxel.13
Figure 1.
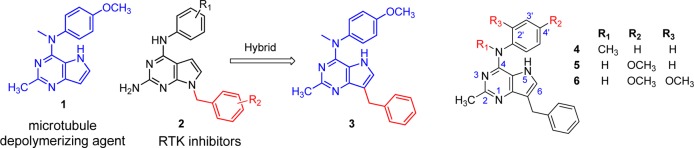
Hybrid design from lead compounds.
Acknowledgments
We thank the NCI for performing the in vitro antitumor evaluation in their 60 tumor preclinical screening program.
Glossary
Abbreviations
- AA
antiangiogenic agents
- VEGFR2
vascular endothelial growth factor receptor-2
- RTK
receptor tyrosine kinase
- Pgp
P-glycoprotein
- CAM
chicken chorioallantoic membrane
- CA4P
combretastatin A-4 phosphate
- BLBCs
basal-like breast cancer cells
Supporting Information Available
Tumor cell inhibitory activity (NCI) GI50 (nM) of 3·HCl, and the Experimental Section. This material is available free of charge via the Internet at http://pubs.acs.org.
This work was supported, in part, by NIH Grants CA136944 (to A.G.), CA114021 (to A.G.), the Duquesne University Adrian Van Kaam Chair in Scholarly Excellence (to A.G.), and NSF equipment grant CHE 0614785 (for the NMR).
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Young R. J.; Reed M. W. Anti-angiogenic therapy: Concept to clinic. Microcirculation 2012, 19, 115–25. [DOI] [PubMed] [Google Scholar]
- Shibuya M. Vascular endothelial growth factor and its receptor system: Physiological functions in angiogenesis and pathological roles in various diseases. J. Biochem. 2013, 153, 13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergers G.; Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat. Rev. Cancer 2008, 8, 592–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain R. K. Normalizing tumor microenvironment to treat cancer: Bench to bedside to biomarkers. J. Clin. Oncol. 2013, 31, 2205–2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangjee A.; Zaware N.; Raghavan S.; Ihnat M.; Shenoy S.; Kisliuk R. L. Single agents with designed combination chemotherapy potential: Synthesis and evaluation of substituted pyrimido[4,5-b]indoles as receptor tyrosine kinase and thymidylate synthase inhibitors and as antitumor agents. J. Med. Chem. 2010, 53, 1563–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangjee A.; Zhao Y.; Ihnat M. A.; Thorpe J. E.; Bailey-Downs L. C.; Kisliuk R. L. Novel tricyclic indeno[2,1-d]pyrimidines with dual antiangiogenic and cytotoxic activities as potent antitumor agents. Bioorg. Med. Chem. 2012, 20, 4217–4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangjee A.; Li W.; Lin L.; Zeng Y.; Ihnat M.; Warnke L. A.; Green D. W.; Cody V.; Pace J.; Queener S. F. Design, synthesis, and X-ray crystal structures of 2,4-diaminofuro[2,3-d]pyrimidines as multireceptor tyrosine kinase and dihydrofolate reductase inhibitors. Bioorg. Med. Chem. 2009, 17, 7324–7336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangjee A.; Zeng Y.; Ihnat M.; Warnke L. A.; Green D. W.; Kisliuk R. L.; Lin F.-T. Novel 5-substituted, 2,4-diaminofuro[2,3-d]pyrimidines as multireceptor tyrosine kinase and dihydrofolate reductase inhibitors with antiangiogenic and antitumor activity. Bioorg. Med. Chem. 2005, 13, 5475–5491. [DOI] [PubMed] [Google Scholar]
- Dumontet C.; Jordan M. A. Microtubule-binding Agents: A dynamic field of cancer therapeutics. Nat. Rev. Drug Discovery 2010, 9, 790–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- www.clinicaltrials.gov.
- Gangjee A.; Pavana R. K.; Li W.; Hamel E.; Westbrook C.; Mooberry S. L. Novel water-soluble substituted pyrrolo[3,2-d]pyrimidines: design, synthesis, and biological evaluation as antitubulin antitumor agents. Pharm. Res. 2012, 29, 3033–3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangjee A.; Zaware N.; Raghavan S.; Yang J.; Thorpe J. E.; Ihnat M. A. N(4)-(3-Bromophenyl)-7-(substituted benzyl) pyrrolo[2,3-d]pyrimidines as potent multiple receptor tyrosine kinase inhibitors: design, synthesis, and in vivo evaluation. Bioorg. Med. Chem. 2012, 20, 2444–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly A.; Cabral F. New insights into mechanisms of resistance to microtubule inhibitors. Biochim. Biophys. Acta 2011, 1816, 164–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott A. J.; Morris P. E.; Petty S. L.; Williams C. H. An improved synthesis of 7-substituted pyrrolo[3,2-d]pyrimidines. J. Org. Chem. 1997, 62, 8071–8075. [DOI] [PubMed] [Google Scholar]
- Luceome Biotechnologies, 1775 S. Pantano Rd, Suite 100, Tucson, AZ 85710.
- Chambers A. F. MDA-MB-435 and M14 cell lines: Identical but not M14 melanoma?. Cancer Res. 2009, 69, 5292–5293. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


